Abstract
Cocaine has long been known to produce an initial “high” followed by an aversive/anxiogenic “crash”. While much is known about the neurobiology of cocaine’s positive/rewarding effects, the mechanisms that give rise to the drug’s negative/anxiogenic actions remain unclear. Recent research has implicated the lateral habenula (LHb) in the encoding of aversive events including the anxiogenic response to cocaine. Of particular interest in this regard are the reciprocal connections between the LHb and the ventral tegmental area (VTA). VTA-DA neurons innervate different subsets of LHb cells that in turn feedback upon and modulate VTA neuronal activity. Here we examined the impact of D2 receptor activation and inhibition on the anxiogenic response to cocaine using a runway model of self-administration that is sensitive to the dual and opposing effects of the drug. Male rats ran a straight alley for IV cocaine (1.0 mg/kg) following bilateral intra-LHb infusions of the D2 receptor antagonist, cis-flupenthixol (0, 7.5 or 15μg/side) or the D2 agonist, sumanirole (0, 5 or 10μg/side). Vehicle-pretreated controls developed approach-avoidance conflict behaviors about goal-box entry reflective of the dual positive and negative effects of cocaine. These behaviors were significantly diminished during LHb-D2 receptor antagonism and increased by the LHb D2 receptor agonist. These results demonstrate that activity at the D2 receptor in the lateral habenula serves to modulate the anxiogenic response to cocaine.
Keywords: cocaine, lateral habenula, runway self-administration, cis-flupenthixol, sumanirole, anxiety
1. Introduction
Human users of cocaine report that the initial “high” felt after taking cocaine is characterized by euphoria, pleasure, and energy (Boutrel & Koob, 2004; Fischman et al., 1976; Gawin, 1991; Resnick & Resnick, 1984). Similarly, laboratory animals will voluntarily self-administer the drug (George & Goldberg, 1989; Woolverton, 1992), and exhibit preferences for places or stimuli associated with the drug (Bardo et al., 1995; Carr, Fibiger & Phillips, 1989; Childress et al., 1988; O’Brien et al., 1998; Rosenow et al., 2007). However, in addition to its euphoric effects, cocaine administration produces strong negative aftereffects. Human users of cocaine report that this “crash” is characterized by dysphoria, anxiety, and craving for the drug (Anthony et al., 1989; Resnick et al., 1977; Rohsenow et al., 2007; Williamson et al., 1997). The anxiogenic effects of acute cocaine administration have also been noted in multiple laboratory animal experiments, including demonstrations that cocaine increases anxious behavior in the elevated plus maze (Rogerio & Takahashi, 1992), increases the latency of an animal to enter an open field (Yang et al., 1992), and can produce “panic-like” responses in both mice and monkeys (Blanchard & Blanchard 1999; Crowley et al., 1992). These dual positive and negative effects of cocaine can be seen in a single animal during a single trial using a runway self-administration test. Here animals are trained to run down a straight alleyway and enter a goal box to earn a single daily IV infusion of cocaine. Over trials, the animals begin to display an approach-avoidance conflict behavior in which they advance toward the goal box, but stop before entering, then turn, and retreat back toward the start box (Ettenberg, 2004; Ettenberg & Geist, 1991; Ettenberg & Geist, 1992). This “retreat” behavior has been shown to stem from the dual positive and negative associations that animals form with the goal box in which they experienced the cocaine (e.g., see reviews by Ettenberg 2004, 2009) and is in fact comparable to behavior observed in animals approaching a goal box previously associated with both food and foot-shock (Geist & Ettenberg, 1997). In addition, while animals develop place preferences for locations paired with the immediate effects of IV cocaine, they develop aversions for places paired with the effects present 15 minutes post-IV injection (Ettenberg, 2004; Jhou et al., 2013). Thus, there is significance in fully understanding the behavioral and underlying biological bases for these opposing effects as any decision to seek the drug must logically involve the individual’s assessment of the cost/benefit (positive versus negative) attributes of the drug experience (Ettenberg et al., 2015).
Previous work has demonstrated that the dopaminergic (DA) neurons that extend from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) play a key role in cocaine’s rewarding effects. Cocaine blocks the presynaptic reuptake of DA, effectively increasing the amount of extracellular DA – an effect thought by many to result in the experience of drug reward (Dackis & O’Brien 2001; Di Chiara & Imperato, 1988; Koob et al. 1994; Roberts et al. 1977; Wise et al. 1995). Additionally, lesions or antagonist drugs that disrupt this system have been shown to attenuate both cocaine-induced conditioned place preferences (Isaac et al., 1989, Morency & Beninger, 1986, Spyraki et al., 1987; Veeneman et al., 2011) as well as operant self-administration of the drug (Ettenberg et al., 1982, Pettit et al., 1984, Roberts et al., 1977; Roberts & Koob, 1982). But what brain structures or circuitry are responsible for cocaine’s delayed anxiogenic effects? While less is known about these negative actions of the drug, one structure that has garnered much recent attention is the lateral habenula (LHb).
