Abstract
6-Formylindolo[3,2-b]carbazole (FICZ) is a potent aryl hydrocarbon receptor (AHR) agonist that is efficiently metabolized by AHR-regulated cytochrome P4501 enzymes. FICZ is a proposed physiological AHR ligand that induces its own degradation as part of a regulatory negative feedback loop. In vitro studies in cells show that CYP1 inhibition in the presence of FICZ results in enhanced AHR activation, suggesting that FICZ accumulates in the cell when its metabolism is blocked. We used zebrafish (Danio rerio) embryos to investigate the in vivo effects of FICZ when CYP1A is knocked down or inhibited. Embryos were injected with morpholino antisense oligonucleotides targeting CYP1A (CYP1A-MO), Ahr2, or a combination of both. FICZ exposure of non-injected embryos or embryos injected with control morpholino had little effect. In CYP1A-MO-injected embryos, however, FICZ dramatically increased mortality, incidence and severity of pericardial edema and circulation failure, reduced hatching frequency, blocked swim bladder inflation, and strongly potentiated expression of Ahr2-regulated genes. These effects were substantially reduced in embryos with a combined knockdown of Ahr2 and CYP1A, indicating that the toxicity was mediated at least partly by Ahr2. Co-exposure to the CYP1 inhibitor alpha-naphtoflavone and FICZ had similar effects as the combination of CYP1A-MO and FICZ. HPLC analysis of FICZ-exposed embryos showed increased levels of FICZ after concomitant CYP1A-MO injection or αNF co-exposure. Together, these results show that a functioning CYP1/AHR feedback loop is crucial for regulation of AHR signaling by a potential physiological ligand in vivo and further highlights the role of CYP1 enzymes in regulating biological effects of FICZ.
Keywords: Aryl hydrocarbon receptor; Cytochrome P4501; 6-Formylindolo[3,2-b]carbazole; enzyme inhibition; zebrafish embryo toxicity; synergistic receptor activation
1. Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that regulates expression of many genes including those for the cytochrome P450 family 1 (CYP1; CYP1A, CYP1B, and CYP1C) enzymes. Both AHR itself and the CYP1 enzymes have been highly conserved during vertebrate evolution [1, 2], suggesting that these systems have conserved physiological functions. This is further corroborated by the presence of AHR in most cell types and during most phases of development [3, 4]. Numerous reports suggest the existence of physiological AHR agonist(s) and suggest that AHR-induced CYP1 activity is important for down-regulating AHR activity by metabolically depleting the agonist, generating a tightly regulated negative feedback loop of receptor signaling. In support of this suggestion, mutant cells lacking functional CYP1A1 enzyme activity and CYP1 triple knockout mice (cyp1a1/1a2/1b1−/−) show elevated AHR activation and CYP1 transcription [5-13]. Together these data suggest that CYP1-inhibiting agents may disrupt physiological AHR signaling by interfering with CYP1/AHR feedback regulation, causing accumulation of endogenous agonist(s) and thus a subsequent prolonged and/or potentiated AHR activation.
The endogenous and proposed physiological AHR ligand 6-formylindolo[3,2-b]carbazole (FICZ) is a high-potency AHR agonist that also has the qualities of a perfect substrate for mammalian CYP1 enzymes, resulting in efficient auto-regulatory feedback of its action and thereby only transient activation of AHR signaling in cell systems [14]. Such transient activation, in contrast to sustained activation by the highly toxic persistent pollutant 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD), may be essential for physiological AHR signaling [15]. The ability of FICZ to activate AHR signaling appears to be evolutionarily conserved. FICZ has been shown to bind and activate AHRs in different vertebrate species in addition to human, including mouse, African clawed frog (Xenopus laevis), zebrafish (Danio rerio) and several avian species [16-20]. Furthermore, recent studies suggest that FICZ-AHR and TCDD-AHR interactions are distinct. For example, avian species known to differ in response to TCDD show no significant differences in sensitivity to FICZ [20], and FICZ differs from TCDD in its ability to bind AHR variants with certain mutated amino acids in the ligand-binding domain [21]. Such results emphasize the robustness of the FICZ-AHR interaction and further suggest that FICZ could have a role as an important natural signaling molecule.
Previously, we described how various agents inhibiting the catalytic activity of CYP1A1 enzymes may generate prolonged and potentiated AHR activation in a human cell line in vitro, by compromising the cellular clearance of FICZ [14, 22, 23]. In line with these reports, a recent paper by Jönsson et al [19] demonstrated an increased AHR activation and enhanced toxicity in chicken embryos after co-exposure to FICZ and ketoconazole, a known inhibitor of CYP1 enzyme activity. However, ketoconazole can inhibit many CYPs, and those studies did not determine whether the observed toxicity was dependent on the AHR; this is a primary aim of the current study. Considering the crucial role of CYP1 enzymes in regulating AHR activation by FICZ in vitro, we hypothesized that the otherwise non-toxic AHR agonist FICZ may cause AHR-dependent toxicity if its metabolic clearance is blocked. To address this, we studied FICZ-mediated phenotypic and transcriptional effects of CYP1A loss of function in zebrafish embryos using morpholino-mediated knockdown of CYP1A (CYP1A-MO), Ahr2 (Ahr2-MO), or combined knockdown of CYP1A and Ahr2 (CYP1A/Ahr2-MO). Alpha-naphthoflavone (αNF), a potent CYP1 inhibitor with IC50 values in the nM range for human CYP1 enzymes [24], was used to investigate effects of chemical inhibition of CYP1. αNF is also considered a weak AHR agonist and partial AHR antagonist [25-28]. Our results show that a functioning CYP1/AHR feedback loop is crucial for regulating biological effects of FICZ in zebrafish embryos in vivo and further support the hypothesis that CYP1 enzymes function to regulate endogenous AHR ligands and receptor activity.
2. Material and methods
2.1. Chemicals
Dimethyl sulfoxide (DMSO) was obtained from Acros Organics (Thermo Fisher Scientific, Pittsburgh, PA, USA) and Sigma-Aldrich (Stockholm, Sweden). FICZ (≥95%) was obtained from Enzo Life Sciences (Farmingdale, NY, USA) and Syntastic AB (Stockholm, Sweden), and αNF (≥98%) was from Sigma-Aldrich (Stockholm, Sweden). Acetonitrile (VWR, Stockholm Sweden) and formic acid (Sigma-Aldrich, Stockholm, Sweden) were of HPLC grade.
2.2. Zebrafish breeding
Zebrafish were maintained under standard light and temperature conditions: 14 h of light, 10 h of dark, at 28.5 °C, with water quality monitored daily. Experiments were performed at two different locations; Woods Hole Oceanographic Institution (morpholino studies) and Karolinska Institutet (αNF studies); hence, zebrafish with two different origins were used. However, exposure regimens were constant throughout all experiments and non-fertilized eggs were removed within the first 10 hours in both settings. Fish embryos that have hatched but not started feeding are technically eleutheroembryos, but for simplicity, in this paper we refer to zebrafish at all stages before 6 dpf as embryos.
Morpholino (MO) studies
For all CYP1A-MO and Ahr2-MO experiments zebrafish from the Tupfel/long fin (TL) mutation wild-type strain were used. Embryos were collected from group matings of at least 30 females and 15 males per tank, and several tanks were used during each breeding event. Eggs were maintained in 0.3× Danieau’s solution (17 mM NaCl, 2 mM KCl, 0.12 mM MgSO4, 1.8 mM Ca(NO3)2, and 1.5 mM HEPES buffer).
α NF studies
All experiments with αNF +/− FICZ were performed using zebrafish from the AB wild type strain. Embryos were collected from group matings of 3 females and 3 males per tank, and only one tank was used during each breeding event. Eggs were maintained in E3 embryo medium (5.0 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4).
2.3. Morpholino mediated knockdown
Zebrafish embryos were injected with morpholino antisense oligonucleotides blocking CYP1A or Ahr2 translation, or with a combination of both morpholinos, at the 1-4 cell stage as previously described [29]. Morpholinos targeting the transcriptional start site of CYP1A (CYP1A-MO; 5- TGGATACTTTCCAGTTCTCAGCTCT -3) [30] or Ahr2 (Ahr2-MO; 5- TGTACCGATACCCGCCGACATGGTT-3) [31] and control morpholinos (Ctrl-MO; 5- CCTCTTACCTCAGTTACAATTTATA-3) were obtained from Gene Tools (Philomath, OR, USA). The morpholinos were diluted in sterile-filtered deionized water to 0.15 mM (CYP1A-MO), 0.18 mM (Ahr2-MO) and 0.15/0.18 mM (Ctrl-MO) respectively. For injection of the combination of CYP1A-MO and Ahr2-MO morpholinos with twice the concentration was prepared. A Narishige IM-300 microinjector (Narishige, Tokyo, Japan) with a fine glass needle was used to inject 3-5 nL of morpholinos into the yolk of the embryos. All morpholinos were fluorescein-tagged and embryos were screened at 6-8 hpf by fluorescence microscopy to verify successful incorporation. Any damaged embryos or those not displaying homogenous fluorescence were removed. In addition to the MO-injected groups, groups of non-injected (NI) embryos were used.
