Abstract
Background
Ca2+ channel blockers (CCB) and verapamil in particular prevented β-cell apoptosis and enhanced endogenous insulin levels in recent studies of mouse models of diabetes. Verapamil's effect on serum glucose levels in humans with diabetes is not described.
Methods
We used data from the REasons for Geographic and Racial Differences in Stroke (REGARDS), a national cohort study of community-dwelling middle-aged and older adults, enrolled between 2003 and 2007 from the continental United States. We examined associations of CCB and verapamil use with fasting serum glucose among 4978 adults with diabetes, controlling for covariates in generalized linear models (GLM).
Findings
The sample included 1484 (29.6%) CCB users, of which 174 (3.4%) were verapamil users. In fully adjusted GLMs, CCB users had 5 mg/dL lower serum glucose compared to non-users. Verapamil users had on average 10 mg/dL lower serum glucose compared to CCB non-users with substantially greater differences among insulin users: 24 mg/dL lower serum glucose among users of insulin in combination with oral agents and 37 mg/dL lower among users of insulin alone.
Interpretation
CCB and in particular verapamil use was associated with lower fasting blood glucose levels among REGARDS participants with diabetes.
Funding
UO1NS041588 from the National Institute of Neurological Disorders and Stroke, NIH; K24HL111154 and R01HL080477 from the National Heart, Lung, and Blood Institute.
Keywords: Diabetes, Verapamil, Ca2+ channel blockers
1. Introduction
Type 1 and type 2 diabetes have distinct pathophysiology [1], but both diseases are characterized by apoptotic pancreatic β-cell loss [1–3]. A recent study found that Ca2+ channel blockers (CCB) such as verapamil prevented β-cell apoptosis in mouse models of type 1 and type 2 diabetes [4,5]. Verapamil also enhanced endogenous insulin levels, rescued mice from streptozotocin-induced diabetes, and improved obesity-associated diabetes [4]. These emerging findings raise the intriguing possibility that a familiar widely used drug, verapamil, may have new indications if similar results can be found in humans. However, there are very few data on the effect of verapamil on serum glucose levels in humans with diabetes. The objective of this study was to examine the association between the use of CCB in general and verapamil specifically and fasting serum glucose levels among diabetic participants of the REasons for Geographical and Racial Differences in Stroke (REGARDS) national cohort study. We hypothesized that CCB and, in particular, verapamil use is associated with lower fasting blood glucose compared to otherwise similar non-users of CCB.
2. Methods
2.1. Study participants and procedures
The REGARDS study is a prospective national cohort of 30,239 community-dwelling adults from the 48 continental US states that examines regional and racial influences on stroke mortality. Details are described elsewhere [6]; briefly, participants were enrolled between 2003 and 2007 using commercially available lists combining mail and telephone contacts to recruit English-speaking adults aged 45 years and older, who were living in the continental US [6]. Severe debilitating conditions and cancer were exclusion criteria [6]. Race and gender were balanced by design, with oversampling from the Southeastern US; the final cohort composition was 58% women and 42% African American [6]. Baseline data collection included computer-assisted telephone interviews on socio-demographics, health history and health status. In-home examinations by trained staff followed standardized, quality-controlled protocols to collect fasting blood and urine samples; electrocardiograms; blood pressure (BP); anthropometric measures; and medication use by pill bottle review. Blood and urine samples were centrally analyzed at the University of Vermont. Electrocardiograms were centrally analyzed at Wake Forest University. Diabetes status was defined as taking insulin or oral antidiabetic agents, or a fasting blood glucose ≥126 mg/dL. The study protocol was reviewed and approved by the Institutional Review Board and all participants provided written informed consent.
3. Measures
The main outcome measure was fasting serum glucose (mg/dL). Glucose was measured using colorimetric reflectance spectrophotometry on the Ortho Vitros 950 IRC Clinical Analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, NY) with a coefficient of variation of 1% [7].
The main predictor variable was any CCB use, ascertained by the medication bottle review during the in-home study visit. The following CCBs were included: amlodipine, aranidipine, azelnidipine, barnidipine, benidipine, cilnidipine, clevidipine, efonidipine, felodipine, lacidipine, lercanidipine, manidipine, nicardipine, nifedipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, pranidipine, verapamil, gallopamil, and diltiazem. Verapamil was also assessed separately from other CCB.