The LHb receives projections from a multitude of brain sites that have been implicated in the production of both rewarding and aversive states (e.g., see reviews by Lecca et al., 2014; Meye et al., 2013; Velasquez et al., 2014) and has itself been implicated in the behavioral response to numerous anxiogenic and aversive stimuli (Amat et al., 2001; Caldecott-Hazard et al., 1988; Gao et al., 1996; Murphy et al., 1996; Stamatakis & Stuber, 2012). For example, in recording studies, researchers have found that the lateral habenula is activated by both the presentation of aversive stimuli as well as by the absence of an expected reward (Bromberg-Martin & Hikosaka, 2011; Matsumoto & Hikosaka, 2007). Recent studies have proposed that the LHb may facilitate the onset of cocaine’s negative properties, possibly by inhibiting the action of midbrain DA neurons in the VTA (Friedman et al., 2010; Jhou et al., 2009). The mechanism through which this occurs is thought to be the rostro-medial tegmental nucleus (RMTg) – a small cluster of cells situated at the tail end of the VTA that send inhibitory GABAergic projections into the VTA (Balcita-Pedicino et al., 2011; Jhou et al., 2009a; Jhou et al., 2009b; Kaufling et al., 2009). It appears then that activation of cells within the LHb results in a glutamatergic activation of the RMTg that in turn inhibits cell firing within the VTA (Good et al., 2013; Jhou et al., 2009a; Jhou et al., 2009b; Kowski et al., 2009). To complete the circuit DA cells within the VTA have themselves been shown to project to the LHb (Gruber et al., 2007; Skagerberg et al., 1984) where D2 and D4 receptors have been identified (Aizawa et al., 2012; Good et al., 2013; Jhou et al., 2013; Weiner et al., 1991) and DA receptor activation has been shown to alter of LHb neuronal activity (Kowski et al., 2009).
It would seem then that activity in this LHb-RMTg-VTA circuit is anatomically and functionally well suited to play a significant role in the affective response to cocaine. Indeed, Jhou et al. (2013) have shown that a subset of LHb neurons exhibit biphasic responses to IV cocaine with an initial suppression of neuronal firing (thereby removing the LHb-RMTg inhibition of the VTA and permitting the reward signal to prevail) followed by a delayed excitation whose timing parallels cocaine’s shift from rewarding to aversive (Ettenberg et al., 1999; Ettenberg, 2004). Others have also reported that cocaine actions within the LHb are implicated in cocaine-seeking behavior and the reinstatement of cocaine-seeking following cessation of drug self-administration (e.g., Friedman et al., 2010; Mahler & Aston-Jones, 2012; Morteaux & Mameli, 2012; Neumann et al., 2015; Zuo et al., 2013). In this context, the current study was devised to contribute to this body of work by examining the effects of D2 activation and inhibition within the LHb on the dual positive and negative effects of cocaine as measured in a unique operant runway model of drug self-administration.
2. Methods
2.1 Subjects
Subjects were 87 male Sprague-Dawley albino rats obtained from Charles River Laboratories (Wilmington, MA, USA) and weighing between 300-500g at the time of surgery. Animals were pair-housed in a temperature-controlled (23°C), reverse light-cycle room which alternated 12 hours of light and 12 hours of darkness (lights on at 8:00pm). Food and water were provided ad libitum and rats were gentled through daily handling for one week prior to surgery.
2.2 Surgery
Each rat was surgically implanted with an IV catheter (13mm long, 0.3mm inner tubing and 0.64mm outer tubing diameters, Dow Corning Corp, Midland, MI, USA) under deep anesthesia induced by an intra-muscular injection of a combined solution of ketamine and xylazine (56.5 and 7.5mg/kg, respectively). Subjects were also given a subcutaneous (SC) injection of the non-opioid analgesic, flunixin meglumine (1.3 mg/kg in 0.06 ml/kg FluMeglumine; Phoenix Pharmaceuticals, Belmont, CA) to reduce post-operative pain. One end of the catheter was inserted into the jugular vein and secured in place by suture while the other end was affixed to a guide cannula (Plastics One, Roanoke, VA) that was passed subcutaneously to a small opening on the midline of the rat’s back. The guide cannula was itself affixed to a small square of Marlex mesh (Bard; Warwick, RI) with the cannula positioned to protrude through the small opening in the skin and the Marlex mesh laid flat and subcutaneously on the animal’s back. Following implantation, catheters were flushed with ticarcillin-clavulanate (50 mg in 0.25 ml IV) followed by heparinized saline (6.0 IU heparin in 0.1 ml 0.9% physiological saline IV) to ensure catheter patency and reduce the risk of microbial infection. Rats were then given a SC injection of 3.0 ml of 0.9% physiological saline to prevent dehydration. During the same surgical session as the catheter implantation, rats were stabilized in a stereotaxic unit, and fitted with bilateral chronic indwelling guide cannula (22-gauge, 5mm, Plastics One, Roanoke, VA, USA) aimed 2 mm above the target site. Cannula were secured with acrylic dental cement and four stainless steel screws mounted to the animal’s skull. The following LHb coordinates were derived from Jhou et al. (2013) and confirmed in pilot studies: tooth-bar set at 10°; AP −3.4; ML ±1.5; DV −4.2mm from bregma.
