Abstract
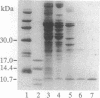
We have identified a 10-kDa stress-inducible mitochondrial protein. The protein is synthesized at elevated rates in cultured rat hepatoma cells challenged with heat shock or amino acid analogues and, therefore, designated heat shock protein 10 (Hsp10). Hsp10 was purified to homogeneity from rat liver and found to exhibit a native molecular mass of 65 kDa, as opposed to a monomeric molecular mass of 10,813.4 +/- 0.41 Da. The amino acid sequence of rat Hsp10 disclosed extensive sequence similarity with bacterial chaperonin (Cpn) 10. Rat Hsp10 and Escherichia coli Cpn60 were used to reconstitute functional trimeric rat ornithine transcarbamoylase from a chemically denatured state with high efficiency. This process depended completely upon rat Hsp10 and was abolished in the presence of a nonhydrolyzable ATP analogue. We conclude that Hsp10 is a eukaryotic Cpn10 homologue and, therefore, together with Cpn60 essential for mitochondrial protein biogenesis. The Cpn-mediated protein-folding apparatus, thus, exhibits a high degree of conservation between prokaryotes and mitochondria of higher eukaryotes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Van Kersen I., Kraft P. E., Hahn G. M. Biochemical analysis of heat-resistant mouse tumor cell strains: a new member of the HSP70 family. Mol Cell Biol. 1989 Aug;9(8):3509–3516. doi: 10.1128/mcb.9.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. A major antigen from Mycobacterium tuberculosis which is homologous to the heat shock proteins groES from E. coli and the htpA gene product of Coxiella burneti. Nucleic Acids Res. 1988 Sep 26;16(18):9047–9047. doi: 10.1093/nar/16.18.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Cerrone M. C., Ma J. J., Stephens R. S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991 Jan;59(1):79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby A. A., Ellis R. J. Chaperone function: the assembly of ribulose bisphosphate carboxylase-oxygenase. Annu Rev Cell Biol. 1990;6:125–149. doi: 10.1146/annurev.cb.06.110190.001013. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hindersson P., Høiby N., Bangsborg J. Sequence analysis of the Legionella micdadei groELS operon. FEMS Microbiol Lett. 1991 Jan 1;61(1):31–38. doi: 10.1016/0378-1097(91)90009-y. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Svendsen I., Scheller H. V., Møller B. L. Identification of a chloroplast-encoded 9-kDa polypeptide as a 2[4Fe-4S] protein carrying centers A and B of photosystem I. J Biol Chem. 1987 Sep 15;262(26):12676–12684. [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Laminet A. A., Ziegelhoffer T., Georgopoulos C., Plückthun A. The Escherichia coli heat shock proteins GroEL and GroES modulate the folding of the beta-lactamase precursor. EMBO J. 1990 Jul;9(7):2315–2319. doi: 10.1002/j.1460-2075.1990.tb07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach K. R., Graf L. J., Dunn A. R., Hoogenraad N. J. Effect of deletions within the leader peptide of pre-ornithine transcarbamylase on mitochondrial import. Eur J Biochem. 1986 Nov 17;161(1):19–23. doi: 10.1111/j.1432-1033.1986.tb10119.x. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Ristevski S., Höj P., Hoogenraad N. High-level expression of a mitochondrial enzyme, ornithine transcarbamylase from rat liver, in a baculovirus expression system. DNA Cell Biol. 1991 Jul-Aug;10(6):443–449. doi: 10.1089/dna.1991.10.443. [DOI] [PubMed] [Google Scholar]
- Lubben T. H., Gatenby A. A., Donaldson G. K., Lorimer G. H., Viitanen P. V. Identification of a groES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7683–7687. doi: 10.1073/pnas.87.19.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Chang C., Garrels J. I., Welch W. J. Identification, characterization, and purification of two mammalian stress proteins present in mitochondria, grp 75, a member of the hsp 70 family and hsp 58, a homolog of the bacterial groEL protein. J Biol Chem. 1989 Dec 5;264(34):20664–20675. [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tokunaga F., Iwanaga S., Mori M. Presequence does not prevent folding of a purified mitochondrial precursor protein and is essential for association with a reticulocyte cytosolic factor(s). J Biochem. 1990 Aug;108(2):207–214. doi: 10.1093/oxfordjournals.jbchem.a123182. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Peralta D., Hartman D. J., McIntosh A. M., Hoogenraad N. J., Høj P. B. cDNA and deduced amino acid sequence of rat liver prehsp60 (chaperonin-60). Nucleic Acids Res. 1990 Dec 11;18(23):7162–7162. doi: 10.1093/nar/18.23.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sipos A., Klocke M., Frosch M. Cloning and sequencing of the genes coding for the 10- and 60-kDa heat shock proteins from Pseudomonas aeruginosa and mapping of a species-specific epitope. Infect Immun. 1991 Sep;59(9):3219–3226. doi: 10.1128/iai.59.9.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Tamada H., Ohta T., Hamamoto T., Otawara-Hamamoto Y., Yanagi M., Hiraiwa H., Hirata H., Kagawa Y. Gene structure of heat shock proteins 61KDa and 12KDa (thermophilic chaperonins) of thermophilic bacterium PS3. Biochem Biophys Res Commun. 1991 Aug 30;179(1):565–571. doi: 10.1016/0006-291x(91)91408-5. [DOI] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Vijayasarathy C., Bhat N. R., Avadhani N. G. Intramitochondrial fatty acylation of a cytoplasmic imported protein in animal cells. J Biol Chem. 1989 May 15;264(14):7772–7775. [PubMed] [Google Scholar]
- Yon J. M., Betton J. M. Protein folding in vitro and in the cellular environment. Biol Cell. 1991;71(1-2):17–23. doi: 10.1016/0248-4900(91)90047-q. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]