4. Covariates
Age, race, gender, annual household income, and educational attainment were self-reported. Annual income was dichotomized at <$35,000 and education was dichotomized at having a high school diploma. Self-reported cigarette smoking was categorized as current (now) vs. past (smoking at least 100 cigarettes in a lifetime) or none. Self-reported alcohol use was categorized as moderate (one to seven drinks per week for women or one to fourteen drinks per week for men) vs. heavy (any more than moderate alcohol use) or none, according to the National Institute on Alcohol Abuse and Alcoholism criteria [6]. Physical activity was assessed by self-report of any exercise enough to work up a sweat vs. none during a regular week. Self-reported medication adherence was ascertained using the four-item Morisky scale [8], and defined as perfect adherence (score = 0) vs. non-adherence (score ≥ 1) [8]. BP was the average of 2 measures using an aneroid sphygmomanometer taken after a 5 min seated rest. Hypertension was defined as BP ≥140/≥90 mmHg or report of current use of antihypertensive medication. Waist circumference was obtained during the in-home visit. Serum concentrations of total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured using colorimetric reflectance spectrophotometry. Estimated glomerular filtration rate (eGFR) was calculated according to the 2012 Chronic Kidney Diseases Epidemiology Collaboration (CKD EPI) equation, which includes both creatinine and cystatin C, and urine albumin concentrations [9]. Serum creatinine was measured and calibrated to isotope dilution mass spectrometry-traceable methods [9]. Cystatin C was measured by particle-enhanced immunonephelometry (N Latex Cystatin C on the BNII, Formerly, Dade Behring, Now Siemens AG, Munich, Germany) [9]. Urine albumin was measured by nephelometry using the BNII ProSpec nephelometer (Now Siemaless AG), and urine creatinine was measured by the rate Jaffe' method using the Modular-P chemistry analyzer (Roche/Hitachi, Basel, Switzerland) [9]. Urinary albumin-to-creatinine ratio (ACR) was used in analyses. Heart rate was obtained from the electrocardiogram. History of coronary heart disease (CHD) was defined as electrocardiographic evidence of myocardial infarction (MI) or self-reported history of coronary artery bypass surgery, percutaneous coronary intervention, or MI. History of other cardiovascular disease (CVD) included self-reported diagnosis or intervention procedure for peripheral arterial disease, aortic aneurism, and/or stroke. Use of antihypertensive medications, oral antidiabetic agents, and insulin was determined via medication bottle review during the in-home visit or by self-report.
5. Statistical analysis
Baseline characteristics of diabetic REGARDS participants who used CCB were compared to those who did not. The Chi-square test was used for categorical variables and Student's t-tests—for continuous variables. Sequentially adjusted General Linear Regression Models (GLM) examined cross-sectional associations between CCB use and fasting glucose. A partially adjusted model included age, gender, region of residence, annual family income, and education. The fully adjusted model added waist circumference, systolic BP, total and HDL-cholesterol, logarithmically transformed ACR, eGFR, history of CHD and CVD, hypertension, smoking, alcohol use, physical activity, medication adherence, insulin and/or oral diabetic medication use, and heart rate. Similar sets of models were constructed to examine the association of verapamil use and fasting serum glucose.
Subgroup analyses examined only individuals on anti-hypertensive medication and then, separately, participants on different diabetes treatment modalities. For the latter we examined separately those not on any pharmacological diabetes treatment; those only on oral antidiabetic agents; those on insulin or insulin-oral antidiabetic agent combinations; and finally those who were only on insulin. All analyses were performed using SAS Version 9.4 (SAS Inc, Cary, NC).
6. Role of the funding source
Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data.