Animals were allowed to recover for one week before behavioral testing. Catheter patency was established by assessing the loss of the righting reflex after IV injection of the fast-acting barbiturate methohexital (Brevital, 2.0 mg/kg/0.1 ml). Animals that were unresponsive to Brevital before testing were re-implanted with a new catheter using the left jugular vein and given additional days for recovery. If catheter patency failed during the course of behavioral testing, that animal was removed from data analysis; a total of five animals were removed due to catheter failure.
2.3 Drugs
Cocaine hydrochloride (provided by the National Institute of Drug Abuse) was dissolved in a vehicle solution of 0.9% physiological saline and administered IV in a dose of 1.0 mg/kg (in a volume of 0.1 ml delivered over a period of 4.5 s) via a 10 ml syringe seated in a motorized syringe pump (Razel Scientific Instruments, St. Albans, Vermont, USA). This cocaine dose was selected to produce an optimal behavioral response (highest positive relative to negative impact), as shown in previous work (e.g., Raven et al., 2000).
For intracranial (IC) administration, the DA antagonist cis-flupenthixol (0, 7.5, or 15μg/injection) or the D2 agonist sumanirole (0, 5, or 10μg/inj) were each dissolved in 0.9% physiological saline and administered bilaterally in volumes of 0.25 μl delivered over 75 s.
2.4 Apparatus
Runway Apparatus
Two identical runway apparatus were utilized in the current experiment each consisting of a straight alleyway (155 cm L×15 cm W×40 cm H) that connected the “start box” (24×25×40 cm) at one end to the “goal box” (24×25×40 cm) at the opposite end. The floor of each apparatus was composed of small diameter steel rods aligned perpendicular to the side walls of the alley. Retractable doors gated the entrance to both the start and goal box. Thirteen infrared photodetector-emitter pairs were positioned along the length of the runway walls approximately 16 cm apart and 5 cm above the floor. Input from these photocells was fed through an ANY-maze interface (Stoelting Co., Wood Dale, IL) to a laptop computer running customized software that served to control the start/goal doors and drug infusions, and to record the animal’s position in the alley in real time.
Running lengthwise above each runway was a pair of magnetic tracks between which was positioned a free-moving swivel (375-22PS; Instech Laboratories Inc., Plymouth Meeting, PA). The swivel penetrated (and was cemented into) the middle of a flat 4-inch Plexiglas disk (a “skirt”) that prevented the swivel from falling between the rails. Affixed to the bottom of the “skirt” was a pot magnetic whose polarity was arranged to repel that of the tracks and thereby permitted the entire assembly to float a few centimeters above the rails. PE20 tubing connected the IV catheter on the animal’s back to the swivel, and the swivel to a 10 ml syringe seated within a computer-controlled syringe pump. This set-up allowed for the precise automated IV administration of drug upon the subject’s goal-box entry, while allowing the rat to move about freely within the alley (for a more detailed description of the runway apparatus see Geist & Ettenberg, 1990).
Locomotor apparatus
Locomotor activity was measured in 12 identical Plexiglas chambers (each 20 cm L × 40 cm W × 20 cm H; Kinder Scientific, San Diego, CA). Embedded in the walls 8 cm above the floor of each chamber were 15 infrared photodetector-emitter pairs evenly spaced along the long axis and 7 more along their narrow axis. Movement within the chamber produced infrared-beam interruptions that were recorded by a desktop computer running custom software (Kinder Scientific). The data were collected as the distance travelled by each subject in cm.