2.4. Exposure and sampling
All exposures were initiated at 24 hours post fertilization and performed in glass petri dishes. Groups of embryos, with or without concomitant morpholino treatment, were exposed to various concentrations of FICZ (0.001 to 100 nM), αNF (0.1 μM to 2.5μM), combinations of FICZ and αNF, or corresponding amount of vehicle (0.01% DMSO, v/v) at a density of 1 embryo / ml Danieau’s solution (0.3×) or E3 medium. Exposures were started by adding the compound directly into the water at a position distant from the eggs and then immediately mixed through gentle but thorough swirling and the embryos were kept in the same exposure medium throughout the experiment. An additional experiment where the exposure medium was replenished every 8 h was included for comparison. For morphological assessment, embryos were scored for edema and circulation failure using a stereomicroscope as described below. Samples for quantitative real time PCR (qRT-PCR) analysis were collected as group replicates composed of 7-10 pooled embryos at indicated time points and stored in RNAlater (Ambion Inc., Austin, TX, USA) at 4°C for up to a week then at -20°C until RNA extraction. Samples for chemical analysis using high performance liquid chromatography (HPLC) were collected as group replicates of 10 pooled embryos in 1.5 ml centrifuge tubes and washed once with clean E3 medium. The E3 medium was aspirated and the samples were stored at − 20°C until extraction and analysis.
2.5. Morphological assessment
Dead embryos were counted and discarded every day, and cumulative mortality was calculated at 2 or 3 days post treatment (dpt).
Morpholino experiments
At 1-3 dpt embryos were examined for phenotypic effects using a Zeiss Stemi 2000-CS microscope (Zeiss, Thornwood, NY, USA). Severity of pericardial edema and circulatory failure was determined at 2 or 3 dpt (3 or 4 dpf), and hatching frequency and swim bladder inflation was determined at 3 dpt. Severity of pericardial edema and circulatory failure was scored between 0-3 with “0” being no effect and “3” being a severe effect (see Figure 1D). Severity of circulatory failure is defined as different degrees of reduced heart rate and circulation of blood cells through the trunk vessels. Frequency of hatching, swim bladder inflation and mortality was determined as the percentage of embryos showing those effects compared to the total number at exposure start. For the dose-response study (Figure 3) exposure vessels were randomized in order to avoid subjective scoring. αNF experiments: At 3 dpt (4 dpf) embryos were sedated using tricaine (150 mg/ml; Sigma-Aldrich, Stockholm, Sweden), photographed using a Leica MZ16F microscope and Leica Application Suite V4.1. (Leica, Stockholm, Sweden) and scored for incidence of pericardial and/or yolk sac edema and swim bladder inflation. The incidence of edema and swim bladder inflation was determined as the percentage of embryos showing those effects compared to the total number at exposure start. Other effects such as craniofacial malformations, tube-shaped heart and reduced body length was noted but not scored.
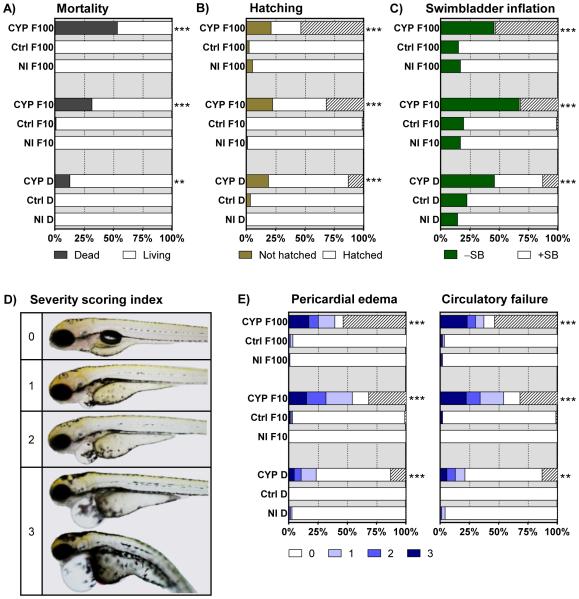
Figure 1. Impact of CYP1A knockdown on FICZ-mediated effects on mortality and phenotype.
Groups of embryos, non-injected (NI) or injected with Ctrl-MO (Ctrl) or CYP1A-MO (CYP), were exposed to vehicle control (DMSO; D), or 10 or 100 nM FICZ (F10, F100). All endpoints were determined at 3 dpt (4 dpf); A) cumulative mortality, B) hatching rates, C) swim bladder inflation and E) rates of pericardial edema and circulatory failure. Severity of pericardial edema and circulatory failure was graded 0-3, “0” representing no effect and “1-3” representing effects of increasing severity. The severity scoring index for pericardial edema is shown in D). In B), C) and E) the hatched part of a bar indicates the mortality rate. Results are shown as % of total number of embryos at exposure start based on three separate experiments (n=69-99). Statistically significant differences in numbers of affected and unaffected embryos between the MO injected groups (Ctrl or CYP) and the corresponding NI group were determined by Fisher's exact test with Bonferroni's correction and are shown by asterisks (** p<0.01 and *** p<0.001). For the statistical analysis of data in E) “affected embryos” are represent by the pooled number of all living embryos with severity score 1-3 in a group.
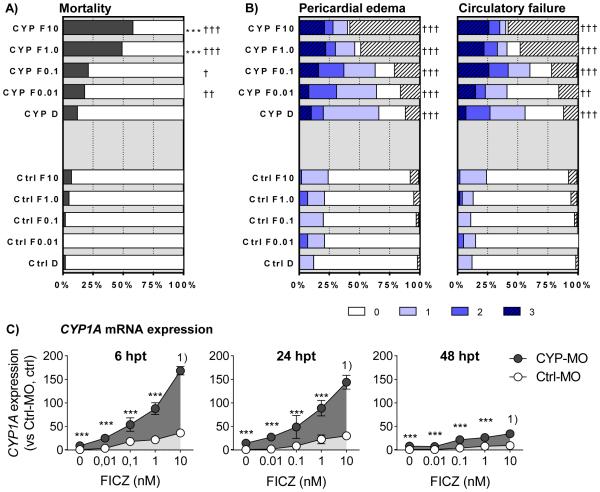
Figure 3. FICZ concentration dependent phenotypic and transcriptional effects.
Groups of embryos, injected with Ctrl-MO (Ctrl or Ctrl-MO) or CYP1A-MO (CYP or CYP-MO), were exposed to vehicle control (DMSO; D or 0) or various concentrations of FICZ (0.01 to 10 nM; F0.01, F0.1, F1.0, and F10). Exposure vessels were randomized in order to avoid subjective scoring of the different dose-groups. At 48 hpt rates of A) cumulative mortality and B) pericardial edema (PE) and circulatory failure (CF) were determined. Severity of PE and CF was scored as in Fig. 1D. In B) the hatched part of a bar indicates the mortality rate. Levels of CYP1A transcription were determined at 6, 24, and 48 hpt (C). Data in A) and B) are shown as % of total number of embryos at exposure start based on three separate experiments (n=75-105) and statistically significant differences were determined by Fisher's exact test with Bonferroni's correction. Asterisks indicate differences between DMSO control and FICZ-exposed groups of CYP1A-MO injected embryos (*** p<0.001). Daggers designate differences between the Ctrl-MO and CYP1A-MO injected groups at the same FICZ concentration († p<0.05, †† p<0.01, and ††† p<0.001). In Figure C) results are shown as means ± SEM of data from 2 or 3 pooled experiments (n= 4-8, except for the Ctrl-MO injected groups treated with 10 nM FICZ, which were composed of 2 replicates each). Relative levels of CYP1A transcription was calculated using l13 as a reference gene and the Ctrl-MO injected DMSO controls at each time-point as calibrators. Differences in transcription between Ctrl-MO and CYP1A-MO injected groups at the same FICZ concentration were determined using One-way ANOVA followed by Bonferroni’s post-hoc test with selected pairs and with log transformed data. Statistically significant differences are shown by asterisks (*** p<0.001). Footnote: 1) These groups were not statistically analyzed since n=2 in the Ctrl-MO groups.