7. Results
7.1. Participants characteristics
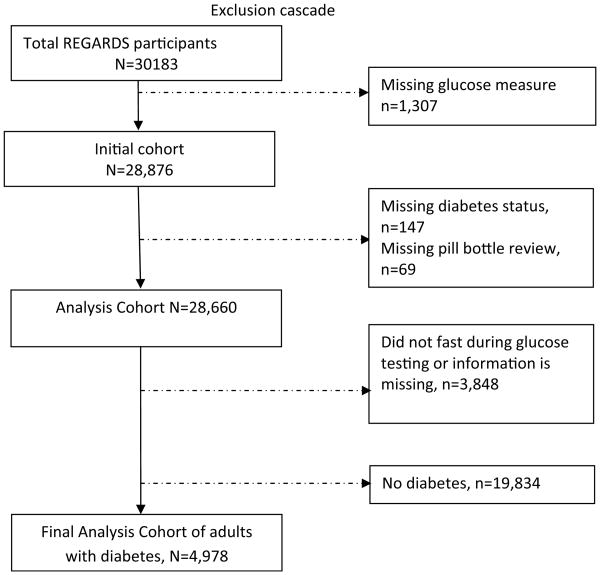
After excluding participants with missing data on serum glucose, diabetes status, and/or medication review; those who did not fast before blood sampling; and those who did not have diabetes; 4978 adults with diabetes were included in the final analytic sample (Fig. 1). Of these 1484 (29.6%) were CCB users, of which 174 (3.4%—of the analytic sample) were verapamil users.
Fig. 1. Exclusion cascade presents selection process of the analytic sample for the study.
Table 1 presents comparisons of non-users of any CCB with CCB users and verapamil users, respectively. Compared to non-users, both CCB users and verapamil users were slightly older (mean age 64.9 years vs. 66.2 and 66.1, respectively). CCB and verapamil users were more likely to be African American (67.9%, 69.5% vs. 51.4%, respectively) and women (56.6%, 62.6% vs. 50.7%, respectively). Both CCB and verapamil users had lower education and income levels. CCB and verapamil users were also more likely to suffer from hypertension (95.0% and 96.6%, respectively, vs. 70.7% among non-users) and to have higher mean systolic BP (134.3 and 139.3, respectively, vs. 130.7 mm Hg among non-users). Additionally, CCB and verapamil users had higher microalbuminuria levels, (median ACR 16.7 and 17.9, respectively, vs. 11.0 for non-users). CCB and verapamil users tended to have slightly larger mean waist circumference (105.8 and 106.6 vs. 104.5 cm among non-users). Both CCB and verapamil users were more likely to receive pharmacological treatment for diabetes than non-users (80% and 83% vs. 73%, respectively). There were no significant differences in the rates of smoking or non-adherence to medications.
Table 1. Baseline characteristics of REGARDS participants with diabetes by the use of Calcium Chanel Blockers (CCB), n = 4978.
| Characteristics | Non-users, n = 3494 | CCB users, n = 1484 | Verapamil users, n = 174 | ||
|---|---|---|---|---|---|
|
|
|
||||
| n (%) | n (%) | P | n (%) | P | |
| Socio-demographics | |||||
| Age, mean years, SD | 64.9 ± 8.8 | 66.2 ± 8.5 | <0.001 | 66.1 ± 8.7 | 0.09 |
| African American | 1795 (51.4) | 1007 (67.9) | <0.001 | 121 (69.5) | <0.001 |
| Female | 1771 (50.7) | 840 (56.6) | <0.001 | 109 (62.6) | 0.002 |
| Geographical region | 0.70 | 0.45 | |||
| Stroke belt | 1256 (35.9) | 515 (34.7) | 70 (40.2) | ||
| Stroke buckle | 811 (23.2) | 352 (23.7) | 35 (20.1) | ||
| Non-belt | 1427 (40.8) | 617 (41.6) | 69 (39.7) | ||
| Did not graduate from high school | 605 (17.3) | 324 (21.9) | <0.001 | 40 (23.0) | 0.001 |
| Income < $35,000 | 1743 (49.9) | 830 (55.9) | <0.001 | 108 (62.1) | 0.06 |
| Behaviors | |||||
| Current smoking | 490 (14.1) | 198 (13.4) | 0.83 | 22 (12.6) | 0.62 |
| Any alcohol use | 909 (26.8) | 344(23.6) | 0.03 | 41(24.0) | 0.37 |
| Not physically active | 1354 (39.3) | 645 (44.2) | <0.001 | 74 (43.3) | 0.30 |
| Medication non-adherence | 1034 (31.0) | 459 (31.7) | 0.66 | 56 (32.7) | 0.67 |
| Other medical conditions | |||||
| CHD | 868 (24.8) | 439 (29.6) | <0.001 | 42 (24.1) | 0.83 |
| Other CVD | 418 (12.0) | 233 (15.7) | <0.001 | 16 (9.2) | 0.27 |