2.5 Procedure
Runway self-administration procedure
One week post-surgery, animals were habituated to the runway apparatus (with the goal door closed) for a single 10-min session. Testing then began the next day and consisted of 15 consecutive once-daily trials. Prior to each trial, subjects were administered IC bilateral LHb infusions of either the D2 antagonist cis-flupenthixol (0, 7.5, or 15μg/injection/side; n = 7, 11 and 11 respectively) or the D2 agonist sumanirole (0, 5, or 10μg/injection/side; n = 12, 12 and 16 respectively). Doses were selected on the basis of prior behavioral studies with each of these compounds (Duvauchelle et al., 1992; Fernando et al., 2012; Koffarnus et al., 2011; Moscarello et al., 2010; Weber et al., 2010). Following a 5-min wait to account for drug diffusion, subjects were connected to the IV drug-delivery system and placed into the runway start box. The trial was initiated 5 s later with the opening of the start door. Upon the animal’s entry into the goal box the goal door was automatically closed and a single IV infusion of cocaine (1.0 mg/kg) was delivered. The rat remained in the goal box for 5-min after which it was returned to its home cage. The following dependent measures were recorded on every trial: start latency - the time it took the animal to leave the start box once the start door was opened; run time - the time elapsed between the animal’s departure from the start box and entry into the goal box; and retreat frequency - the number of times the animal stopped its forward locomotion, turned, and retraced its path toward the start box.
Locomotor procedure
Locomotor behavior was assessed to determine the effects of cis-flupenthixol and sumanirole on spontaneous movement. Testing was conducted over the course of one day during which the distance traveled by each subject (in cm) served as an index of locomotor activity. Animals were first habituated to the chamber for 50 minutes, then removed from the chamber, given an IC injection of cis-flupenthixol, sumanirole, or vehicle (same concentrations as in runway) and returned to the locomotor chambers for 15 min during which their locomotor activity (distance traveled) was recorded.
3. Results
3.1 Histology
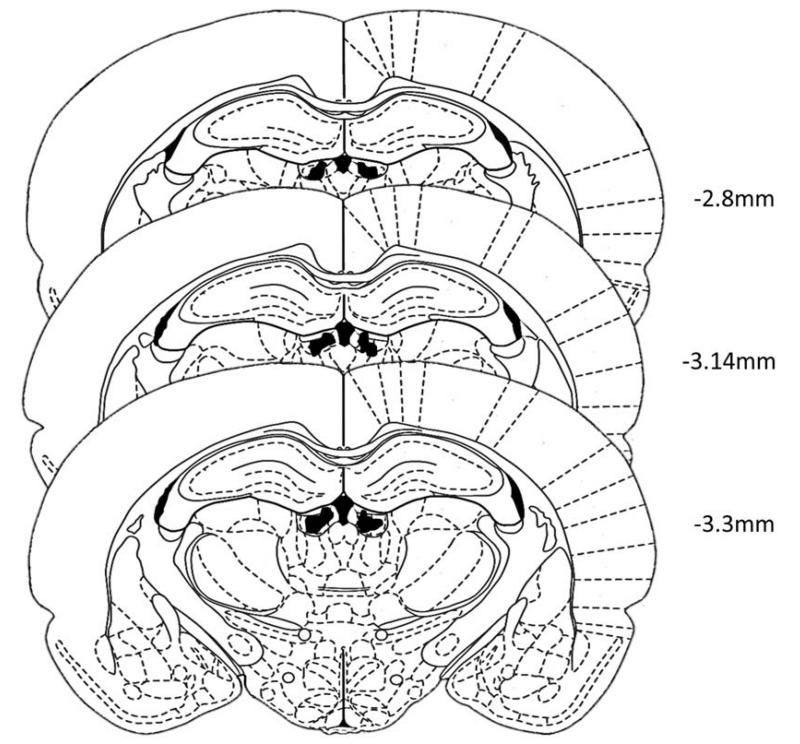
Figure 1 shows the brain regions within which the injection cannula were located in the LHb. A total of 13 animals were removed from the study because their cannula lay outside the target region.
Figure 1.
Bilateral cannula targeted to the lateral habenula. Shaded areas on either side of the third ventricle indicate the regions within the LHb where histological analyses confirmed the location of intracranial cannulae. “mm” represent distance posterior to bregma. Figure adapted from Paxinos & Watson (1998). Animals whose cannula were found to be outside the region of the habenula (n=13) were removed from the data analyses.
