Abstract
Aging is characterized by a progressive loss of tissue function and an increased susceptibility to injury and disease. Many age-associated pathologies manifest an inflammatory component, and this has led to the speculation that aging is at least in part caused by some form of inflammation. However, whether or not inflammation is truly a cause of aging, or is a consequence of the aging process is unknown. Recent work using Drosophila has uncovered a mechanism where the progressive loss of lamin-B in the fat body upon aging triggers systemic inflammation. This inflammatory response perturbs the local immune response of the neighboring gut tissue and leads to hyperplasia. Here, we will discuss the literature connecting lamins to aging and inflammation.
Introduction
Chronic systemic inflammation without apparent infection in elderly humans is often referred to as the “inflammaging” phenotype, and is primarily characterized by elevated levels of circulating pro-inflammatory cytokines[1–6]. Epidemiological studies have found a strong correlation between the inflammaging phenotype and the presence of several aging-associated pathologies[7–9]. Genome-wide association studies have implicated several genes that function in immune, inflammatory and stress responses as being modifiers of longevity and health span[10].
Understanding the contribution of inflammation to aging has been pursued for many years with much effort aimed at identifying biomarkers of aging and cellular events that might serve as triggers for inflammatory responses. While conceptually straightforward, identifying cellular events has been very difficult, and is still largely correlative. Further, understanding the consequences of inflammation on aging presents its own challenges because, in part, many age-related diseases such as cardiovascular disease and osteoarthritis invoke strong inflammatory responses[11].
Recent studies have implicated the involvement of the nuclear intermediate filament proteins, the lamins, in aging-related inflammation. Here we will first introduce the general functions of lamins and then discuss the studies connecting these proteins to inflammation and aging.
Nuclear Lamins
Nuclear lamins are classed as type V intermediate filaments. There are two lamin subtypes, A-type and B-type, which are distinguished by their protein sequences, physical properties and expression profiles. In humans, the A-type lamin is encoded by LMNA, while two separate genes, LMNB1 and LMNB2, encode the B-type lamins. In Drosophila, the subject of some discussion below, the A- and B-type lamins (LAMC and LAM) are encoded by LamC and Lam, respectively.
Alternative splicing of the human LMNA transcript produces the major lamin-A and lamin-C isoforms. Lamin-C lacks the final two exons found in lamin-A, and is not post-translationally processed like either lamin-A or the B-type lamins. Post-translational processing of this latter group involves a carboxyl-terminal farnesylation motif (CaaX). The cysteine residue of this motif is first farnesylated. A proteolytic event removes the “aaX” and then the farnesylcysteine is methylated. For B-type lamins, there is no further processing and the farnesylation is a permanent feature of the protein. In the case of lamin-A, however, the carboxyl-terminus undergoes a second proteolytic cleavage to produce a mature unfarnesylated protein[12]. A zinc metalloprotease known as Zmpste24 performs both proteolytic processing steps required for production of mature unfarnesylated lamin-A while the “aaX” in B-type lamin is removed by a protease known as Rce1[13–15].
The lamins are believed to assemble into a dense meshwork underneath the inner nuclear envelope. This meshwork can serve as an interaction node for chromatin and proteins of the nuclear periphery[16,17]. Considering the diversity of these interactions, it is not surprising that lamins function in different nuclear activities such as chromatin organization, DNA replication, transcriptional regulation, signal transduction, and nuclear shape maintenance[12,18–20]. Consequently, lamins are viewed as housekeeping proteins that are essential for cell viability.
