Abstract
Introduction
Patients treated with stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC) are subject to locoregional and distant recurrence, as well as the formation of second primary lung cancers (SPLC). The optimal surveillance regimen for patients treated with SBRT for early-stage NSCLC remains unclear, and herein we investigated the post-treatment recurrence patterns and development of SPLC.
Methods
Three-hundred and sixty-six patients with pathologically proven inoperable early-stage NSCLC treated with SBRT between 2006 and 2013 were assessed. Patients underwent a CT scan of the chest every 3 months during years 1 and 2, every 6 months during years 3 and 4, and annually thereafter. Competing risks analysis was used for all time-to-event analyses.
Results
With a median follow up of 23 months, the 2-year cumulative incidence of local, nodal and distant failures were 12.2%, 16.1%, and 15.5%, respectively. Of patients with disease progression post-SBRT (n=108), 84% (n=91) occurred within the first two years. Five percent (n=19) of patients developed a SPLC. The median time to development of SPLC was 16.5 months (range 6.5 to 71.1 months), with 33% (n=6) of these patients developing SPLCs after two years. None of the never smokers, but 4% of former and 15% of current tobacco smokers developed a SPLC (p=0.005).
Conclusion
Close monitoring with routine CT scans within the first 2 years after SBRT is effective in detecting early disease progression. In contrast, the risk for developing a SPLC remains elevated beyond 2 years, particularly in former and current smokers.
Keywords: Stereotactic body radiotherapy (SBRT), Non-small cell lung cancer, surveillance, early stage
MicroAbstract
Patients treated with SBRT for early-stage NSCLC the optimal surveillance regimen remains unclear. We assessed 366 early-stage NSCLC patients treated with SBRT. Of patients with progression post-SBRT, 84% occurred within the first two years. Of patients with formation of second primary lung cancers, 33% occurred after two years. Close monitoring beyond 2-years may be necessary in patients treated with SBRT.
Introduction
Stereotactic body radiotherapy (SBRT) has resulted in unprecedented local control in patients with inoperable early stage non-small cell lung cancer (NSCLC). This has significantly shifted practice patterns, resulting in an exponential rise in the use of SBRT which has been associated with improvements in overall survival.1,2 In parallel, CT screening in the high risk population has resulted in an increase in detection of early-stage NSCLC and associated improvement in overall survival.3 For these reasons it is likely that the use of SBRT for early stage NSCLC will continue to rise.
SBRT consistently results in long-term local control rates of approximately 90%, and surveillance is important not only to monitor the primary tumor site, but also to detect the considerable number of regional and distant failures that occur in this population.2 Furthermore, second primary lung cancers (SPLC) develop at an estimated crude rate of approximately 6%.4 Early detection and intervention of SPLC is likely important for these patients, as even early stage lung cancer results in a median survival time of only 14 months when left untreated.5 Thus it is critical to understand temporal development of SPLC. However, due to the rapid adoption of this technology, the optimal post-treatment surveillance regimen has yet to be established.
To address the void in data on timing of recurrence and formation of SPLC, we aimed to investigate and understand the appropriate surveillance regimen for patients treated with SBRT. Herein, we report a detailed analysis of recurrence and the development of new second primary lung cancers in a large cohort of patients with NSCLC uniformly treated with SBRT at a single institution.
METHODS
Patient and Study Details
This study was approved by the institution review board at XXXXX Cancer Center. We identified 366 consecutive patients who received SBRT for inoperable, biopsy-proven stage I NSCLC (T1–2aN0M0) at our institution between 2006 and 2013. Pathologic confirmation is required before SBRT for all patients.