2.6. RNA purification and quantitative real time RT-PCR
Total RNA was isolated using the Aurum™ Total RNA Fatty and Fibrous Tissue kit followed by cDNA synthesis using the iScript cDNA Synthesis kit, both from Bio-Rad (Stockholm, Sweden), according to the manufacturer’s instructions. The concentration and quality of RNA was determined spectrophotometrically using a NanoDrop 1000 (Thermo Scientific, Stockholm, Sweden). Quantification of gene expression was performed using Power SYBR® Green qPCR Master Mix (Thermo Fisher Scientific, Stockholm, Sweden) with detection on an Applied Biosystems 7500 Real-Time PCR system (Thermo Fischer Scientific, Stockholm, Sweden). Relative gene expression quantification was based on the comparative threshold cycle method (2−ΔΔCt). l13 was chosen as reference gene based on comparisons of temporal- and exposure dependent effects on expression of commonly used housekeeping genes (not shown). Quantitative real-time PCR (qRT-PCR) analysis was performed with the following protocol: 95 °C for 10 min, followed by 35-40 cycles of 95 °C for 15 s and 62 °C for 45 s. Melt curve analysis was performed at the end of each PCR run to ensure that a single product was amplified. Gene-specific primers for qRT-PCR were synthesized by Sigma-Aldrich (Stockholm, Sweden) and sequences for the gene-specific primers are given in table 1.
Table 1.
Zebrafish specific primer sequences used in the study.
| Gene | Sequence |
|---|---|
| zfCYP1A1 | F: 5’-GCATTACGATACGTTCGATAAGGAC |
| R: 5’-GCTCCGAATAGGTCATTGACGAT | |
| zfCYP1C1 | F: 5’-AGTGGCACAGTCTACTTTGAGAG |
| R: 5’-TCGTCCATCAGCACTCAG | |
| zfCYP1C2 | F: 5’-GTGGTGGAGCACAGACTAAG |
| R: 5’-TTCAGTATGAGCCTCAGTCAAAC | |
| zfAHRRA | F: 5’-AGAACCAGCAGGACACACCA |
| R: 5’-TGGTGGAAGCCCAGGTAATCA | |
| zfAHRRB | F: 5’-AGGACACTGATGAGGATGTTGTG |
| R: 5’-GTAAGAAACGGAGTCAATTCAGGAG | |
| zfL13 | F: 5’-GCTAAGGACGGAGTGAACAAC |
| R: 5’-GCACTCTCTTCTGCCAGTC |
2.7 Extraction and quantification of FICZ
Deionized sterile water (200 μl) was added to each sample and they were then homogenized by sonication on ice (4 × 5 seconds) using a MSE Soniprep 150 equipped with an exponential probe (Measuring and Scientific Equipment, London, UK). The embryo homogenates were extracted by adding acetonitrile (300 μl) and vortexing each sample tube for 20 seconds followed by centrifugation at 13 000 rpm for 15 minutes at 4°C. Chemical analysis of the supernatants was performed using an YL9100 HPLC system equipped with an Agilent 1200 fluorescence detector (both from Dalco ChromTech, Stockholm, Sweden). Separations were achieved on a 150-mm long, 5 μm particle size reverse phase ACE C18-AR column (Scantec Lab, Partille, Sweden) using a 25-min linear mobile phase gradient from 40% B to 100% B (A, 1.5 mM formic acid in H2O; B, 1.5 mM formic acid in acetonitrile) at a flow rate of 0.8 mL/min. The fluorescence of FICZ was determined at excitation/emission wavelengths of 390/525 nm and quantified according to a standard curve of FICZ.
2.8. Statistics
Statistical analysis was performed using Prism 5 from GraphPad Software Inc. (La Jolla, CA, USA). For determining effects on the rates of mortality, hatching, swim bladder inflation, pericardial edema, and circulatory failure we used Fisher’s exact test and, to control family wise error rate at multiple comparisons, Holm-Bonferoni’s method was used [32]. The statistical methods used to determine effects on mRNA expression were Student's t test or one-way ANOVA followed by Tukey's or Dunnett's post hoc tests. Data were log-transformed when variance differed. Values for LC50 of FICZ in prescence of αNF were calculated using the curve fitting routine of GraphPad Prism for nonlinear regression sigmoidal dose-response. Statistical significance of more than additive effects on mRNA expression was determined using Student’s t test followed by Holm-Sidak post hoc test. For this analysis, calculated levels of CYP1A expression, i.e. the sum of the single exposures (FICZ alone + αNF alone), was compared to the observed levels of CYP1A expression after co-exposure to FICZ+αNF. The statistical tests used are also given in the figure legends.
3. Results
3.1. Impact of CYP1A knockdown on FICZ-mediated effects in zebrafish embryos
In order to determine the role of the CYP1A enzyme in regulating FICZ-mediated activities, various endpoints were examined in groups of zebrafish embryos injected with a morpholino targeting CYP1A (CYP1A-MO) and exposed to FICZ (10 or 100 nM) or vehicle (DMSO, 0.01%). Embryos injected with control morpholino (Ctrl-MO) and non-injected embryos (NI) were similarly exposed and included for comparison. Mortality and phenotypic effects were determined at 3 dpt (4 dpf) (Fig. 1), while effects on transcription were examined at 6, 24, and 48 hpt (Fig 2). Because FICZ is rapidly cleared in mammalian cells, we performed an additional experiment in which the exposure medium containing FICZ (100 nM) or DMSO was replenished every 8 h up to 6 dpf, with the purpose of mimicking persistent FICZ exposure and to determine whether this might elicit mortality and phenotypic effects.
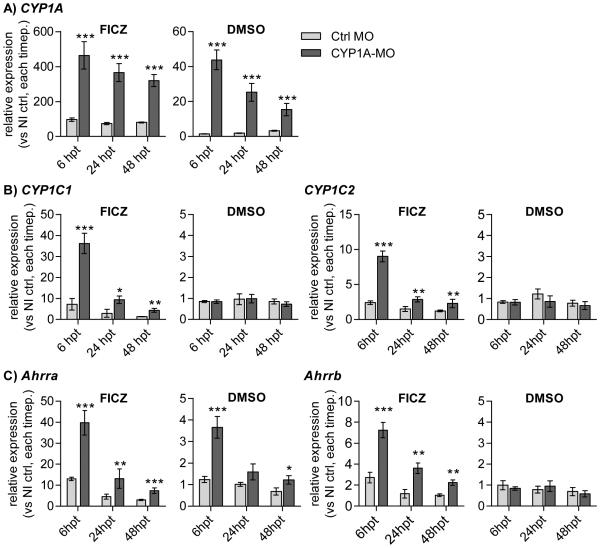
Figure 2. Impact of CYP1A knockdown on FICZ-mediated transcriptional effects.
Groups of embryos, non-injected or injected with Ctrl-MO or CYP1A-MO, were exposed to vehicle control (DMSO) or 100 nM FICZ. Effects on expression of Ahr2-related genes were determined at 6, 24 and 48 hpt. A) CYP1A, B) CYP1C1 and CYP1C2, and C) Ahrra and Ahrrb. Relative transcription was calculated using l13 as a reference gene and data from non-injected DMSO control groups at each time point as calibrators. Statistically significant differences in levels of transcription between paired groups of embryos injected with Ctrl-MO and CYP1A-MO were determined by student’s t test and are shown by asterisks (*** p<0.001). The results are based on two repeated experiments (n=4) and are shown as mean ± SD. Note the different y-axis scales used for FICZ vs DMSO graphs.
3.1.1. Mortality and phenotypic effects
CYP1A morphants exposed to FICZ exhibited increased incidence of mortality (Fig. 1A), non-hatching (Fig. 1B), failure of swim bladder inflation (Fig. 1C), and increased severity of pericardial edema and circulation failure (Fig. 1E) compared to FICZ-exposed NI or Ctrl-MO-injected embryos. The range of severity scores of edema is illustrated in Figure 1D.
Increased toxicity was observed with both concentrations of FICZ, although FICZ at the highest concentration had the strongest effect (32% and 54 % mortality for 10 and 100 nM FICZ, respectively). Embryos exposed to FICZ in the Ctrl-MO and NI groups displayed no significant incidence of any of the measured endpoints compared with the control groups and no significant difference was observed between similarly exposed Ctrl-MO and NI embryos. Embryos for which the FICZ containing medium was replenished every 8 h appeared normal at 6 dpf (data not shown). CYP1A-MO embryos exposed to vehicle alone displayed a small but statistically significant increase in toxicity (Fig. 1A-E).
3.1.2. Transcriptional effects
Knowing that FICZ is a potent activator of Ahr2 in zebrafish [16] and an excellent substrate for human and rat CYP1 enzymes [14, 33] we next asked whether the toxicity observed after exposure of CYP1A-MO-injected embryos to FICZ was reflected in prolonged or potentiated Ahr2 activation. Induction of CYP1A mRNA was chosen as a marker for Ahr2 transactivation. Exposure of Ctrl-MO-injected and NI embryos to FICZ (100 nM) resulted in CYP1A induction as expected (Fig. 2A) and the two groups showed no difference in induction level (not shown). However, CYP1A-MO-injected embryos showed a strongly increased CYP1A induction by FICZ compared to Ctrl-MO-injected embryos that were exposed to FICZ at all time-points (5.0-, 4.5- and 4.0-fold at 6, 24 and 48 hpt, respectively; Fig. 2A). Other AhR2 regulated genes (CYP1C1, CYP1C2, Ahrra and Ahrrb) also were strongly induced by FICZ in the CYP1A-MO groups but not in the Ctrl-MO groups, with CYP1C1 and Ahrra showing the strongest induction (Fig. 2B and 2C). CYP1A knockdown and exposure to vehicle alone resulted in a statistically significant increase in CYP1A expression at all time-points (29-, 15-, and 15-fold higher expression compared to Ctrl-MO injected embryos at 6, 24 and 48 hpt, respectively). A similar trend was observed for Ahrra expression.