More recent studies, however, have shown that mouse embryonic stem cells (mESCs) completely lacking lamin proteins can self-renew and differentiate in vitro[21]. Thus, lamins are not required for the survival of at least this cell type. The study of lamins in different model organisms suggests that lamins are required for the proper development of several organs (e.g., brain, the diaphragm, and the testis)[22–25]. This idea is also supported by the discovery of tissue-specific diseases caused by mutations in lamin genes. For example, different mutations in LMNA cause a spectrum of disorders ranging from dilated cardiomyopathy, to partial lipodystrophy, and to the segmental premature aging syndrome, Progeria[26–28]. To date, there are no reported mutations in LMNB1 that cause disease; however, duplication of this gene causes an adult-onset form of leukodystrophy[29]. LMNB2 has also recently been linked to a progressive form of epilepsy[30]. How lamins cause tissue-specific diseases remains unclear.
Lamin-A: aging and inflammation
Hutchinson-Gilford Progeria Syndrome (HGPS) is an exceptionally rare disorder that resembles premature aging. Most HGPS patients harbor the same LMNA mutation, a de novo C1824T change that enhances the use of a cryptic splice site and leads to the production of a permanently farnesylated form of lamin-A[31,32]. This aberrant form of lamin-A, termed progerin, causes nuclear blebbing, down-regulation of some nuclear envelope proteins, accumulation of DNA damage and accelerated cellular senescence[31,33,34]. Surprisingly, progerin mRNA has been detected at low level in both young and aged individuals, and some, but not all studies on the subject indicate that the progerin product appears more abundantly in select tissues from aged individuals[35–38]. While it is uncertain if there is any function for the progerin present in these tissues, progerin can be induced upon UV damage and might represent an extension of the DNA damage response[39].
Markers of inflammation have been examined in HGPS patient samples and mouse models for this disorder. Cells derived from HGPS patients show an elevated NF-κβ transcriptional response profile[40]. The overexpression of an atypical-HGPS LMNA mutant also increases mRNA levels of certain inflammatory cytokines[41]. Further, inflammatory markers are elevated in the arteries, liver and skin of Progeria mouse models (both Lmna and Zmpste24)[38,42–44]. While the basis of this is not well understood, inflammation appears to be a part of the accelerated aging phenotype caused by LMNA mutations.
Lamin B: aging and inflammation
Human and mouse primary cell lines have a finite replicative lifespan (Hayflick limit) when cultured in vitro. This phenomenon, more commonly called replicative senescence, is characterized by cessation of cell division, increased secretion of inflammatory factors and changes in cell morphology and chromatin organization[45,46]. Recent studies have found that the replicative senescence of cultured mammalian fibroblasts is accompanied by lamin-B1 reduction[47–49]. The induction of senescence in cell culture by expression of oncogenic Ras, or activation of the downstream Rb or p53 tumor suppressors also leads to lamin-B1 decline[47,48]. Additionally, lamin-B1 reduction was detected in senescence-prone fibroblasts derived from Progeria patients and in cells with shortened telomeres or other forms of DNA damage[34,48–51]. In vivo, the irradiation of mice leads to cell senescence and lamin-B1 loss in the liver[48]. The loss of lamin-B1 appears to be regulated at both mRNA and protein levels[48,49,52,53]. There are, however, studies that show the association of increased lamin-B1 protein level and senescence[49,54]. In these studies, the overexpression of lamin-B1 in primary fibroblasts was sufficient to drive cells towards senescence whereas depletion resulted in proliferative arrest[49,54]. While there is no clear rationale for why lamin-B1 levels change under any of these conditions, lamin-B1, whether it is a reduction or increase in protein level, appears to be a marker of cellular senescence and various forms of cellular stress[48,54].