Radiotherapy Treatment
Our radiotherapy treatment methods have been previously described.6 Briefly, patients were immobilized with an alpha cradle with their arms raised above their head and underwent a 4D-CT simulation. Treatment planning was performed using our in-house treatment planning software. Target delineation was based on standard ICRU definitions. The gross tumor volume was defined on the free-breathing CT and modified based on the 4D-CT scan to create an internal target volume for all patients. The internal target volume was expanded with a 2- to 3-mm margin for microscopic disease extension to create a clinical target volume, which was then expanded 5 mm to the planning target volume. Dose was prescribed to the 100% isodose line surrounding the PTV using inhomogeneity corrections. The median dose prescribed was 48 Gy, ranging from 45 to 60 Gy in three to five fractions. Tumors were typically treated in a riskadapted approach with 9–10 Gy × 5 fractions (n = 94) for tumors within 2 cm from the proximal bronchial tree, 12 Gy × 4 fractions (n = 123) for tumors within 1 cm from the chest wall, 18 to 20 Gy × 3 fractions (n = 135) for all other peripherally located tumors, and 14 patients received alternative dose/fractionation schedules. All patients had treatment delivered every other day. Treatment setup was verified using a cone-beam CT scan, and adjustments and shifts were performed for optimal alignment.
Follow-up
Patients typically underwent a CT scan of the chest and upper abdomen including the adrenal glands and liver every 3 months during years 1 and 2, every 6 months during years 3 and 4, and annually thereafter. 18F-FDG-PET/CT scans were performed at baseline, and only done again if there was radiographic suspicion of recurrence on CT scan. Recurrences were documented by biopsy unless there was clear evidence of metastatic disease on CT or PET/CT scan.
Endpoints and Statistics
Recurrences were defined as local, with failure of the irradiated primary tumor in or adjacent to the PTV; nodal, with failure in intrathoracic, mediastinal or supraclavicular lymph nodes; or distant, with failure at all other sites. Second primary tumors were defined using all available radiologic and pathologic information according to a modified version of the criteria of Martini and Melamed. We defined them as a new pulmonary malignancy occurring in a different lobe or lung than the first tumor with no intervening lymph nodes and no evidence of metastases, different histology or subtype and/or molecular genomic differences.7 However, we did not mandate a minimum interval of 2 years between the first and SPLC. We also considered second primary tumors in the same lobe of the lung when they were of different histology or outside the previous radiation field.
The associations between factors and the risk of local failure, nodal failure, distant failure, and SPLC, were evaluated using competing-risks analyses. The risk of each event was estimated using a cumulative incidence function that accounted for death without the event of interest. The analysis of SPLC included a second competing event, other progression. Cumulative incidence comparisons across subgroups were analyzed using Gray’s test. The Fine and Gray method was used for multivariate analyses. Kaplan-Meier analysis was used to estimate overall survival (OS), and patients who were still alive were censored at the date of last available follow-up. All endpoints were determined from the date of the last fraction of SBRT=. Candidate factors with p < 0.20 on univariate analysis (UVA) were incorporated into a multivariate model (MVA) for each endpoint. Statistical significance for all analyses was two-sided and used a 5% significance level (p<0.05). Statistical analyses were performed using R (version 3.0.1; R Development Core Team) with the “survival” and “cmprsk” packages.
RESULTS
The median age of the cohort was 77 years old (range, 50–95), 170 (46%) were men, and 196 (54%) were women (Table 1). The majority of patients (n=263; 72%) had a KPS of ≥80. Most patients presented with a T1 tumor (n=297; 81%), and 257 (70%) had adenocarcinoma histology. The median tumor size was 2 cm (range, <0.5 cm to 5 cm). Thirty-seven (10%) patients were never smokers, 290 (79%) former tobacco smokers, and 39 (11%) current tobacco smokers. A total of 121 patients had prior lung surgery for a previous primary NSCLC, 30 had prior conventionally fractionated RT (29 for previous NSCLC and 1 for previous esophageal cancer).
Table 1.