3.2. Dose-dependent effects of FICZ
Due to the high toxicity observed in CYP1A-MO-injected embryos exposed to 10 or 100 nM FICZ, we next set out to determine the lowest concentration of FICZ able to cause toxicity and AHR-related transcriptional responses in these morphants. Zebrafish embryos injected with CYP1A-MO were exposed to a wide range of FICZ concentrations (0.01 to 10 nM) or vehicle (DMSO, 0.01%). Similarly exposed embryos injected with Ctrl-MO or not injected (NI) were included for comparison. Mortality and effects on pericardial edema and circulatory function were examined at 48 hpt (3 dpf), while effects on CYP1A transcription were examined between 6 and 48 hpt (Fig 3).
3.2.1. Mortality and phenotypic effects
In CYP1A-MO-injected embryos exposure of 1 or 10 nM FICZ gave a higher mortality rate than exposure to DMSO (Fig. 3A). With exposure of CYP1A MO-injected embryos to vehicle alone there was a small tendency (but not statistically significant) for increase in mortality (Fig. 3A). For incidence of pericardial edema and circulatory failure in the CYP1-MO-injected embryos, there were no significant differences between the FICZ-exposed groups and the vehicle control (DMSO) (Fig. 3B and 3C). For the same endpoints, all CYP1A MO-injected embryos, e.g. both the DMSO-exposed and FICZ-exposed groups, showed significant increase compared to exposed Ctrl-MO-injected embryos (Fig. 3B and 3C). Results with NI embryos did not differ significantly from those in embryos injected with Ctrl-MO (not shown).
3.2.2. Transcriptional effects
CYP1A-MO-injection resulted in an increase in CYP1A expression in all FICZ-exposed groups and the DMSO-exposed group at all time-points tested compared to the corresponding Ctrl-MO-injected group (Fig. 3C). However, the effect of FICZ was substantially reduced at 48 hpf.
3.3. AHR2-dependent effects of FICZ
The increase in transcriptional responses in parallel with the increased toxicity resulting from FICZ with CYP1A-MO led us to ask whether the toxic effects were AHR2-dependent. Zebrafish embryos injected with the CYP1A-MO, AHR2-MO, or a combination of CYP1A-MO and AHR2-MO were exposed to FICZ (10 nM) or vehicle (DMSO, 0.01%). Similarly exposed embryos injected with Ctrl-MO and NI embryos were included for comparison. Mortality, incidences of pericardial edema and circulatory failure were examined at 2 dpt (3 dpf) (Fig. 4A-B), and effects on CYP1A transcription were examined at 6, 24 and 48 hpt (Fig 4C).
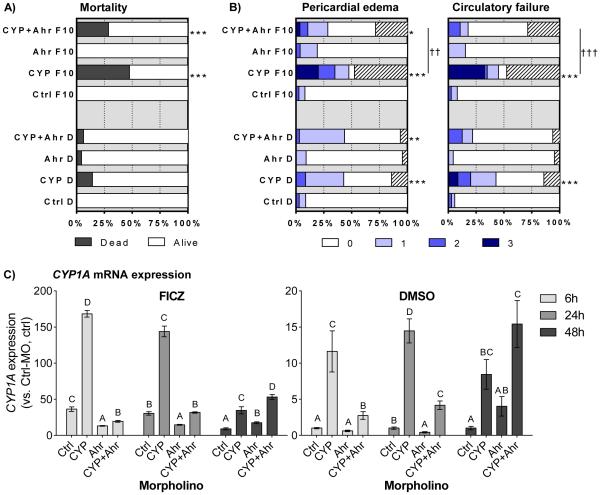
Figure 4. Role of Ahr2 in FICZ-mediated effects.
Groups of embryos injected with Ctrl-MO (Ctrl), CYP1A-MO (CYP), Ahr2-MO (Ahr), or a combination of CYP1A-MO and AHR2-MO (CYP+Ahr), were exposed to vehicle control (DMSO; D) or 10 nM FICZ (F10). Exposure vessels were randomized in order to avoid subjective scoring of the different morpholino groups. At 48 hpt, rates of A) cumulative mortality and B) pericardial edema (PE) and circulatory failure (CF) were determined. Severity of PE and CF was scored as shown in Fig. 1D. In B) the hatched part of a bar indicates the mortality rate. Relative levels of CYP1A mRNA expression were determined at 6, 24, and 48 hpt (C). All data were normalized to il13 as housekeeping gene and calibrated to DMSO-exposed Ctrl-MO embryos at respective time-point. Data in A) and B) are shown as % of total number of embryos at exposure start based on three separate experiments (n=75-90). Statistically significant differences between the Ctrl-MO injected groups and the CYP1A-MO, Ahr2-MO, or CYP1A+Ahr2-MO injected groups were determined by Fisher's exact test with Bonferroni's correction and are shown by asterisks (*p<0.05, ** p<0.01 and *** p<0.001). Daggers designate differences between indicated bars (†† p<0.01 and ††† p<0.001). For the statistical analysis “affected embryos” are represent by the pooled number of all living embryos with severity score 1-3 in a group. Results for CYP1A expression are from 2 or 3 replicate experiments (n= 5-7) and are shown as mean ± SEM. Transcript levels are calculated using l13 as a reference gene and the DMSO-exposed Ctrl-MO groups at each time-point as calibrators. Statistical differences among groups were determined by One-way ANOVA followed by Tukey’s post-hoc test and are indicated by different letters (p<0.05).
3.3.1. Mortality and phenotypic effects
Exposure to FICZ had no impact on the phenotype in embryos injected with Ahr2-MO or Ctrl-MO alone (Fig. 4A-B). In agreement with results in the other experiments (shown in Fig. 1 and Fig. 3), CYP1A-MO-injected embryos exposed to 10 nM FICZ showed a significant increase in mortality (Fig. 4A) and pericardial edema and circulatory failure (Fig. 4B) as compared to FICZ-exposed embryos injected with Ctrl-MO. Co-injection of Ahr2-MO significantly reduced, but did not eliminate, the increases in pericardial edema and circulatory failure seen in FICZ-exposed, CYP1A-MO-injected embryos (Fig. 4B). The group co-injected with Ahr2-MO and CYP1A-MO and exposed to FICZ also showed a tendency for reduction of mortality from 48% (CYP1A-MO, FICZ) to 29% (AHR2-MO + CYP1A-MO, FICZ), but this was not statistically significant. The CYP1A-MO injected DMSO-exposed group displayed a slight but significantly higher incidence of circulatory failure compared with the Ctrl-MO injected DMSO group; this was not observed in the combined CYP1A/Ahr2-MO-injection group. Results with NI embryos did not differ significantly from those with Ctrl-MO-injected embryos and thus are not included in the figure.
3.3.2. Transcriptional effects
In the Ctrl-MO groups, exposure to 10 nM FICZ generated a 36-, 30- and 9-fold induction of CYP1A at 6, 24 and 48 hpt respectively, while FICZ exposure of Ahr2-MO embryos resulted in a lower CYP1A induction at 6 and 24 hpt and a somewhat higher induction at 48 hpt than in the Ctrl-MO group (Fig. 4C). In CYP1A-MO groups, CYP1A induction by FICZ was strongly enhanced relative to the levels in the Ctrl-MO groups at all time-points, reaching levels of 168-, 134- and 35-fold the DMSO-exposed, Ctrl-MO-injected control values at 6, 24 and 48 hpt, respectively (and 4.7-, 4.7-, and 3.9-fold as compared to the FICZ-exposed, Ctrl-MO-injected control values at 6, 24 and 48 hpt, respectively). Most importantly, the level of CYP1A induction by FICZ in the Ahr2-MO and CYP1A-MO co-injected group was greatly reduced as compared to the FICZ-exposed CYP1A-MO-injected embryos at 6 and 24 hpt, although by 48 hpt this effect was not seen. Ahr2-MO-injected embryos exposed to DMSO showed no difference in CYP1A expression at 6 hpt compared to Ctrl-MO-injected embryos. The same morpholino treatment and DMSO exposure resulted in a lower level of CYP1A transcript at 24 hpt, while a tendency for increase (4-fold but not significant) was observed at 48 hpt (Fig. 4C). Knock-down of CYP1A resulted in a significant induction of CYP1A expression at all time-points in the vehicle control group. At 6 and 24 hpt, the level of CYP1A expression in the vehicle control was lower in embryos co-injected with Ahr2-MO and CYP1A-MO as compared to those injected with CYP1A-MO alone. At 48 hpt there was a trend for higher CYP1A expression in co-injected embryos compared to embryos injected with CYP1A-MO alone, but this was not statistically significant.