| Hypertension | 2466 (70.7) | 1409 (95.0) | <0.001 | 168 (96.6) | <0.001 |
| Physiological | |||||
| Waist circumference, cm, mean, SD | 104.5 ± 15.4 | 105.8 ± 15.9 | <0.001 | 106.6 ± 15.7 | 0.08 |
| Systolic blood pressure, mmHg, mean, SD | 130.7 ± 16.9 | 134.3 ± 17.3 | <0.001 | 139.3 ± 18.2 | <0.001 |
| Total cholesterol mg/dL, mean, SD | 183.2 ± 42.6 | 178.1 ± 40.8 | <0.001 | 182.8 ± 43.6 | 0.91 |
| HDL-C, mg/dl, mean, SD | 46.3 ± 14.0 | 48.0 ± 14.3 | <0.001 | 49.5 ± 16.0 | 0.004 |
| Albumin to creatinine ratio, median, IQR | 11.0 [5.8–30.4] | 16.7 [7.5–70.8] | <0.001 | 17.9 [6.6–70.8] | <0.001 |
| Heart Rate, beat/min, mean, SD | 71.1 ± 33.8 | 69.6 ± 12.4 | 0.02 | 69.4 ± 11.2 | 0.10 |
| Diabetes management | |||||
| Any anti-diabetic medication or insulin use | 2569 (73.5) | 1188 (80.1) | <0.001 | 145 (83.3) | 0.004 |
Abbreviations: CCB—calcium channel blocker, CHD—coronary heart disease, CVD—cardiovascular disease, IQR—interquartile range, HDL-C—high density lipoprotein cholesterol, SD—standard deviation.
7.2. Effect of CCB and verapamil use on fasting serum glucose
In unadjusted analyses CCB and Verapamil users had lower fasting serum glucose by 7.3 and 9.7 mg/dL, respectively, compared to non-users (Table 2). In the fully adjusted models, this difference decreased for CCB users, but persisted for verapamil users. On average, verapamil users had 9.6 mg/dL lower blood glucose compared to diabetic CCB non-users after controlling for all covariates (Table 2). In sensitivity analyses, race did not modify the association of CCB use (p for interaction = 0.82) or verapamil use (p for interaction = 0.92) on glucose levels.
Table 2. Generalized Linear Models of fasting glucose levels and CCB and verapamil use.
| Fasting glucose (mg/dL) | Fasting glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean difference | P-value for difference | |
| Any CCB users, n = 1484 | Non-users, n = 3494 | |||||
| Crude | 134.7 | 132.1–137.2 | 141.9 | 140.0–143.8 | 7.3 | <.001 |
| LS-mean | (95% CI) | LS-mean | (95% CI) | |||
| Model 1 | 134.6 | 131.7–137.6 | 141.4 | 139.3–143.5 | 6.7 | <.001 |
| Model 2 | 135.2 | 130.3–140.1 | 140.2 | 136.0–144.5 | 5.1 | 0.005 |
| Verapamil users, n = 174 | Non-users of any CCB, n = 3494 | |||||
| Mean | (95% CI) | Mean | (95% CI) | Mean difference | P-value for difference | |
| Crude | 132.3 | 125.7–138.8 | 141.9 | 140.0–143.8 | 9.7 | 0.0056 |
| LS-mean | (95% CI) | LS-mean | (95% CI) | |||
| Model 1 | 132.3 | 124.0–140.7 | 141.8 | 139.5–144.0 | 9.4 | 0.03 |
| Model 2 | 130.2 | 120.7–139.7 | 139.8 | 134.7–144.8 | 9.6 | 0.03 |
Abbreviations: CCB—calcium channel blocker, CHD—coronary heart disease, CVD—other cardiovascular disease, HDL-C—high density lipoprotein cholesterol, LS-mean—least square mean, SBP—systolic blood pressure. Model 1 adjusts for age, race, sex, region, income and education. Model 2 adjusts for model 1 covariates, plus waist circumference, SBP, total and HDL-cholesterol, log of albumin to creatinine ratio, estimated glomerular filtration rate, history of CHD, CVD, hypertension, smoking, alcohol use, physical activity and medication non-adherence, treatment with oral anti-diabetic agents and/or insulin and heart rate.