Baseline characteristics
| Factor | N (%) |
|---|---|
| Gender | |
| Male | 170 (46) |
| Female | 196 (54) |
| KPS | |
| < 80 | 103 (28) |
| ≥ 80 | 263 (72) |
| History of Smoking | |
| Never | 37 (10) |
| Former | 290 (79) |
| Current | 39 (11) |
| Histology | |
| Adenocarcinoma | 257 (70) |
| Squamous cell | 99 (27) |
| NSCLC NOS | 10 (3) |
| T-Stage | |
| T1 | 297 (81) |
| T2 | 69 (19) |
| Age (range) | 77 (50–95) |
| Tumor size (cm) | 2.0 (range <0.5 to 5.0) |
| Median Radiation dose (cGy) |
4800 (IQR 4800 to 5400) |
Local Failure
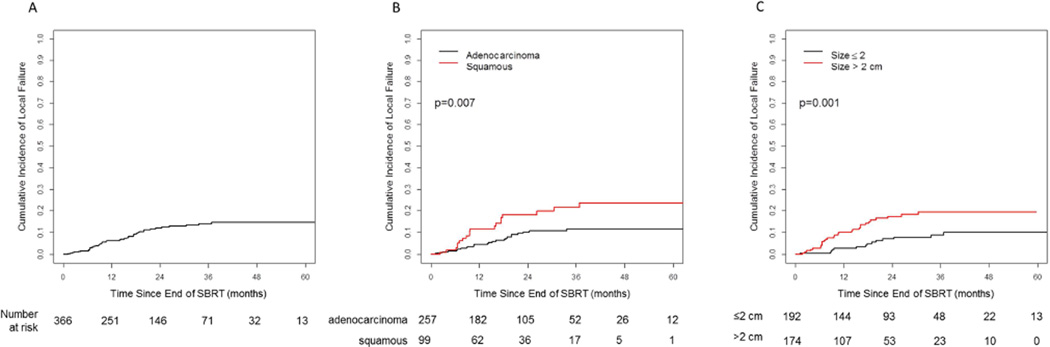
With a median follow up of 23 months, there were 43 local failures of the irradiated primary tumor observed. The cumulative incidence rates for local failure at 12, 24, and 36 months were 6.4%, 12.2%, and 14.2%, respectively (Figure 1a). On univariate analysis (Table 2), significant predictors included histology (squamous vs. adenocarcinoma, HR 2.15 [95%CI 1.18–3.92], p=0.012), tumor size as a continuous variable (HR 1.58 [95%CI 1.24–2.02], p<0.001), and radiotherapy dose (HR 0.94 [95%CI 0.90–0.996], p=0.035). On multivariate analysis only histology (squamous vs adenocarcinoma, HR 2.12 [95%CI 1.15–3.91], p=0.016) and tumor size (HR 1.50 [95%CI 1.15–1.96], p=0.003) remained significant (Table 3). Two-year cumulative incidence for local failure for squamous vs. adenocarcinoma histology was 18.4% vs. 10.4%, and for tumors >2 cm vs. ≤2 cm was 17.6% vs. 7.2% (p=0.007), respectively (Figure 1b and 1c).
Fig. 1.
Cumulative incidence of local failure; A) total cohort, B) comparison of adenocarcinoma and squamous cell histologies, and C) comparison of primary tumor size >2 cm and ≤2 cm.
Table 2.