3.2 Runway Self-Administration
Cis-flupenthixol
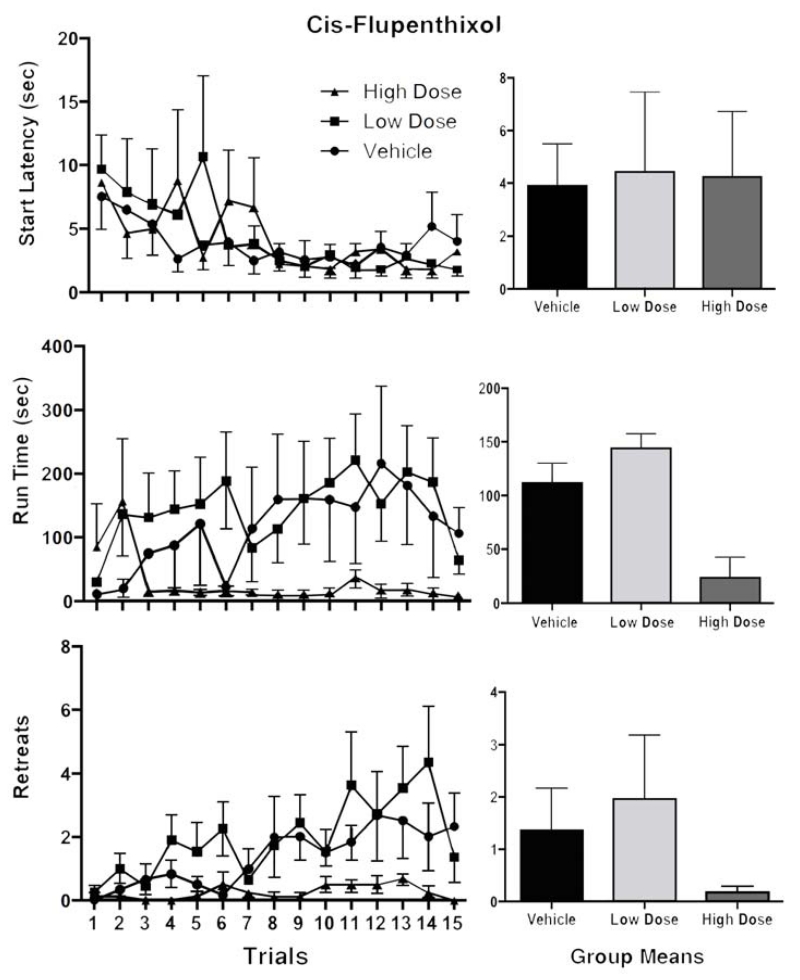
The runway behavior of animals treated with the D2 antagonist, cis-flupenthixol, are depicted in Figure 2. Start latencies (top panels of Fig 2), which are presumed to reflect the positive incentive properties of the goal-box experience, decreased comparably across all three groups as trials progressed. A two-factor Group × Trial Analysis of Variance (ANOVA) performed on these data confirmed a statistically reliable decrease in start latencies over trials (F (14, 364)=2.05, p < 0.02) but no main effect of Group nor any Group × Trial interactions (p > 0.05). In contrast, the middle and bottom panels of Figure 2 suggest that animals treated with cis-flupenthixol behaved differently than vehicle-treated controls. The ANOVAs computed on the data depicted in the figure confirmed the presence of a statistically reliable main effect for run time (F(2,26) = 3.38, p <.05) and for retreat frequency significant effects were obtained for Trial (F (14, 364) = 2.32, p < 0.001), Group (F (2, 26) = 5.63, p < 0.01), and a marginal Group × Trial interaction (F(28,364) = 1.38, p = 0.09) results all clearly stemming from the virtual absence of approach-avoidance retreats observed in the high dose group.
Figure 2.
Mean (+/−SEM) Start Latencies (top panels), Run Times (middle panels) and Retreat frequency (bottom panels) for rats running for IVcocaine following bilateral intra-LHb infusions of 0.0, 7.5 or 15.0 μg/side of the D2 antagonist, cis-flupenthixol. The bar graphs depict the average scores (+/− SEM) for each group across all trials.