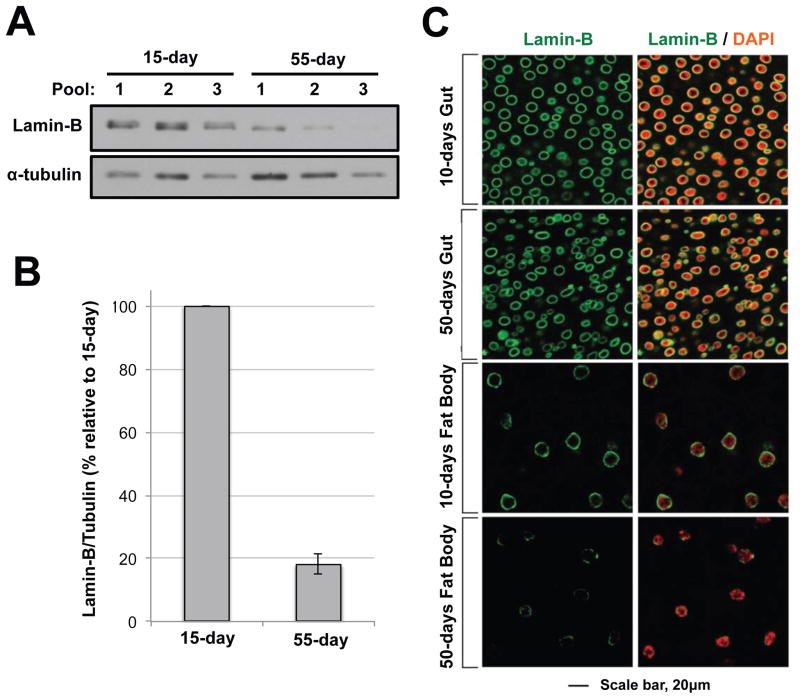
The human skin is the only organ reported to naturally have an age-related loss of lamin-B1[49]. In an effort to explore this potential relationship in other tissues, we analyzed lamin levels in different organs from young and old Drosophila[55]. Drosophila is a tractable model for studies of aging, inflammation and lamins as this model is relatively short-lived and is historically important in the immunity field[56,57]. Further, the Drosophila system is armed with an extensive set of genetic tools and methods that facilitate tissue-specific in vivo experimentation[56]. We found that B-type lamin (LAM), but not A-type lamin (LAMC), was reduced in the brain (Figure 1A, B, unpublished observations) and the fat body (Figure 1C)[55]. Notably, not all tissues (e.g., gut, heart) lose LAM upon aging (Figure 1C). One possibility is that gut epithelium was continuously replaced by intestinal stem cells and so senescent cells were simply replaced. However, in post mitotic heart cells and oenocytes, which persist throughout the life of the fly, lamin loss was not apparent[55]. The simplest interpretation from these observations is that age-related loss of B-type lamin occurs in select tissues. What makes these tissues susceptible to lamin-B loss is unknown.
Figure 1. Reduction of lamin-B in the brain and fat body but not in the midgut upon aging.
(a) Lamin-B reduction in old fly brains. Brains were dissected from 15-day or 55-day fruit flies. Five brains were pooled for each sample. Alpha tubulin was used as a loading control. (b) Quantitation of lamin-B western blots. Lamin-B signal was normalized against alpha tubulin, and the levels in the 55-day sample were compared relative to the 15-day sample. (c) Lamin B immunostaining of the midgut (top) and fat body (bottom) from 10 and 50-day old flies. Cells from the 50-day old fat body show reduced and patchy lamin-B staining.
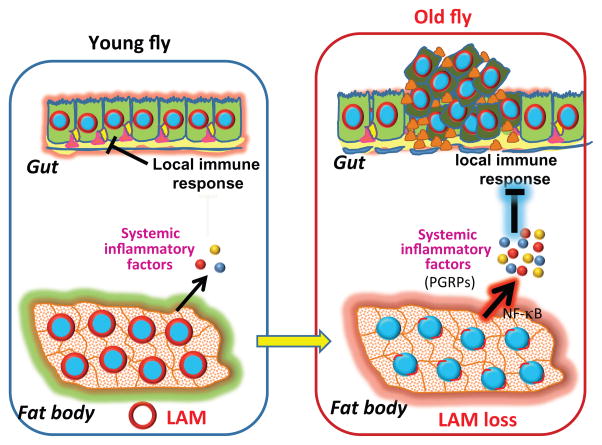
While there is some evidence that links B-type lamin and inflammation, this aspect has not been thoroughly explored. In cell culture, the induction of Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1)-related coactivator (PRC)-mediated inflammatory stress by a topoisomerase inhibitor resulted in cell senescence and loss of lamin-B1[58]. Lamin-B1 mRNA was also stimulated by treatment of arthritic synovium macrophages with the anti-inflammatory cytokine, IL-10[59]. Using Drosophila, we showed that aged fat bodies with LAM reduction or young fat bodies with RNAi-mediated depletion of LAM had significant inflammation mediated by the up-regulation of peptidoglycan recognition proteins (PGRPs) of the immune deficiency (IMD) pathway. Elevated PGRPs repressed IMD activity in the midgut and contributed to over-proliferation of intestinal stem cells and gut hyperplasia. Thus, lamin loss in one tissue (fat body) can affect the function of other tissues (gut) and this is mediated in part by systemic inflammation (Figure 3) [55]. Further studies will be needed to investigate how the fat body secreted PGRPs influence the gut immune system and whether or not the old fat body cells are senescent.