Table 3 presents results of the adjusted GLMs examining the verapamil-glucose association among different subgroups according to diabetes treatment modality. There were no statistically significant differences in serum glucose between verapamil users and non-users among those not receiving any glucose-lowering medications or those only on oral antidiabetic agents. However, among participants on insulin or insulin-oral antidiabetic agent combinations, verapamil users had 24.1 mg/dL lower glucose compared to non-users (p = 0.039). The difference in serum glucose between verapamil users and non-users was more pronounced for those only on insulin (mean difference 37.4 mg/dL), but did not reach statistical significance (p = 0.06).
Table 3. Subgroup analysis: Generalized Linear Models of fasting glucose levels and verapamil use among various subgroups of adults with diabetes.
| Fasting glucose (mg/dL) | Fasting glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|
| a. Restricted to those not on any anti-diabetic medications* | ||||||
| Verapamil users, n = 15 | Non-users of any CCB, n = 646 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model | 153.8 | 132.2–175.6 | 155.0 | 150.8–159.1 | 1.1 | 0.91 |
| b. Restricted to those only on oral anti-diabetics | ||||||
| Verapamil users, n = 116 | Non-users of any CCB, n = 2049 | |||||
| LS-mean | (95%CI) | LS-mean | (95%CI) | Mean difference | P-value for difference | |
| Model | 129.8 | 120.7–138.9 | 135.7 | 133.1–138.4 | 6.0 | 0.19 |
| c. Restricted to those on insulin (± oral anti-diabetics) | ||||||
| Verapamil users, n = 43 | Non-users of any CCB, n = 799 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model | 131.7 | 109.1–154.4 | 155.9 | 149.0–162.7 | 24.1 | 0.039 |
| d. Restricted to those only on insulin | ||||||
| Verapamil users, n = 15 | Non-users of any CCB, n = 319 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model | 118.8 | 80.0–157.7 | 156.3 | 145.5–167.0 | 37.4 | 0.06 |
Abbreviations: ACR—albumin to creatinine ratio, CCB—calcium channel blocker, CHD—coronary heart disease; LS-mean—least square mean. Model adjusts for age, race, education, systolic blood pressure, total and HDL-cholesterol, log of ACR, baseline CHD, waist circumference, medication adherence, smoking, heart rate and hypertension (only variables significant in bivariate comparisons).
Antidiabetic medication defined by self-report or pill bottle review.
The results of the analogous set of subgroup analyses for CCB users are presented in Table 4. On average, CCB users had 9 mg/dL lower serum glucose than non-users among those not on any diabetes medication and those on insulin or insulin-oral antidiabetic agent combinations after controlling for covariates. Similar to the verapamil subgroup analysis, there was no statistically significant association between CCB use and fasting serum glucose among participants only on oral anti-diabetic agents.
Table 4. Subgroup analysis: Generalized Linear Models of fasting glucose levels and CCB use among various subgroups of adults with diabetes.
| Fasting glucose (mg/dL) | Fasting glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|
| a. Restricted to those not on any anti-diabetic medications* | ||||||
| CCB users, n = 176 | Non-users, n = 646 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model 2 | 147.3 | 140.1–154.6 | 156.0 | 152.1–159.9 | 8.6 | 0.03 |
| b. Restricted to those only on oral anti-diabetics | ||||||
| CCB users, n = 886 | Non-users, n = 2049 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model 2 | 131.2 | 127.9–135.8 | 135.2 | 132.6–137.8 | 3.4 | 0.10 |
| c. Restricted to those on insulin (± oral anti-diabetics) | ||||||
| CCB Users, n = 422 | Non-users n = 799 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model 2 | 145.7 | 137.0–154.4 | 155.2 | 148.9–161.6 | 9.5 | 0.04 |
| d. Restricted to those only on insulin | ||||||
| CCB users, n = 180 | Non-users n = 319 | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | Mean difference | P-value for difference | |
| Model 2 | 146.3 | 132.1–160.4 | 156.1 | 145.9–166.4 | 9.9 | 0.20 |
Abbreviations: ACR—albumin to creatinine ratio, CCB—calcium channel blocker, CHD—coronary heart disease; LS-mean—least square mean. Model adjusts for age, race, education, systolic blood pressure, total and HDL-cholesterol, log of ACR, baseline CHD, waist circumference, medication adherence, smoking, heart rate and hypertension (only variables significant in bivariate comparisons).