Univariate analysis for predictors of recurrence and survival
| Local (LFFS) | Nodal (NFFS) | Distant (DFFS) | Overall Survival (OS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (continuous) | 1.00 | (0.97, 1.04) | 0.84 | 0.99 | (0.97, 1.02) | 0.62 | 1.00 | (0.97, 1.02) | 0.88 | 1.03 | (1.01, 1.05) | 0.003 |
| Gender (Female vs. Male) | 0.80 | (0.44, 1.46) | 0.47 | 0.57 | (0.33, 0.97) | 0.038 | 0.83 | (0.51, 1.35) | 0.45 | 0.74 | (0.54, 1.01) | 0.059 |
| KPS (≥ 80 vs. < 80) | 1.28 | (0.63, 2.58) | 0.50 | 1.37 | (0.72, 2.61) | 0.33 | 1.52 | (0.83, 2.81) | 0.18 | 0.54 | (0.39, 0.75) | < 0.001 |
| Smoking (Never vs. Current) | 2.16 | (0.64, 7.33) | 0.22 | 1.10 | (0.32, 3.80) | 0.88 | 1.28 | (0.47, 3.50) | 0.64 | 1.12 | (0.57, 2.18) | 0.75 |
| Smoking (Former vs. Current) | 1.09 | (0.40, 3.01) | 0.86 | 1.21 | (0.48, 3.03) | 0.68 | 0.96 | (0.43, 2.12) | 0.92 | 1.10 | (0.67, 1.80) | 0.72 |
| Histology (Squamous vs. Adeno, n=358) |
2.15 | (1.18, 3.92) | 0.012 | 0.81 | (0.43, 1.52) | 0.51 | 0.64 | (0.35, 1.18) | 0.15 | 1.74 | (1.25, 2.41) | 0.001 |
| Tumor size (continuous) | 1.58 | (1.24, 2.02) | < 0.001 | 1.17 | (0.92, 1.50) | 0.20 | 1.25 | (0.98, 1.59) | 0.073 | 1.43 | (1.23, 1.65) | < 0.001 |
| Radiation Dose (Gy, continuous) |
0.94 | (0.90, 0.996) | 0.035 | 1.01 | (0.97, 1.06) | 0.57 | 1.02 | (0.98, 1.06) | 0.34 | 0.97 | (0.95, 0.999) | 0.045 |
Table 3.
Multivariate analysis for predictors of recurrence and survival
| Endpoint | Variable | HR (95% CI) | p-value |
|---|---|---|---|
| LFFS | Histology (Squamous vs adeno-Ca) | 2.12 (1.15, 3.91) | 0.016 |
| Tumor size (continuous) | 1.50 (1.15, 1.96) | 0.003 | |
| Radiation dose (continuous) | 0.96 (0.91, 1.01) | 0.16 | |
| NFFS | Gender (Female vs Male) | 0.58 (0.34, 0.99) | 0.045 |
| Tumor size (continuous) | 1.16 (0.90, 1.49) | 0.25 | |
| DFFS | KPS (≥80 vs <80) | 1.51 (0.81, 2.83) | 0.20 |
| Histology (Squamous vs adeno-Ca) | 0.65 (0.35, 1.20) | 0.17 | |
| Tumor size (continuous) | 1.28 (1.01, 1.64) | 0.045 | |
| OS | Age (continuous) | 1.02 (1.003, 1.05) | 0.022 |
| Gender (Female vs Male) | 0.77 (0.56, 1.07) | 0.12 | |
| KPS (≥80 vs <80) | 0.64 (0.45, 0.89) | 0.009 | |
| Histology (Squamous vs adeno-Ca) | 1.50 (1.07, 2.11) | 0.020 | |
| Tumor size (continuous) | 1.30 (1.09, 1.54) | 0.003 | |
| Radiation dose (continuous) | 0.99 (0.96, 1.02) | 0.49 | |
Abbreviations: DFFS, distant failure-free survival; LFFS, local failure-free survival; NFFS, nodal failure-free survival; OS, overall survival.