Figure 3. Model for systemic inflammation induced by age-related lamin-B reduction.
Old fat bodies exhibit loss of LAM, which then increases the expression of inflammatory factor (PGRPs) secreted into the circulation. These factors then block the local immune response of the gut and lead to over-proliferation and hyperplasia of this tissue.
Lamin-B may maintain tissue homeostasis by controlling gene expression
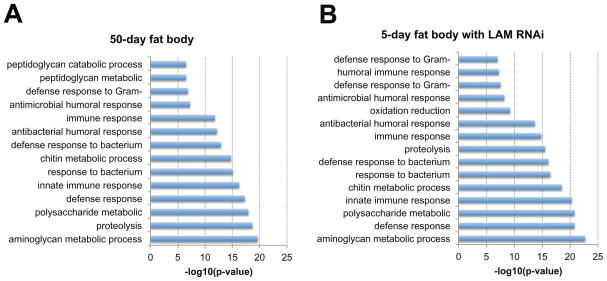
To gain insight into the role of lamin-B in maintaining the fat body organ, we performed RNA-seq to identify gene expression differences in fat bodies from young (5 days), old (50 days) and 5-day fat bodies that had LAM prematurely depleted by a tissue-specific RNAi[55]. Gene ontology (GO) analyses revealed that age-associated LAM loss was characterized by changes in genes primarily belonging to immune, metabolic, proteolysis, and oxidative pathways (Figure 2A). Depletion of LAM in young fat body yielded a gene expression profile (Figure 2B) that was remarkably similar to old fat bodies (~86% concordance). While immune response genes were significantly up regulated, genes required for cell fate determination and cell-cell adhesion were significantly down regulated upon LAM loss in both old and LAM RNAi young fat bodies. This dichotomy indicates that lamin-B can either activate or repress genes important for the fat body organ.
Figure 2. Gene Ontology (GO) analyses of old wild type fat body and young fat body depleted of lamin-B.
GO terms were determined by DAVID Bioinformatics Resources 6.7. Shown are significant GO terms determined from genes that were up-regulated by equal or greater than 2 fold in (a) old fat bodies (50-day, wild type w1118) or (b) young fat bodies depleted of lamin-B (5-day, Cg-Gal4/+;UAS-lamin-B RNAi/tub-Gal80ts).
DNA adenine methyltransferase identification (DamID) and chromatin immunoprecipitation (ChIP)-sequencing methods have been used to map chromatin-lamin interaction in a number of cell types [22,60,61]. These lamin-B1 associated chromatin domains (also called LADs) were enriched for immune response genes and since lamins can modulate gene expression, it was feasible that LAM in the Drosophila fat body could repress these immune response genes in the absence of infection. Consistent with this idea, a global reduction of staining for heterochromatin protein 1 (HP1) and histone H3 lysine 9 trimethylation (H3K9me3) was observed both in old fat bodies and in young fat bodies depleted of LAM. Moreover, supplementing HP1 partially reduced the inflammatory response triggered by LAM reduction in the fat body. Direct analyses using ChIP-qPCR of fat body tissue revealed that depletion of LAM reduced H3K9me3 on several immune response genes. Thus, lamin-B represses the expression of immune response genes and inflammation in fat bodies by maintaining the heterochromatic state of these genes[55].