3.4. Combined exposures of zebrafish embryos to FICZ and αNF
To test whether a chemical CYP1 inhibitor could mimic the effects observed with the morpholino-mediated CYP1A knockdown, we performed a series of experiments exposing zebrafish embryos to the CYP1A inhibitor αNF (0.1 to 2.5 μM) and FICZ (0.001 to 10 nM) separately or in combination. DMSO (0.01%) was used as the vehicle control. Embryos were exposed from 24 hpf and incidences of mortality, edemas and non-inflated swim bladder were examined at 3 dpt (4 dpf). Typical phenotypic effects observed with the different exposures and statistical analyses of these effects are shown in Figure 5. The impact on CYP1A transcription was examined at times up to 48 hpt (Fig. 6). To determine if the observed effects were Ahr2-dependent, similar exposures were performed with Ahr2-MO-injected embryos.
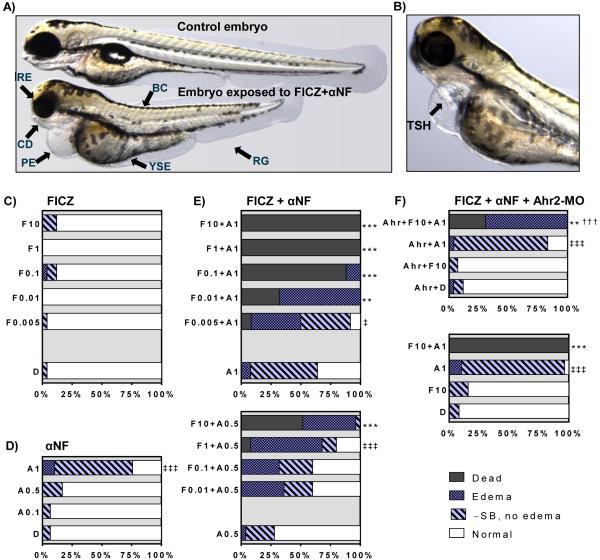
Figure 5. Phenotypic and quantitated effects of FICZ and αNF alone and in combination.
Groups of 1 dpf embryos were exposed to different doses of FICZ or vehicle control (DMSO) in combination with αNF starting at 1 dpf. A-B) Illustrative pictures of phenotypic effects; A) Control embryo (top) and embryo treated with FICZ (1.0 nM) + αNF (0.5 μM) (bottom). B) Embryo treated with FICZ (0.005 nM) + αNF (1.0 μM). Abbreviations: reduced growth (RG), body curvature (BC), craniofacial deformities (CD), reduced eye size (RE), pericardial- and yolk sac edema (PE, YSE), and tube-shaped heart (TSH). All pictures were taken at 3 dpt (4 dpf). C-F) quantitated data on lethality and phenotypic effects after exposure to C) FICZ (0.005 to 10 nM; F0.005 etc), or D) αNF (0.1 to 1 μ M; A0.1 etc), or E) combinations of αNF and FICZ. F) Embryos injected with Ahr2-MO (Ahr) were exposed to FICZ (10 nM), αNF (1 μM), or a combination of FICZ and αNF. DMSO (D) was used as a vehicle control for all exposures. At 3 dpt (4 dpf) frequencies of cumulative mortality (Dead), pericardial- or yolk sac edema (Edema), and failure of swim-bladder inflation (- SB, no edema) were determined as % of total number of embryos at exposure start based on one representative experiment (n=25-30). Embryos with none of the effects listed were scored as normal. Statistically significant differences among groups were determined by Fisher's exact test with Bonferroni's correction. A difference in mortality rate is indicated by asterisks (** p<0.01; *** p<0.001) between a treatment group and the reference group (D, A1, A0.5, or Ahr+D) and by daggers (††† p<0.001) between an Ahr2-MO group and its corresponding non-injected group. For embryo groups where no significant mortality was observed, the difference in rates of any effect between an exposed group and the reference group is indicated by double daggers (‡ p<0.05; ‡‡‡ p<0.001).
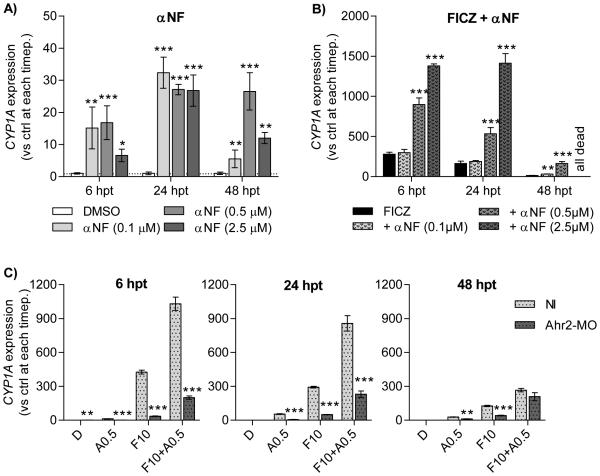
Figure 6. Effects of αNF on FICZ-mediated CYP1A transcription and role of Ahr2.
Groups of 1-dpf embryos were exposed to αNF (0.1 to 2.5μ M) A) alone or B) in combination with FICZ (10nM). C) Embryos injected with Ahr2-MO were exposed to 0.5 μM αNF (A0.5), 10 nM FICZ (F10) or the combination of αNF and FICZ. All data were normalized to il13 as housekeeping gene and calibrated to DMSO-exposed non-injected embryos at respective time-point. Results in A-B) are from one experiment (n=3) and statistical differences among groups were determined by One-way ANOVA followed by Dunnett’s test. Results in C) is from a one experiment (n=3) and statistical differences between non-injected and Ahr2-MO injected groups at the same exposure were determined by student’s t test. Statistically significant differences are indicated by asterisks (*p<0.05, ** p<0.01 and *** p<0.001).
3.4.1. Mortality and phenotypic effects
Exposure to DMSO, FICZ or 0.1 or 0.5 μM αNF alone did not cause any mortality or significant increase in phenotypic deformities (Fig. 5C,D). At a 1μ M dose, αNF caused a low incidence of edema (10 %) and a greater incidence of non-inflated swim bladder (65 %) than appeared in the DMSO control (Fig. 5D). In contrast, numerous adverse effects were observed after the combined exposures to FICZ and αNF, including pericardial- and yolk sac edema, lack of swim bladder inflation, craniofacial malformations, reduced eye size, body curvature, retarded growth and tube-shaped heart (Fig. 5A-B).
The combination of FICZ with 1 μM αNF caused 88 to 100 % mortality with the three highest doses of FICZ (0.1, 1.0 and 10 nM) while a lower mortality (32 and 8 %) was observed after co-exposure to 0.01 and 0.005 nM FICZ respectively (Fig. 5E). After exposure to FICZ combined with 1 μM αNF the incidence of mortality was higher than the control in all but the group exposed to the lowest concentration of FICZ (0.005 nM), and at all FICZ concentrations except this one all remaining live embryos had edema. The combined exposure to FICZ and 0.5 μM αNF resulted in an increase in mortality only with the highest FICZ concentration (10 nM; 52 %), while the lower FICZ concentrations (0.01 – 1 nM) caused an increased edema but no mortality (Fig. 5E). LC50 values for FICZ in combination with 1.0 μ M or 0.5 μM αNF calculated from the concentration-response data included in Figure 6 were around 50 times higher with the lower αNF concentration than with the higher αNF concentration (9.8 and 0.02 nM FICZ, respectively), showing that αNF sensitized embryos to FICZ.
To determine if the observed toxicity from combined treatment with FICZ + αNF is Ahr2-dependent, embryos injected with Ahr2-MO were exposed to 10 nM FICZ or 1 μM αNF alone or in combination. As seen in Figure 5F, Ahr2 knockdown reduced the mortality caused by co-exposure to 10 nM FICZ and 1 μM αNF to 31%, compared to 100% mortality observed in the NI group exposed to these two compounds; however, all live embryos displayed edema. Notably, the high incidence of non-inflated swim bladders observed with embryos exposed to 1 μ M αNF alone was not reduced upon Ahr2-MO injection. Ctrl-MO embryos included for comparison showed no difference compared to NI embryos for any of the exposures (not shown).
3.4.2. Transcriptional effects
Zebrafish embryos were exposed to αNF or FICZ separately or in combination as above. Exposure to αNF alone caused an increase in CYP1A expression in a time- and dose-dependent manner; at 24 hpt all αNF concentrations had induced CYP1A to about 30-fold the DMSO control (Fig. 6A). Exposure to FICZ resulted in a transient induction of CYP1A expression with highest level, 280-times the DMSO control, after 6 h of treatment, which was followed by a time-dependent reduction in expression (Fig. 6B).