Nodal Failure
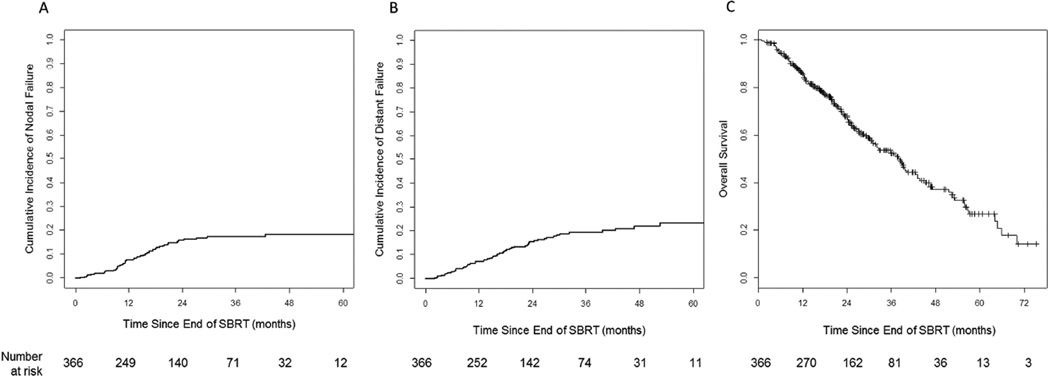
The cumulative incidence rates for nodal failure at 12, 24, and 36 months were 7.7%, 16.1%, and 17.4%, respectively (Figure 2a). On univariate analysis (Table 2), the only significant predictor was gender (female vs. male, HR 0.57 [95% CI 0.33–0.97], p=0.038), and this remained significant on multivariate analysis (HR 0.58 [95%CI 0.34–0.99], p=0.045; Table 3). Histology and tumor size were not significant predictors of nodal failure
Fig. 2.
A) Cumulative incidence of nodal failure, B) Cumulative incidence of distant failure, and C) Kaplan-Meier estimate for overall survival (OS).
Distant Failure
The cumulative incidence rates for distant failure at 12, 24, and 36 months were 7.3%, 15.5%, and 19.5%, respectively (Figure 2b). On univariate analysis (Table 2), there were no significant predictors with the exception of a trend (p=0.073) for tumor size. However, when adjusting for KPS and histology on multivariate analysis, tumor size became an independently significant predictor for distant failure (HR 1.28 [95%CI 1.01–1.64], p=0.045; Table 3). Cumulative incidence rates by histology and tumor size are shown in Supplementary Figure 1a and 1b.
Overall Survival
The 2-year OS was 67.6% (Figure 2c). On univariate analysis significant predictors for OS included age, KPS, histology, tumor size, and radiation therapy dose (Table 2). On multivariate analysis, age, KPS, histology, and tumor size were independently associated with OS (Table 3). OS by histology and tumor size shown in Supplementary Figure 2a and 2b.
Second Primary Lung Cancers
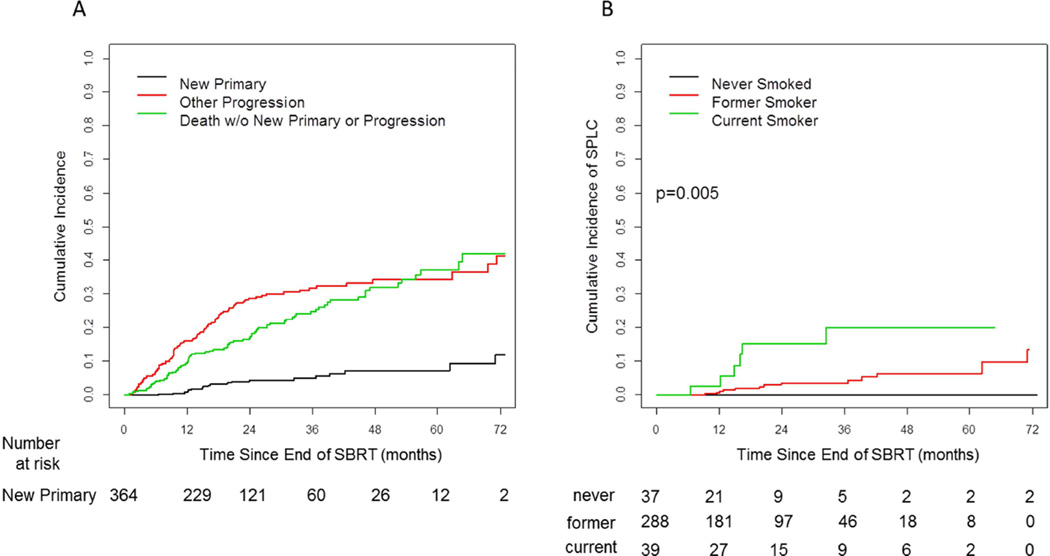
Of patients with disease progression post-SBRT (n=108), 84% (n=91) occurred within the first two years, 7.4% (n=8) during year three, and 8.3% (n=9) progressed after year three (Figure 3a). 5.2% (n=19) of patients developed a SPLC, with a median time to development of SPLC of 16.5 months (range, 6.5 to 71.1 months), with 32% (n=6) of these patients developing SPLCs after 2 years. These were biopsy-proven in 11out of 19 patients. All but one SPLC were in a different lobe or contralateral side of the lung. One SPLC was in the same lobe as the initial primary but of a different histology (small cell lung carcinoma). Five non-biopsied SPLCs were in a different lobe than the initial primary. Of the remaining three SPLCs one occurred after an interval of >2 years and has not recurred again after a second course of definitive SBRT, one occurred in a location peripheral to the initial primary tumor, and one was difficult to distinguish from the primary tumor, as it was a squamous cell carcinoma that was subsequently surgically resected. Of the 19 patients with SPLC, 9 received SBRT for their SPLC, one had a wedge resection, one had definitive fractionated radiotherapy, four had palliative treatment, and 3 had no further treatment. Nineteen percent of SPLC were located in the involved ipsilateral lobe, 26% in the uninvolved ipsilateral lobe, and the remaining in