Sumanirole
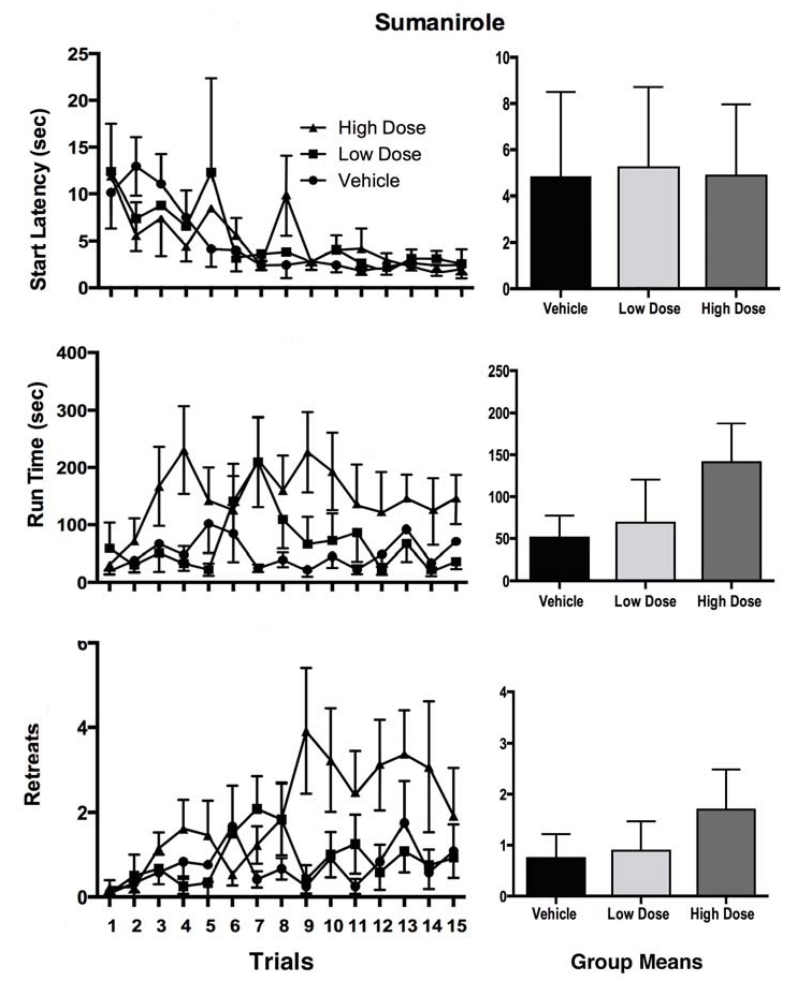
The runway performance of rats treated with the D2 agonist, sumanirole, is shown in Figure 3. Once again, two-factor Group × Trial ANOVA were computed on each of the three dependent measures examined in the study. As was the case for cis-flupenthixol, sumanirole-treated subjects exhibited decreases in start latencies as testing progressed (a main effect of Trial; F (14,476) = 3.78, p<0.001), with no main effect of Group and no Group × Trial interaction (p>.05). In contrast to cis-flupenthixol, the D2 agonist reliably increased run times over trials (F (14, 518) = 2.39, p < 0.004) and produced a reliable Group × Trial interaction (F (28, 518) = 1.58, p < 0.04) indicating that treated subjects behaved differently over trials. Indeed, as the figure shows, while all groups tended to slow their run times over trials, the effect was most pronounced in the high dose group. The bottom panels of Fig. 3 depict the retreat frequencies of the sumanirole-treated rats. When averaged across all three groups, approach-avoidance behavior tended to increase over trials (F (14, 518) = 2.09, p < 0.02), and while the main effect for Group was only marginally reliable (F(2,37) = 23.91, p =0.06) the ANOVA identified a statistically significant Group × Trial interaction (F(28,518) = 1.54, p < 0.05) a result that clearly stemmed from the fact that while all groups exhibited few and comparable numbers of retreats at the outset of testing, over trials only the high dose group exhibited an elevation in approach-avoidance behavior.
Figure 3.
Mean (+/−SEM) Start Latencies (top panels), Run Times (middle panels) and Retreat frequency (bottom panels) for rats running for IV cocaine following bilateral intra-LHb infusions of 0.0, 5.0 or 10.0 μg/side of the D2 agonist, sumanirole. The bar graphs depict the average scores (+/− SEM) for each group across all trials.
3.3 Locomotor Test
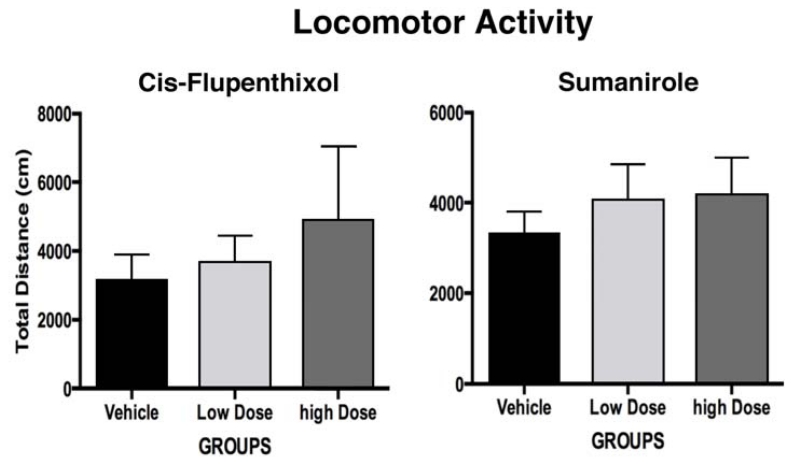
To ensure that drug-induced changed in runway performance were not due to any non-specific motoric effects of the pretreatments, animals underwent spontaneous locomotor activity testing during challenge with cis-flupenthixol or sumanirole. One way-ANOVA confirmed that, for either drug, there were no differences in initial locomotor behavior during the pre-injection baseline/habituation period p>.05). The locomotor data obtained during the 15-min test session (i.e., following IC infusions) are shown in Figure 4. One-way ANOVA computed on the data for each drug again revealed no reliable group differences in locomotor activity following either dose of cis-flupenthixol or sumanirole (p>.05).