Co-exposure to FICZ and 0.1-2.5 μM αNF resulted in dose- and time-dependent enhanced CYP1A expression. To investigate if these effects were more-than additive (synergistic) we performed statistical analysis of the observed CYP1A mRNA levels after co-exposure to FICZ with αNF as compared to the calculated sum of CYP1A mRNA levels after exposure to FICZ alone and αNF alone (Table 2). The level of CYP1A expression in embryos co-exposed to FICZ and 0.1 μ M αNF did not differ from that in the group exposed to FICZ alone at 6 or 24 hpt but was increased to more than additive levels at 48 hpt (Fig. 6B and Table 2). Co-exposure to FICZ and 0.5 or 2.5 μM αNF strongly enhanced CYP1A expression up to 5 and 9 times the level induced by FICZ alone at 6 and 24 hpt, respectively, resulting in more-than additive CYP1A expression levels (Table 2). At 48 hpt CYP1A expression in response to FICZ + 0.5 μM αNF was much lower than at 24 hpt, but still 12 times higher compared to FICZ alone (Figure 6B and Table 2), and all embryos exposed to FICZ + 2.5 μM αNF were dead. This means that the effect of FICZ in combination with αNF is synergistic.
Table 2.
Statistical analysis of CYP1A expression after combined exposure to FICZ and αNF
| FICZ | αNF 0.1 μM | αNF 0.5 μM | αNF 2.5 μM | ||||||
| hpt | observed | observed | observed | observed | |||||
| 6 | 279±25 | 15±7 | 17±5 | 7±2 | |||||
| 24 | 163±30 | 32±5 | 27±2 | 27±2 | |||||
| 48 | 13±2 | 6±3 | 27±6 | 12±3 | |||||
| FICZ + αNF 0.1 μM | FICZ + αNF 0.5 μM | FICZ + αNF 2.5 μM | |||||||
| hpt | observed | calculated | p value† | observed | calculated | p value | observed | calculated | p value |
| 6 | 298±68 | 294±26 | 0.9387 | 898±142 | 296±26 | 0.0019 | 1379±43 | 286±26 | < 0.0001 |
| 24 | 189±19 | 195±30 | 0.8027 | 534±131 | 190±29 | 0.0115 | 1413±207 | 189±30 | 0.0005 |
| 48 | 31±4 | 19±3 | 0.0152 | 163±43 | 40±6 | 0.0085 | all embryos dead | - | |
P values of differences between observed CYP1A mRNA levels after co-exposure to FICZ with αNF and calculated sum of CYP1A mRNA levels after exposure to FICZ alone + αNF alone were determined by Student’s t test followed by Holm-Sidak post hoc test. Observed and calculated values are based on one experiment (n=3) and given as mean ± SD. CYP1A expression is given as relative levels compared to DMSO control at each respective time point. P values showing significant more-than additive values (synergistic; P value < 0.05) are shown in bold font.
To determine if the observed effects on CYP1A expression were Ahr2-dependent, Ahr2-MO-injected embryos were exposed to FICZ (10 nM) or αNF (0.5 μM) alone or in combination and compared to non-injected embryos. The levels of CYP1A expression observed with αNF or FICZ alone were significantly lower in the Ahr2-MO-injected embryos as compared to NI embryos at all time-points (Fig. 6C). In a similar manner, the synergistic induction of CYP1A expression observed with the combined exposure to FICZ and αNF was significantly reduced in the Ahr2-MO-injected embryos at 6 and 24 hpt. At 48 hpt the CYP1A transcript levels in Ahr2-MO embryos did not differ from those in the non-injected embryos.
3.5. Levels of FICZ in embryos injected with CYP1A-MO or co-exposed to αNF
To test whether morpholino-mediated knockdown of CYP1A or co-exposure to αNF affects the level of FICZ in the embryos over time we performed chemical analysis of embryos exposed to FICZ using HPLC.
3.5.1. CYP1-MO injected embryos
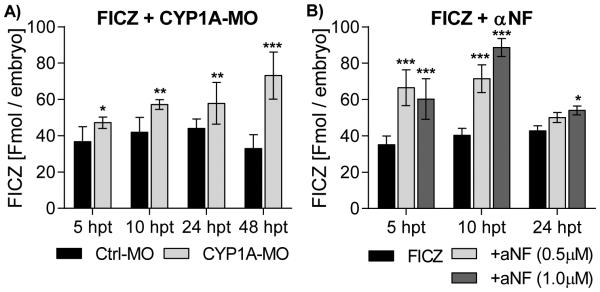
Zebrafish embryos injected with CYP1A-MO or CT-MO were exposed to 10 nM FICZ starting at 24 hpf. Samples were taken at 5, 10, 24 and 48 hpt, extracted and analyzed for levels of FICZ (Fig. 7A). Levels of FICZ in CT-MO injected embryos showed a weak increase in level of FICZ from 37 to 44 fmol/embryo from 5 to 24 hpt, whereas a decrease to 33 fmol/embryo was observed at 48 hpt. CYP1A knockdown resulted in higher levels of FICZ at all time points, with strongest effect observed at 48 hpt (33 and 73 fmol/embryo for Ctrl-MO and CYP1A-MO injected embryos, respectively).
Figure 7. Quantification of FICZ in embryos injected with CYP1A-MO or co-exposed to αNF.
A) Groups of 1-dpf embryos were injected with Ctrl-MO or CYP1A-MO and exposed to FICZ (10 nM) and B) groups of non-injected 1-dpf embryos were exposed to FICZ (10 nM) alone or in combination with αNF (0.5 μM or 1.0 μM). At 5, 10, 24 and 48 h levels of FICZ in the zebrafish embryos were determined using HPLC analysis. All data is given as fmol FICZ per embryo. At each time point, statistical differences between Ctrl-MO and CYP1A-MO injected groups, and between groups of non-injected embryos exposed to FICZ alone and FICZ in combination with αNF were determined by student’s t test. Statistically significant differences are indicated by asterisks (*p<0.05, ** p<0.01 and *** p<0.001).
3.5.2. Co-exposure to αNF
Zebrafish embryos (non-injected) were exposed to 10 nM FICZ alone or to FICZ in combination with 0.5 μ M or 1.0 μ M αNF starting at 24 hpf. Samples were taken at 5, 10 and 24 hpt, extracted and analyzed for levels of FICZ (Fig. 7B). In combination with 0.5 μM αNF, higher levels of FICZ were observed at 5 and 10 hpt; 33 and 40 fmol/embryo with FICZ alone compared to 66 and 71 fmol/embryo in embryos co-exposed to αNF. Similar effects were observed at 5 and 10 hpt after co-exposure to 1.0 μM αNF. This embryo group showed higher levels of FICZ also at 24 hpt (43 and 54 fmol/embryo, respectively).
4. Discussion
In this study we show that in zebrafish embryos, Ahr2 activation by FICZ was enhanced by interference with CYP1A expression or function, resulting in Ahr2-dependent toxicity. Thus, morpholino knockdown of CYP1A strongly increased the mortality, pericardial edema and circulation failure caused by the endogenous Ahr agonist FICZ, while the knockdown of Ahr2 strongly reduced the toxicity resulting from exposure of CYP1A-MO-injected embryos to FICZ. Several studies have indicated a role for CYP1A1 in feedback-regulation of AHR signaling in mammals and suggest that this feedback mechanism is essential for the appropriate timing, duration, and amplitude of AHR-regulated cellular functions [13, 34]. In this context, elevated systemic levels of endogenous, rapidly metabolized AHR ligands such as FICZ could result from reduced catalytic activity of CYP1 enzymes, which may contribute to adverse biological effects of potent CYP1 inhibiting agents [35, 36]. In support of this, morpholino-mediated knockdown of CYP1A as well as co-exposure to the potent CYP1A/CYP1B1 inhibitor αNF was shown to increase the levels of FICZ in the zebrafish embryos (Fig. 7). In the present study, CYP1A knockdown alone also resulted in increased CYP1A expression, possibly revealing the presence of endogenous AHR activators dependent on CYP1 for metabolic clearance. Concomitant Ahr2 knockdown significantly reduced the increase in CYP1A expression in the CYP1A-MO-injected embryos, confirming an increased Ahr2 activation upon CYP1A knockdown. This is consistent with the hypothesis that a CYP1/AHR feedback system is crucial for regulation of endogenous AHR signaling.
We also observed a somewhat increased toxicity by CYP1A knockdown alone, an effect that has not been reported in previous zebrafish embryo studies [30, 37]. A possible explanation for this could be the use of different zebrafish strains; in our study the TL strain was used whereas previous work has used the AB strain. However, the toxicity observed in CYP1A-MO injected embryos was not clearly reversed by Ahr2 knockdown and thus Ahr2-independent toxicity or nonspecific toxicity by the morpholino (i.e. toxicity not related to the CYP1A knockdown) cannot be ruled out.