the contralateral lung. The cumulative incidence of SPLC continued to rise up to 6 years from the end of SBRT. The 18-month and 2-year cumulative incidence rate of SPLC were 3.3% and 4.5%, respectively. None of the never smokers, but 4% (n=13) of former and 15% (n=6) of current tobacco smokers developed a SPLC (p=0.005). The 2-year cumulative incidence rate for SPLC was 0% for non-smokers, 3.5% for former tobacco smokers, and 15.1% for current tobacco smokers (Figure 3b). Seventy-six percent of patients who developed a SPLC were candidates for curative treatment for their SPLC.
Fig. 3.
A) Cumulative incidence of new primary disease (second primary lung cancer), any other progression, and death without any new primary or progression. B) Cumulative incidence of second primary lung cancers by smoking status.
DISCUSSION
Herein we report a large analysis of temporal patterns of recurrence and formation of SPLC in a cohort of pathologically proven inoperable early stage NSCLC patients treated with SBRT. A thorough understanding of recurrence patterns and the development of SPLC allows for improved insight into optimal surveillance strategies. This is especially relevant with the introduction and rapid adoption of SBRT. Current National Comprehensive Cancer Network (NCCN) surveillance guidelines for patients treated with either SBRT or surgical resection for early stage NSCLC recommend a chest CT with or without contrast every 6–12 months for the first two years post-treatment, followed by annual chest CT thereafter.8 However, recent RTOG studies such as RTOG 0236 have followed patients with a closer surveillance regimen.9 Similarly, our institutional surveillance regimen differs for patients treated with SBRT in that we recommend a CT chest with contrast every 3 months during years 1 and 2 and every 6 months during years 3 and 4. Data from our study and others lend support for a more frequent screening regimen then the current NCCN recommendations.4,8,10
The majority of data to support the current NCCN recommendations comes from CT screening trials or large cohorts of patients treated for early stage NSCLC with surgical resection.10–11 However, even the granular supporting data for the current guidelines are unclear regarding the optimal surveillance regimen. One such study reported by Lou and colleagues analyzed 1,294 patients treated with surgical resection who were followed as per current NCCN guidelines. They found that scheduled surveillance scans detected only 61% of recurrences, and that in the first 4 years there was a 6–10% per year recurrence rate. This suggests that nearly 40% of recurrences were detected by symptomatic progression in between routine surveillance imaging. Furthermore, even when only assessing loco-regional recurrences within the field of view of routine CT chest imaging, the current surveillance recommendations fail to detect almost 20% of loco-regional recurrences that were detected most commonly from symptomatic progression between surveillance scans.