Figure 4.
Effects of bilateral IC infusions of the vehicle, low dose, or high dose of cis-flupenthixol or sumanirole into the LHb on the mean (+/− SEM) distance traveled (in cm) during a 15-min locomotor activity test
4. Discussion
Our current results confirm what we have previously reported in that animals running an alley once a day for a single IV injection of cocaine developed an ambivalence about goal box entry that increases in strength over the course of testing (Ettenberg, 2004, 2009; Ettenberg et al., 2011, 2015; Ettenberg & Geist, 1991, 1992). This ambivalence was reflected in the development of an approach-avoidance conflict (retreat behaviors) that results from the subjects’ dual positive (rewarding) and negative (anxiogenic) associations with the cocaine-paired goal box (Ettenberg 2004, 2009; Geist & Ettenberg, 1997). Here, we demonstrated that bilateral local LHb administration of the DA antagonist, cis-flupenthixol, reliably reduced the anxiogenic response to cocaine, while infusion of the D2 agonist, sumanirole, produced the opposite effect. While, cis-flupenthixol is a potent antagonist at both the D1 and D2 dopamine receptor (Creese et al., 1983; Murrin, 1983), the distribution of D1 receptors in the LHb has been shown to be extremely low relative to the D2 receptor (Wamsley et al., 1989, Weiner et al., 1991) suggesting that flupenthixol’s effects in the LHb are likely attributable to an action at D2 receptors. Consistent with this conclusion is the fact that sumanirole has an affinity for the D2 receptor that is reportedly more than 200-fold greater than it has for other DA receptor subtypes (McCall et al., 2005). Hence, the demonstration that cis-flupenthixol and sumanirole had opposing actions in the current study leads us to conclude that the changes in the behavioral anxiogenic response to cocaine observed in the present study are due to treatment-induced modulations of activity at the D2 receptor within the LHb.
These results add to a growing literature demonstrating that the administration of cocaine alters the neuronal activity of the LHb and that such activity in turn modulates the affective response to the drug. For example, Friedman et al. (2010) have reported that deep brain electrical stimulation of the LHb produces an enduring inhibitory effect on cocaine-seeking behavior (Friedman et al., 2010) and suggest that elevations in LHb neuronal activity are associated with an enhanced anxiogenic response to the drug that acts to suppress cocaine-reinforced responding. The notion that LHb activity is associated with a suppression of cocaine-reinforced behavior is supported by the findings of Mahler and Aston-Jones (2012) who observed increases in LHb Fos immunoreactivity (a marker of neuronal activity) in cocaine self-administering rats during negative states - i.e., when the reinforcer was removed (i.e., during extinction trials) and upon presentation of a light/tone stimulus (a CS-) predicting cocaine’s non-availability. Other researchers have provided evidence that cocaine activates glutamatergic LHb projections to the RMTg and thereby facilitates the expression of the drug’s anxiogenic effects via an indirect inhibition of the reward elements within the VTA (e.g., Jhou et al., 2013; Meye et al., 2015; Zuo et al., 2013). The current results support this view in that modulation of the D2 receptor in the LHb produced reliable alterations in the anxiogenic response to cocaine.
Note that nonspecific impairments in the motoric capacity of our subjects cannot easily account for the observed changes in runway behavior during LHb-D2 receptor modulation since neither the antagonist nor agonist treatments produced any reliable changes in locomotor behavior (See Fig. 4). It is also noteworthy that neither cis-flupenthixol nor sumanirole altered the start latencies of the subjects relative to vehicle controls. In all groups, animals improved their start latencies (initiated responding faster) over trials suggesting that the “approach” component of the behavior – i.e., the positive incentive properties of the cocaine – remained intact even while retreat behaviors were developing. So while cis-flupenthixol and sumanirole had different and opposite effects on retreat frequency, neither treatment appears to have altered the subjects’ motivation to seek the cocaine. This suggests that the impact of D2-LHb modulations was selective for the aversive/anxiogenic effects of the cocaine, while leaving the positive incentive/rewarding properties of the drug relatively unaffected. It may therefore be that different subsets of cells within the VTA are responsive to the positive and negative properties of incentive stimuli and that the latter selectively impacts LHb neurons. Indeed, consistent with this view are the recent findings of Stamatakis et al., (2013) suggesting that the neurons projecting to the LHb are anatomically distinct from those reward-related DA neurons projecting to the nucleus accumbens.