Antidiabetic medication defined by self-report or pill bottle review.
7.3. Antihypertensive medication use and the verapamil-serum glucose association
Table 5 in the online supplement displays the results of the analysis of fasting serum glucose and CCB use among participants taking anti-hypertensive medications. Consistent with the main results, CCB users and verapamil users taking anti-hypertensive medications had significantly lower blood glucose levels compared to non-users, controlling for all covariates. In sensitivity analyses, we also performed GLM models that separately adjusted for the use of angiotensin converting enzyme (ACE)-inhibitors and angiotensin 2-type 1 receptor antagonists, number of anti-hypertensive medications, and use of corticosteroid medications in addition to the above-mentioned covariates. These additional adjustments did not produce any significant change in the magnitude of the verapamil effect or CCB's effect on fasting blood glucose levels (data not shown).
8. Discussion
Using a large national population-based cohort, we observed lower blood glucose levels associated with CCB use generally and verapamil use specifically in adults with diabetes. After adjusting for potential confounders, verapamil users had on average 10 mg/dL lower serum glucose compared to non-users. These differences were substantially greater among insulin users, with differences of 24 mg/dL among those on insulin in combination with oral agents but 37 mg/dL among those using insulin alone. Insulin use reflects either type 1 or late stage type 2 diabetes, both conditions characterized by β-cell apoptosis. The association between verapamil use and lower blood glucose was robust across a variety of adjustments for potential confounders. Similar but potentially less pronounced differences in blood glucose were observed for the whole class of CCB.
Verapamil, an L-type CCB, has been used widely in clinical practice to treat hypertension [10], cardiac arrhythmias [11], cluster headaches [12], and migraines [13], but not to lower blood glucose for diabetes. Since the previous evidence of a hypoglycemic effect of verapamil comes from animal studies, there are few data in humans that can be compared with our findings. The INternational VErapamil SR-Trandolapril STtudy (INVEST) trial examined predictors of the development of type 2 diabetes among 16,176 adults aged 50 years and older with clinically stable coronary artery disease and treated for high blood pressure [14,15]. At the 24-month follow-up, participants who were treated with verapamil and trandolapril were less likely to develop diabetes than those treated with atenolol (hazard ratio 0.85 [95% CI 0.76–0.95]) [14,15]. While not directly comparable to our study, this study's findings are concordant with our findings of lower blood glucose levels among verapamil users in diabetic REGARDS participants.
The lower glucose levels among verapamil users with diabetes reported here are consistent with previously reported animal studies and can be explained by several molecular mechanisms. Shalev et al. reported that overexpression of thioredoxin interacting protein (TXNIP) induces β-cell apoptosis, and its suppression improves β-cell survival as well as prevents streptozotocin-induced and severe obesity-related diabetes in mouse models [5,16–18]. Interestingly, the group further found that CCB and in particular verapamil decreased TXNIP expression in mouse as well as in human islet β-cells [4,5]. Moreover, in in vivo mouse studies, orally administered verapamil reduced TXNIP expression and β-cell apoptosis, increased β-cell mass, enhanced endogenous insulin levels, rescued mice from streptozotocin-induced diabetes, and improved obesity-associated diabetes [4]. Thus, verapamil had a protective effect on β-cell survival and function in mice and also decreased pro-apoptotic TXNIP in human islets [4]. Our finding of a larger difference in blood glucose among verapamil and CCB users who were insulin-dependent suggests that these same mechanisms could be operating in humans.