Conclusions and future considerations
Aging is multifactorial, and different theories that center on cellular damage have been proposed to explain this phenomenon[62]. While the relationship between the nuclear lamina and various forms of cellular damage has not been extensive explored, there are examples in the literature that suggest that protein homeostatic mechanisms, such as autophagy and the Ubiquitin Proteasome System, are particularly relevant. For instance, autophagy activation with either rapamycin or temsirolimus improved the cell morphology of HGPS fibroblasts, and heart function of LMNA mutant animals[63–65]. Two recent studies showed that autophagy is involved in the turnover of lamin-B1 upon induction of senescence[52,53]. Further, proteasome activity appears to be reduced in dermal fibroblasts derived from Progeria patients[66]. Maintaining the homeostasis of cellular proteins, a process often referred to as proteostasis, appears to be intertwined with many known pathways of lifespan extension, including dietary restriction, enhanced autophagy, and reduced insulin signaling[67]. Studies have shown the decline in a cell’s ability to manage the misfolded and/or aggregated proteins occurs upon aging[67]. It is possible that the nuclear proteins such as B-type lamin and some nuclear pore complex (NPCs) components, which are particularly long-lived, accumulate damage and decline as a result[68,69]. Since both lamin-B and the components of NPCs can regulate gene expression, even subtle damage to these proteins may lead to positive feedback that increases aging-related factors, and perhaps furthers the aging process[70]. Considering the emerging connections, future research on the various roles of lamins may provide us with a clearer picture as to why we age.
Acknowledgments
This work was supported by the Ellison Medical Foundation and by NIH grants GM056312 and GM106023.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Day MJ. Ageing, immunosenescence and inflammageing in the dog and cat. J Comp Pathol. 2010;142(Suppl 1):S60–69. doi: 10.1016/j.jcpa.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar AP, Sarkar FH. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009;2:174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11:537–550. [PubMed] [Google Scholar]
- 5.Khatami M. Inflammation, aging, and cancer: tumoricidal versus tumorigenesis of immunity: a common denominator mapping chronic diseases. Cell Biochem Biophys. 2009;55:55–79. doi: 10.1007/s12013-009-9059-2. [DOI] [PubMed] [Google Scholar]
- 6.Morrisette-Thomas V, Cohen AA, Fulop T, Riesco E, Legault V, Li Q, Milot E, Dusseault-Belanger F, Ferrucci L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi: 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbaraly TN, Hamer M, Ferrie JE, Lowe G, Batty GD, Hagger-Johnson G, Singh-Manoux A, Shipley MJ, Kivimaki M. Chronic inflammation as a determinant of future aging phenotypes. CMAJ. 2013;185:E763–770. doi: 10.1503/cmaj.122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadyab AH, LaCroix AZ. Genetic factors associated with longevity: a review of recent findings. Ageing Res Rev. 2015;19:1–7. doi: 10.1016/j.arr.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- 14.Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, Sinensky MS. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem J. 2005;387:129–138. doi: 10.1042/BJ20041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maske CP, Hollinshead MS, Higbee NC, Bergo MO, Young SG, Vaux DJ. A carboxyl-terminal interaction of lamin B1 is dependent on the CAAX endoprotease Rce1 and carboxymethylation. J Cell Biol. 2003;162:1223–1232. doi: 10.1083/jcb.200303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 17*.Korfali N, Wilkie GS, Swanson SK, Srsen V, de Las Heras J, Batrakou DG, Malik P, Zuleger N, Kerr AR, Florens L, et al. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3:552–564. doi: 10.4161/nucl.22257. The authors compared proteomic datasets from different tissues and found little overlap between the protein complement of the nuclear envelope. This may help explain the tissue-specific nature of the laminopathies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 19.Smythe C, Jenkins HE, Hutchison CJ. Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs. EMBO J. 2000;19:3918–3931. doi: 10.1093/emboj/19.15.