The greater toxicity of chlorinated dioxins in comparison to FICZ and other readily metabolized compounds is considered to depend on the resistance of many chlorinated dioxins to degradation, leading to sustained AHR activation [38, 39]. Recently, effects of FICZ in mice administered the compound continuously via a micro-osmotic pump were investigated by comparing host responses to infection in cyp1a1 knockout (cyp1a1−/−) and wild type animals [40]. In the cyp1a1−/− mice continuous exposure to FICZ provoked a response similar to that caused by TCDD, whereas continuous FICZ exposure had no effect in wild type mice. Together with the results from the FICZ replenishment- and CYP1A-knockdown experiments present in our study, these data suggest that a functioning CYP1 biotransformation capacity is critical for controlling the toxic effects of FICZ and possibly other labile AHR agonists [41].
TCDD-mediated hyper activation of AHR during embryogenesis leads to embryonic lethality or severe developmental defects in multiple tissues in zebrafish [42]. The molecular mechanisms mediating these effects are still elusive; however, AHR-mediated transcriptional regulation is believed to be crucial for these effects [31]. In the present study, knockdown of Ahr2 strongly reduced the mortality, pericardial edema and circulatory failure observed after exposure to FICZ in CYP1A-MO embryos, indicating that the observed toxicity is at least partly Ahr2-dependent. This was further corroborated by the reduction of CYP1A expression at 6 and 24 hpt in the CYP1A/Ahr2-MO embryos exposed to FICZ, compared to embryos injected only with CYP1A-MO and then exposed to FICZ. The increased CYP1A expression observed at 48 hpt in the combined CYP1A/Ahr2-MO group may be due to reduced Ahr2-MO efficacy over time in combination with accumulation of endogenous Ahr2 ligand(s) as a result of the CYP1A loss of function.
CYP1 enzymes have an established role in bioactivation of procarcinogens as well as in the metabolism of endogenous compounds; thus, induction of CYP1 enzyme activity is a possible contributor to some toxic responses. However, there are several studies suggesting that CYP1A induction is not linked to overt toxicity [35, 43]. The role of CYP1A in AHR-related toxicity is controversial, perhaps in part because different types of effects and effects of different types of substrates are often conflated. Some reports show that induction of CYP1A is a factor mediating toxicity, and more specifically vascular toxicity by TCDD [30, 44]. Such effects could involve reactive oxygen species generated by CYP1s resulting from binding of the planar halogenated compound TCDD [30, 44]. Similarly, the knockout of CYP1A1 protected male mice against toxicity of TCDD [45]. However, other reports show no protective role of CYP1A against systemic toxicity of TCDD [37, 46, 47]. With PAH substrates, on the other hand, there are several reports demonstrating synergistic AHR-mediated toxicity with CYP1A knockdown or exposure to a CYP1-inhibiting PAH in combination with an AHR agonist [28, 41, 47-52].
The findings of enhanced PAH toxicity when in combination with a CYP1A inhibitor suggests that such inhibition may enhance the half-life of toxic PAHs [53]. However, less is known about exogenous inhibitors of CYP1A in combination with natural or endogenous ligands and AHR activators such as FICZ. In this study, αNF was chosen as a chemical CYP1 inhibitor [24, 54]. Co-exposure of αNF and FICZ resulted in synergistic Ahr2 activation, as measured by altered CYP1A gene expression (Table 2) and potentiated toxicity of FICZ. Notably, a doubling of the αNF concentration from 0.5 μM to 1 μM lowered the LC50 of FICZ by 500 times. The αNF dose-dependent differences likely result from more efficient inhibition of CYP1A activity at the higher dose, but may also involve the metabolism [55] and thus a changing internal dose of αNF, and the action of αNF as a partial agonist of Ahr (see below). The phenotypic effects observed with exposure to FICZ+αNF are those associated with classical AHR-mediated toxicity (e.g. bent spine, craniofacial deformities, tube-shaped heart, etc). These effects were at least partly Ahr2-dependent since knockdown of Ahr2 reduced both the toxicity and transcriptional effects. The lack of complete rescue is most likely explained by a less than 100% efficacy of the Ahr2-MO in combination with a highly toxic dose of the FICZ/αNF combination.
The structural diversity of compounds shown to activate AHR signaling raise the question whether all activators are bona fide receptor ligands, or if they may activate through other mechanisms such as the indirect activation through CYP1-inhibition as previously suggested by us [23]. Such a CYP1-inhibition mediated activation in vivo is supported by data on induced AHR signaling in tissues from cyp1-triple KO mice [13], and by the induced Ahr2 signaling observed in the CYP1A-MO-injected embryos reported in the present study. Some compounds may act through several mechanisms of AHR activation. αNF, for example, is considered a weak AHR agonist and partial AHR antagonist [25-27] in addition to being a potent CYP1 inhibitor. In this context, αNF may activate the AHR both as a CYP1-inhibitor and a receptor agonist, depending on the concentration. While its function as a weak agonist could explain the induction of CYP1A expression observed with αNF alone, our recent studies using human cell lines suggest that the AHR-activating capacity of αNF is rather mediated through its role as a CYP1 inhibitor and subsequent accumulation of AHR ligands [14, 23]. Previous studies have confirmed the CYP1 inhibiting effects of αNF in zebrafish embryos [47]. However we cannot conclude that the observed toxicity is solely due to blocked CYP1 function and thereby blocked metabolism of FICZ. While co-exposure to αNF was shown to increase the level of FICZ in the zebrafish embryos, the relatively larger increase in FICZ levels after co-exposure to αNF as compared to in CYP1A-MO injected embryos suggests that αNF is either more effective in blocking FICZ metabolism (e.g. by blocking both CYP1A and activity of other CYP1 enzymes) or that αNF may also increase the uptake of FICZ.
Analysis of the rates of FICZ metabolism by the multiple zebrafish CYP1s may help to clarify this. Further, other studies have demonstrated that αNF can, for example, inhibit peroxidase activity [56], repress p38MAPK signaling pathway [57] and inhibit aromatase (CYP19) activity [58]. Whether such “off-target” effects or crosstalk with other signaling pathways contribute to the toxicity observed in our study is yet to be determined
Thus, the mechanism by which a chemical may activate AHR signaling will depend on qualities of the compound, the biological dose, and the cell type, since cells differ in AHR inducibility and metabolic capacity.
In conclusion, our data confirm the hypothesis that CYP1 enzymes are important for regulating systemic levels and biological effects of FICZ in vivo, and further support the suggestion that CYP1 inhibitors may contribute to AHR-mediated toxicity by disrupting CYP1-dependent feedback regulation of endogenous AHR agonists, causing prolonged and/or reinforced receptor activation. The list of known CYP1 inhibitors is extensive, ranging from common environmental pollutants such as PAHs and PCBs, pharmaceutical drugs such as ketoconazole, to bacterial endotoxins (e.g. LPS) and physical agents (e.g. hypoxia and UV radiation). Considering the combined everyday exposure to AHR activators (diet, pollutants, and endogenous ligands) and CYP1 inhibitors, this points to a mode of toxic action previously not accounted for.
Acknowledgement
The research at Swetox was supported by Stockholm County Council, Knut & Alice Wallenberg Foundation, and Swedish Research Council FORMAS.