Should these findings be confirmed in other studies, they could usher in a new therapeutic option for glycemic control in a particularly challenging subset of type 2 diabetes patients. Once such patients become dependent on insulin, glycemic control can be difficult to achieve. Although HbAc was not measured in this study, the size of the difference in glucose we described among verapamil users who were using insulin is clinically important, corresponding approximately to one HbA1c percentage point. This benefit is similar to that expected by adding an oral antidiabetic agent, which in a recent review was estimated to range from 0.5–1.5% [19]. Furthermore, verapamil may be an exciting new strategy for delaying β-cell death in type 1 diabetes.
The strengths of this study include the large sample from a national cohort including almost 5000 individuals with diabetes, the population-based recruitment strategy, a rich variety of covariates, and rigorous laboratory measurement methods. Limitations include the observational, cross-sectional design and non-availability of dose and duration of CCB use, therefore, causal inferences should not be drawn from our study. Data on duration and severity of diabetes and long-term glycemic control were also not available in REGARDS, although insulin use in part reflects both duration and severity of type 2 diabetes. Some measures such as medication adherence were self-reported and could not be verified by pharmacy refill data or medical records. Finally, despite adjustment for a wide variety of participant characteristics, there is still a possibility of unmeasured confounding as in all observational settings.
9. Conclusion
CCB use and, specifically, verapamil use, was associated with lower fasting blood glucose levels among REGARDS participants with diabetes. While prospective studies of verapamil in type 1 diabetes are ongoing (ClinicalTrials.gov: NCT02372253), additional studies are needed to determine the therapeutic potential of verapamil or other CCBs in the treatment of severe type 2 diabetes mellitus.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement UO1NS041588 from the National Institute of Neurological Disorders and Stroke, NIH; K24HL111154 from the National Heart, Lung, and Blood Institute; and R01HL080477 from the National Heart, Lung, and Blood Institute. A.S. is the recipient of JDRF grant 3-SRA-2014-302-M-R and NIH awards R01DK-078752 and UC4DK104204. Dr. Carson received additional support from K01DK095928. The study contents are solely the responsibility of the authors and do not necessarily represent the official vies of NIH, the National Institute of Neurological Disorders and Stroke, or the National Heart, Lung, and Blood Institute. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript. Drs. Monika Safford and Yulia Khodneva had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of interest statement: Drs. Khodneva, Shalev and Frank have nothing to disclose.
Drs. Carson and Safford report receiving salary support from Amgen.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.diabres.2016.01.021
References
- 1.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 2.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–8. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandrup-Poulsen T. Beta-cell apoptosis: stimuli and signaling. Diabetes. 2001;50(1):S58–63. doi: 10.2337/diabetes.50.2007.s58. [DOI] [PubMed] [Google Scholar]
- 4.Xu G, Chen J, Jing G, Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848–56. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296(5):E1133–9. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 7.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–6. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545–52. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens. 2011;13(9):687–9. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar M, Tchou P, Jazayeri M. Use of calcium channel entry blockers in the treatment of cardiac arrhythmias. Circulation. 1989;80(6):IV31–9. [PubMed] [Google Scholar]
- 12.Capobianco DJ, Dodick DW. Diagnosis and treatment of cluster headache. Semin Neurol. 2006;26(2):242–59. doi: 10.1055/s-2006-939925. [DOI] [PubMed] [Google Scholar]
- 13.Pelzer N, Stam AH, Haan J, Ferrari MD, Terwindt GM. Familial and sporadic hemiplegic migraine: diagnosis and treatment. Curr Treat Opt Neurol. 2013;15(1):13–27. doi: 10.1007/s11940-012-0208-3. [DOI] [PubMed] [Google Scholar]
- 14.Cooper-Dehoff R, Cohen JD, Bakris GL, Messerli FH, Erdine S, Hewkin AC, et al. Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]) Am J Cardiol. 2006;98(7):890–4. doi: 10.1016/j.amjcard.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, Cangiano JL, Garcia-Barreto D, Conti CR, et al. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients—findings from the International Verapamil SR/Trandolapril Study (INVEST) Am Heart J. 2006;151(5):1072–9. doi: 10.1016/j.ahj.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22(10):3581–94. doi: 10.1096/fj.08-111690. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57(4):938–44. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J Biol Chem. 2014;289(17):11807–15. doi: 10.1074/jbc.M113.525022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33(8):1859–64. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.