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foti R, Gnan S, Cornacchia D, Dileep V, Bulut-Karslioglu A, Diehl S, Buness A, Klein FA, Huber W, Johnstone E, et al. Nuclear Architecture Organized by Rif1 Underpins the Replication-Timing Program. Mol Cell. 2015 doi: 10.1016/j.molcel.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Kim Y, Zheng X, Zheng Y. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 2013;23:1420–1423. doi: 10.1038/cr.2013.118. The authors show that lamins are not required for ES cell viability, differentiation into various cell lineages in vitro, and teratoma formation in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–1710. doi: 10.1126/science.1211222. While B-type lamins are required for organismal viability, they are not required for cell survival and differentiation of ES cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Chen X, Zheng Y. The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling. Cell Stem Cell. 2013;13:73–86. doi: 10.1016/j.stem.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci U S A. 2010;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011;22:4683–4693. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattout A, Dechat T, Adam SA, Goldman RD, Gruenbaum Y. Nuclear lamins, diseases and aging. Curr Opin Cell Biol. 2006;18:335–341. doi: 10.1016/j.ceb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 28.Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- 29.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 30.Damiano JA, Afawi Z, Bahlo M, Mauermann M, Misk A, Arsov T, Oliver KL, Dahl HH, Shearer AE, Smith RJ, et al. Mutation of the nuclear lamin gene LMNB2 in progressive myoclonus epilepsy with early ataxia. Hum Mol Genet. 2015;24:4483–4490. doi: 10.1093/hmg/ddv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. Please see [32] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. These two studies reported the C1824T mutation in LMNA as the cause of HGPS, and stimulated the subsequent investigation of the nuclear lamina in the context of aging. [DOI] [PubMed] [Google Scholar]
- 33.Chojnowski A, Ong PF, Wong ES, Lim JS, Mutalif RA, Navasankari R, Dutta B, Yang H, Liow YY, Sze SK, et al. Progerin reduces LAP2alpha-telomere association in Hutchinson-Gilford progeria. Elife. 2015;4 doi: 10.7554/eLife.07759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–445. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. The authors discovered an HGPS-like cellular phenotype and the production of progerin in skin fibroblasts derived from normal old individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo YB, Mitrpant C, Johnsen RD, Fabian VA, Fletcher S, Mastaglia FL, Wilton SD. Investigation of age-related changes in LMNA splicing and expression of progerin in human skeletal muscles. Int J Clin Exp Pathol. 2013;6:2778–2786. [PMC free article] [PubMed] [Google Scholar]
- 38.Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Takeuchi H, Runger TM. Longwave UV light induces the aging-associated progerin. J Invest Dermatol. 2013;133:1857–1862. doi: 10.1038/jid.2013.71. This study reported that progerin production in fibroblasts was induced by UV-light damage. This work suggests that progerin might be a response to some form of cellular stress. [DOI] [PubMed] [Google Scholar]
- 40.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afonso P, Auclair M, Boccara F, Vantyghem MC, Katlama C, Capeau J, Vigouroux C, Caron-Debarle M. LMNA mutations resulting in lipodystrophy and HIV protease inhibitors trigger vascular smooth muscle cell senescence and calcification: Role of ZMPSTE24 downregulation. Atherosclerosis. 2015;245:200–211. doi: 10.1016/j.atherosclerosis.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 42**.Osorio FG, Barcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JM, Lopez-Otin C. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26:2311–2324. doi: 10.1101/gad.197954.112. HGPS mouse models show elevated NF-κβ activity and increased inflammatory cytokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenna T, Rosengardten Y, Viceconte N, Baek JH, Grochova D, Eriksson M. Embryonic expression of the common progeroid lamin A splice mutation arrests postnatal skin development. Aging Cell. 2014;13:292–302. doi: 10.1111/acel.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosengardten Y, McKenna T, Grochova D, Eriksson M. Stem cell depletion in Hutchinson-Gilford progeria syndrome. Aging Cell. 2011;10:1011–1020. doi: 10.1111/j.1474-9726.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- 45.