A systematic review and meta-analysis has been performed to identify the value of more intensive surveillance.11 Calman et al meta-analyzed nine studies comprised of 1,669 patients treated with curative resection for stage I–III NSCLC and palliative cohorts of patients treated for small cell lung cancer. Among six of these studies of patients treated with curative intent, the authors found a near significant improvement in survival from more intensive follow-up programs (HR 0.83 [95%CI 0.66–1.05]). Furthermore, the authors identified a significant improvement in survival when recurrences were detected by CT screening (asymptomatic) compared to symptomatically prompted identification of recurrent disease (HR 0.61 [95%CI 0.50–0.74]). This would suggest that especially during the first 3 years when nearly all recurrences in our experience are detected, a more rigorous surveillance schedule may be appropriate.
The previous studies have all examined surveillance regimens in surgically treated cohorts. Important patient and potentially biologic differences exist between patients treated SBRT compared to surgical resection. First, by definition patients undergoing SBRT are usually inoperable due to medical comorbidities, which are often related to severe chronic obstructive pulmonary disease. Second, patients treated with SBRT have a low dose bath of radiation therapy to select volumes of adjacent lung parenchyma which may contribute to excellent local control, but may make the detection of recurrences or nearby SPLC more difficult. Third, patients treated with a segmentectomy or lobectomy by definition have removed adjacent lung tissue and approximately 20% of SPLC occur in the same lobe.4 Lastly, nodal staging for surgical patients is generally more comprehensive with either mediastinoscopy or endobronchial ultrasound and mediastinal lymph node dissection. For these reasons it is unclear if surveillance regimens for all patients should be identical.
Select reports on recurrence rates and the formation of SPLC in patients undergoing SBRT for early stage lung cancer have been investigated.4 Senthi et al reported on 676 patients treated with SBRT for early stage NSCLC. Their surveillance regimen consisted of CT chest imaging at 3, 6, and 12 months post-treatment, then at yearly intervals thereafter. Unfortunately, only 35% of the patients had pathologically confirmed disease which limits the estimates, especially for SPLC. Nonetheless, they reported a 6% incidence of SPLC at a median time of 18.0 months post-treatment, similar to our reported rate of 5.2% at a median time of 18.9 months post-treatment. Importantly, the majority of patients with SPLC was eligible for curative treatment for their SPLC. As it has been demonstrated that treatment (surgery or radiotherapy) improves outcomes over untreated early stage lung cancer, their data as well as ours lend support for continued long-term surveillance post-treatment for the early detection and intervention for SPLC.4,5
A key finding in our study relates to the correlation of smoking status with the formation of SPLC, with none of the never smokers, 4% of former smokers, and 15% of current smokers develop a SPLC (p=0.005). This finding confirms the hypothesis that tobacco use induces mutations stochastically in all exposed lung parenchyma, thus putting the entire lung at risk of developing cancer. However, a risk-adapted surveillance approach may be warranted based on tobacco use, analogous to current CT screening recommendations.3 Our study is consistent with the prior literature in that all recurrences (excluding SPLC) plateau around 24 to 30 months post-treatment, thus warranting close surveillance prior to this time period. However, after 2 years the increase in recurrent disease is minimal while the risk for SPLC continues to rise, and annual CT surveillance is appropriate. This is especially important for current and former smokers, but it remains a valid open question if non-smokers, especially with adenocarcinoma histology, need similar imaging surveillance due to limited likelihood of developing recurrent disease after 2 years and the exceedingly rare formation of SPLC.