Funding
This work was supported by Swedish Research Council FORMAS grants 2011-963 (EW) and 2008-1249 (MJ), by a European Commission Horizon 2020 grant, Project ID 634880 (MJ), by a National Institute of Environmental Health Sciences (NIEHS) grant P42ES007381 (JJS- and MEH), R01ES006272 (MEH) and F32ES017585 (ART-L), by Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad 194313 (AK), by Grant-in-Aids for Research Activity Start-up 26881001 (AK) and for Young Scientists (A) 15H05334 (AK). The funding sources had no role in designing interpreting or summarizing the study, or in the decision of where to publish the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci U S A. 1997;94:13743–8. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goldstone JV, Goldstone HM, Morrison AM, Tarrant A, Kern SE, Woodin BR, et al. Cytochrome P450 1 genes in early deuterostomes (tunicates and sea urchins) and vertebrates (chicken and frog): origin and diversification of the CYP1 gene family. Mol Biol Evol. 2007;24:2619–31. doi: 10.1093/molbev/msm200. [DOI] [PubMed] [Google Scholar]
- [3].Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–43. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- [4].Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci. 2002;68:403–19. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- [5].Gonzalez FJ, Nebert DW. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985;13:7269–88. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hankinson O, Andersen RD, Birren BW, Sander F, Negishi M, Nebert DW. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985;260:1790–5. [PubMed] [Google Scholar]
- [7].Puga A, Raychaudhuri B, Salata K, Zhang YH, Nebert DW. Stable expression of mouse Cyp1a1 and human CYP1A2 cDNAs transfected into mouse hepatoma cells lacking detectable P450 enzyme activity. DNA Cell Biol. 1990;9:425–36. doi: 10.1089/dna.1990.9.425. [DOI] [PubMed] [Google Scholar]
- [8].RayChaudhuri B, Nebert DW, Puga A. The murine Cyp1a-1 gene negatively regulates its own transcription and that of other members of the aromatic hydrocarbon-responsive [Ah] gene battery. Mol Endocrinol. 1990;4:1773–81. doi: 10.1210/mend-4-12-1773. [DOI] [PubMed] [Google Scholar]
- [9].Vasiliou V, Puga A, Nebert DW. Negative regulation of the murine cytosolic aldehyde dehydrogenase-3 (Aldh-3c) gene by functional CYP1A1 and CYP1A2 proteins. Biochem Biophys Res Commun. 1992;187:413–9. doi: 10.1016/s0006-291x(05)81508-6. [DOI] [PubMed] [Google Scholar]
- [10].Singh SS, Hord NG, Perdew GH. Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys. 1996;329:47–55. doi: 10.1006/abbi.1996.0190. [DOI] [PubMed] [Google Scholar]
- [11].Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 1998;18:525–35. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shiizaki K, Ohsako S, Koyama T, Nagata R, Yonemoto J, Tohyama C. Lack of CYP1A1 expression is involved in unresponsiveness of the human hepatoma cell line SK-HEP-1 to dioxin. Toxicol Lett. 2005;160:22–33. doi: 10.1016/j.toxlet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [13].Dragin N, Shi Z, Madan R, Karp CL, Sartor MA, Chen C, et al. Phenotype of the Cyp1a1/1a2/1b1−/− triple-knockout mouse. Mol Pharmacol. 2008;73:1844–56. doi: 10.1124/mol.108.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–6. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- [15].Bock KW. The human Ah receptor: hints from dioxin toxicities to deregulated target genes and physiologic functions. Biol Chem. 2013 doi: 10.1515/hsz-2012-0340. [DOI] [PubMed] [Google Scholar]
- [16].Jönsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, et al. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem Biol Interact. 2009;181:447–54. doi: 10.1016/j.cbi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laub LB, Jones BD, Powell WH. Responsiveness of a Xenopus laevis cell line to the aryl hydrocarbon receptor ligands 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Chem Biol Interact. 2009;183:202–11. doi: 10.1016/j.cbi.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mukai M, Tischkau SA. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 2007;95:172–81. doi: 10.1093/toxsci/kfl126. [DOI] [PubMed] [Google Scholar]
- [19].Jönsson ME, Mattsson A, Shaik S, Brunstrom B. Toxicity and cytochrome P450 1A mRNA induction by 6-formylindolo[3,2-b]carbazole (FICZ) in chicken and Japanese quail embryos. Comparative biochemistry and physiology Toxicology & pharmacology : CBP. 2015;179:125–36. doi: 10.1016/j.cbpc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- [20].Farmahin R, Crump D, Kennedy SW. Sensitivity of avian species to the aryl hydrocarbon receptor ligand 6-formylindolo [3,2-b] carbazole (FICZ) Chem Biol Interact. 2014;221:61–9. doi: 10.1016/j.cbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- [21].Soshilov AA, Denison MS. Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol Cell Biol. 2014;34:1707–19. doi: 10.1128/MCB.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR) Chem Res Toxicol. 2012;25:1878–84. doi: 10.1021/tx300169e. [DOI] [PubMed] [Google Scholar]
- [23].Wincent E, Bengtsson J, Mohammadi Bardbori A, Alsberg T, Luecke S, Rannug U, et al. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2012;109:4479–84. doi: 10.1073/pnas.1118467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–56. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- [25].Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol. 1993;120:179–85. doi: 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- [26].Santostefano M, Merchant M, Arellano L, Morrison V, Denison MS, Safe S. alpha-Naphthoflavone-induced CYP1A1 gene expression and cytosolic aryl hydrocarbon receptor transformation. Mol Pharmacol. 1993;43:200–6. [PubMed] [Google Scholar]
- [27].Blank JA, Tucker AN, Sweatlock J, Gasiewicz TA, Luster MI. alpha-Naphthoflavone antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced murine lymphocyte ethoxyresorufin-O-deethylase activity and immunosuppression. Mol Pharmacol. 1987;32:169–72. [PubMed] [Google Scholar]
- [28].Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquatic toxicology. 2007;85:241–50. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Timme-Laragy AR, Karchner SI, Hahn ME. Gene knockdown by morpholino-modified oligonucleotides in the zebrafish (Danio rerio) model: applications for developmental toxicology. Methods in molecular biology. 2012;889:51–71. doi: 10.1007/978-1-61779-867-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, et al. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem Biophys Res Commun. 2003;304:223–8. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- [31].Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, et al. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol Sci. 2003;76:138–50. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- [32].Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- [33].Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 2004;149:151–64. doi: 10.1016/j.cbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [34].Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 2007;72:1369–79. doi: 10.1124/mol.107.038968. [DOI] [PubMed] [Google Scholar]
- [35].Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–16. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- [36].Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–50. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- [37].Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol Pharmacol. 2004;66:512–21. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- [38].Mitchell KA, Elferink CJ. Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol. 2009;77:947–56. doi: 10.1016/j.bcp.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Riddick DS, Huang Y, Harper PA, Okey AB. 2,3,7,8-Tetrachlorodibenzo-p-dioxin versus 3- methylcholanthrene: comparative studies of Ah receptor binding, transformation, and induction of CYP1A1. J Biol Chem. 1994;269:12118–28. [PubMed] [Google Scholar]
- [40].Wheeler JL, Martin KC, Resseguie E, Lawrence BP. Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol Sci. 2014;137:324–34. doi: 10.1093/toxsci/kft255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brown DR, Clark BW, Garner LV, Di Giulio RT. Zebrafish cardiotoxicity: the effects of CYP1A inhibition and AHR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists. Environmental science and pollution research international. 2015;22:8329–38. doi: 10.1007/s11356-014-3969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol Appl Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- [43].Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicol Sci. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117:537–46. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, et al. Cyp1a1(−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–21. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- [46].Jonsson ME, Kubota A, Timme-Laragy AR, Woodin B, Stegeman JJ. Ahr2-dependence of PCB126 effects on the swim bladder in relation to expression of CYP1 and cox-2 genes in developing zebrafish. Toxicol Appl Pharmacol. 2012;265:166–74. doi: 10.1016/j.taap.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci. 2006;92:526–36. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- [48].Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–64. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004;58:163–8. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [50].Wassenberg DM, Nerlinger AL, Battle LP, Di Giulio RT. Effects of the polycyclic aromatic hydrocarbon heterocycles, carbazole and dibenzothiophene, on in vivo and in vitro CYP1A activity and polycyclic aromatic hydrocarbon-derived embryonic deformities. Environmental toxicology and chemistry / SETAC. 2005;24:2526–32. doi: 10.1897/04-440r1.1. [DOI] [PubMed] [Google Scholar]
- [51].Fleming CR, Di Giulio RT. The role of CYP1A inhibition in the embryotoxic interactions between hypoxia and polycyclic aromatic hydrocarbons (PAHs) and PAH mixtures in zebrafish (Danio rerio) Ecotoxicology. 2011;20:1300–14. doi: 10.1007/s10646-011-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol Appl Pharmacol. 2001;177:264–71. doi: 10.1006/taap.2001.9296. [DOI] [PubMed] [Google Scholar]
- [53].Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV. Altering cytochrome P4501A activity affects polycyclic aromatic hydrocarbon metabolism and toxicity in rainbow trout (Oncorhynchus mykiss) Environmental toxicology and chemistry / SETAC. 2002;21:1845–53. [PubMed] [Google Scholar]
- [54].Miranda CL, Henderson MC, Buhler DR. Evaluation of chemicals as inhibitors of trout cytochrome P450s. Toxicol Appl Pharmacol. 1998;148:237–44. doi: 10.1006/taap.1997.8341. [DOI] [PubMed] [Google Scholar]
- [55].Stegeman JJ, Woodin BR. The metabolism of alpha-naphthoflavone (7,8-benzoflavone) by hepatic microsomes from the marine fish Stenotomus versicolor. Biochem Biophys Res Commun. 1980;95:328–33. doi: 10.1016/0006-291x(80)90742-1. [DOI] [PubMed] [Google Scholar]
- [56].Martinkova M, Kubickova B, Stiborova M. Effects of cytochrome P450 inhibitors on peroxidase activity. Neuro endocrinology letters. 2012;33(Suppl 3):33–40. [PubMed] [Google Scholar]
- [57].He Q, Huang C, Zhao L, Feng J, Shi Q, Wang D, et al. alpha-Naphthoflavone inhibits 3T3-L1 pre-adipocytes differentiation via modulating p38MAPK signaling. International journal of clinical and experimental pathology. 2013;6:168–78. [PMC free article] [PubMed] [Google Scholar]
- [58].Kellis JT, Jr., Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225:1032–4. doi: 10.1126/science.6474163. [DOI] [PubMed] [Google Scholar]