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. Please see [48] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. These studies examined lamin-B1 in the context of cellular senescence. [44] found that silencing lamin-B1 in human fibroblasts induced senescence. [45] demonstrated lamin-B1 decline during replicative and stress-induced senescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, Lane EB, Lee SJ, Vardy LA, Stewart CL, et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200:605–617. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Dreesen O, Ong PF, Chojnowski A, Colman A. The contrasting roles of lamin B1 in cellular aging and human disease. Nucleus. 2013;4:283–290. doi: 10.4161/nucl.25808. Please see [54] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, Jaeger VM, Munck M, Wehnert M, Muller CR, Zhou Z, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum Mol Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]
- 52**.Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, et al. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–109. doi: 10.1038/nature15548. Please see [53] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Lenain C, Gusyatiner O, Douma S, van den Broek B, Peeper DS. Autophagy-mediated degradation of nuclear envelope proteins during oncogene-induced senescence. Carcinogenesis. 2015;36:1263–1274. doi: 10.1093/carcin/bgv124. The authors of these two studies show that autophagy is responsible for the turnover of lamin-B1 upon induction of cellular senescence. [DOI] [PubMed] [Google Scholar]
- 54**.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–1094. doi: 10.1038/emboj.2011.492. These studies show that lamin-B1 overexpression leads to cellular senescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Chen H, Zheng X, Zheng Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell. 2014;159:829–843. doi: 10.1016/j.cell.2014.10.028. Using an in vivo model, the authors show that age-associated reduction of lamin-B induced an inflammatory response in the fat body organ that affected the gut tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wangler MF, Yamamoto S, Bellen HJ. Fruit flies in biomedical research. Genetics. 2015;199:639–653. doi: 10.1534/genetics.114.171785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helfand SL, Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu Rev Genet. 2003;37:329–348. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 58.Gleyzer N, Scarpulla RC. Activation of a PGC-1-related coactivator (PRC)-dependent inflammatory stress program linked to apoptosis and premature senescence. J Biol Chem. 2013;288:8004–8015. doi: 10.1074/jbc.M112.426841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antoniv TT, Ivashkiv LB. Dysregulation of interleukin-10-dependent gene expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54:2711–2721. doi: 10.1002/art.22055. [DOI] [PubMed] [Google Scholar]
- 60.van Bemmel JG, Pagie L, Braunschweig U, Brugman W, Meuleman W, Kerkhoven RM, van Steensel B. The insulator protein SU(HW) fine-tunes nuclear lamina interactions of the Drosophila genome. PLoS One. 2010;5:e15013. doi: 10.1371/journal.pone.0015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 63.Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346. [DOI] [PubMed] [Google Scholar]
- 64.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi JC, Worman HJ. Reactivation of autophagy ameliorates LMNA cardiomyopathy. Autophagy. 2013;9:110–111. doi: 10.4161/auto.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viteri G, Chung YW, Stadtman ER. Effect of progerin on the accumulation of oxidized proteins in fibroblasts from Hutchinson Gilford progeria patients. Mech Ageing Dev. 2010;131:2–8. doi: 10.1016/j.mad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68**.Savas JN, Toyama BH, Xu T, Yates JR, 3rd, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. Please see [69] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. The authors discovered that some protein components of the nuclear pore complex, histones, and B-type lamins are very long-lived in post-mitotic rat brain neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van de Vosse DW, Wan Y, Wozniak RW, Aitchison JD. Role of the nuclear envelope in genome organization and gene expression. Wiley Interdiscip Rev Syst Biol Med. 2011;3:147–166. doi: 10.1002/wsbm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]