Despite our rigorous attempt to quantify failure type (local, nodal, distant, or SPLC) and predictors of failure, limitations of our study are present. Although attempts were made to limit bias, given the retrospective nature of our study inherent bias is likely present. In any population of patients undergoing SBRT, even in our study which had 100% pathologically diagnosed disease, there is a possibility that the primary was in fact a metastatic site from an undocumented NSCLC. Furthermore, although all available data were used to determine if a lesion was a recurrence versus a SPLC, without full evolutionary genomic analytics there is some uncertainty in this distinction. Lastly, our recommendations must be weighed against the risks associated with false-positive detection. Our study was unable to capture the number of added procedures from more frequent screening, which must be factored in when optimizing a surveillance program.
CONCLUSION
Close monitoring with routine CT scans within the first 2 years after SBRT is effective in detecting early disease progression. In contrast, the risk for developing a SPLC remains elevated beyond 2 years. Therefore, we recommend CT surveillance at close intervals in patients treated with SBRT, and stress that long term surveillance is critical, particularly in former and current smokers.
Supplementary Material
Clinical Practice Points.
The use of SBRT for the treatment of early stage NSCLC is rapidly increasing. Current National Comprehensive Cancer Network (NCCN) surveillance guidelines for patients treated with either SBRT or surgical resection for early stage NSCLC are identical, and recommend a chest CT with or without contrast every 6–12 months for the first two years post-treatment, followed by annual chest CT thereafter. However, recent RTOG studies such as RTOG 0236 have followed patients with a closer surveillance regimen. Using close imaging survelliance post-SBRT, CT scan of the chest every 3 months during years 1 and 2, every 6 months during years 3 and 4, and annually thereafter, we demonstrate that close monitoring with routine CT scans within the first 2 years after SBRT is effective in detecting early disease progression. In contrast, the risk for developing a second primary lung cancer (SPLC) remains elevated beyond 2 years. Furthermore, none of the never smokers, but 4% of former and 15% of current smokers developed a SPLC (p=0.005). Therefore, we recommend CT surveillance at closer intervals in patients treated with SBRT than the current NCCN guidelines, and stress that long term surveillance is critical, particularly in former and current smokers.
Acknowledgments
Andreas Rimner is a consultant for Bristol Myers Squibb and has grants for Varian Medical Systems and Boehringer Ingelheim. These financial activities do not relate to the current work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented at the ASTRO 2014 meeting, but has not been submitted for publication elsewhere.
Conflict of interest: All other authors have no conflicts of interest to report.
REFERENCES
- 1.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non–small-cell lung cancer: A population-based time-trend analysis. Journal of clinical oncology. 2010;28(35):5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. The lancet oncology. 2012;13(8):802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 5.McGarry RC, Song G, des Rosiers P, Timmerman R. Observation-Only Management of Early Stage, Medically Inoperable Lung CancerPoor Outcome. CHEST Journal. 2002;121(4):1155–1158. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 6. xxx. [Google Scholar]
- 7.Martini N, Melamed MR. Multiple primary lung cancers. The Journal of thoracic and cardiovascular surgery. 1975 Oct;70(4):606–612. [PubMed] [Google Scholar]
- 8.Ettinger DS, Wood DE, Akerley W, et al. Non–Small Cell Lung Cancer, Version 1.2015. Journal of the National Comprehensive Cancer Network. 2014;12(12):1738–1761. doi: 10.6004/jnccn.2014.0176. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA - Journal of the American Medical Association. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. The Journal of thoracic and cardiovascular surgery. 2013;145(1):75–82. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Calman L, Beaver K, Hind D, Lorigan P, Roberts C, Lloyd-Jones M. Survival benefits from follow-up of patients with lung cancer: a systematic review and meta-analysis. Journal of Thoracic Oncology. 2011;6(12):1993–2004. doi: 10.1097/JTO.0b013e31822b01a1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.