Although our histological analysis was careful to eliminate data from any animals whose cannula were found to be outside the LHb, one may argue that the infusions of cis-flupenthixol and/or sumanirole still managed to diffuse to more ventral areas of the brain. Of particular interest in that regard is the paraventricular nucleus of the thalamus (PVT), which lies immediately below the LHb, is innervated by DA terminals, and contains a high density of D2 receptors (Allen Institute for Brain Science, 2015; Garcia-Allen Cabezas et al., 2009; Lein et al., 2007; Li et al., 2014; Takada et al., 1990). Indeed, DA manipulations of the PVT have been reported to produce changes in rewarding/reinforcing properties of the drug (e.g., Hsu et al., 2014; Millan et al., 2011). That being said, it seems unlikely that the PVT is responsible for the results described herein. Research on the role of the PVT in cocaine reward suggests that the structure contributes to or facilitates drug-seeking behavior in that its inactivation leads to decreases in cocaine-seeking (Browning et al., 2014; James et al., 2010; Matzeu et al., 2015). In the current study, DA antagonism produced the opposite effect – i.e., fewer retreats (less anxiogenic response) and no reductions in the subjects’ motivation to initiate responding (no change in start latencies). Thus while we cannot rule out the possibility that the DA manipulations employed in the current research altered the functionality of PVT D2 receptors, the behavioral results suggest that any such impact, if it occurred, was minimal.
While a role for the LHb in the behavioral response to a variety of stress-inducing, anxiogenic, or aversive stimuli, is not new (e.g., Amat et al., 2001; Caldecott-Hazard et al., 1988; Gao et al., 1996; Matsumoto & Hikosaka, 2007; Murphy et al., 1996; Stamatakis & Stuber, 2012), the precise neurochemical mechanism(s) through which the structure exerts its behavioral functionality remains unclear. Recently, Good et al. (2013) have presented evidence suggesting that the LHb can inhibit VTA neuronal activity (and hence modulate the DA response to rewarding stimuli) via two distinct pathways: a direct glutamatergic excitatory path from LHb to VTA that is inhibited by activity at D2 receptors within in the LHb, and an indirect pathway activated by excitatory D4 receptors and projecting to GABAergic cells in the RMTg that serve to dampen the responding of DA neurons within the VTA. The current data are of course consistent with this model. Intra-LHb application of the D1/D2 antagonist, cis-flupenthixol, decreased the development and frequency of runway retreat behaviors while the selective D2 agonist, sumanirole, had the opposite effect and enhanced the retreat frequency of animals relative to vehicle controls. Thus, based upon the identification of both a VTA projection to the LHb and the existence of post-synaptic D2 and D4 receptors on the cell bodies of LHb neurons, it seems reasonable to conclude that these receptors are responsive to DA released from cells emanating from the VTA (Good et al., 2013; Gruber et al., 2007; Jhou et al., 2013; Meye et al., 2013; Skagerberg et al., 1984). However, more recent research has indicated that the reality is somewhat more complex. Stamatakis et al. (2013), for example, have reported that while optogenetic activation of the VTA-LHb pathway produces conditioned place preferences (presumably by inhibiting the activity of LHb neurons) the application of a cocktail of D1/D2 receptor antagonists did not disrupt the development of such preferences. Additionally, Root et al. (2013) have recently reported that while >90% of the VTA neurons projecting to the LHb contain tyrosine hydroxylase and a subset of these also contain L-aromatic amino decarboxylase, and hence are capable of synthesizing DA, these cells lack the capacity for accumulating DA in synaptic vesicles due to the absence of the vesicular monoamine transporter 2 (Root et al., 2015; see also Li et al., 2013). Indeed, electrical stimulation of the LHb did not evoke DA-like signals using fast-scan cyclic voltammetry (Root et al., 2013), and direct optogenetic activation of the VTA-LHb pathway resulted in no detectable DA release in LHb brain slices (Stamatakis et al., 2013). Stamatakis et al., (2013) have suggested that these VTA-LHb neurons are “hybrid dopaminergic-GABAergic” cells that suppress LHb output via release of GABA, and not DA, during rewarding conditions. This, of course, raises the obvious question: if the VTA terminals in the LHb are not releasing DA, then what endogenous ligands are acting on the D2 and D4 receptors?
A partial answer to this question might stem from the fact that norepinephrine (NE) has previously been shown to act as an agonist at the DA D4 receptor (Newman-Tancredi, 1997) and Root et al., (2015) have recently reported NE release within the LHb. NE fibers have long been know to innervate the LHb from the A6 cell group of the locus coeruleus (Gottesfeld, 1983) and NE release has similarly long been known to occur during periods or stress and anxiety, both factors that potentiate drug-seeking and induce relapse of drug-seeking behavior after withdrawal (Aston-Jones & Harris, 2004; Aston-Jones & Kalivas, 2008; Smith & Aston-Jones, 2008). So in times of stress or anxiety, NE release within the LHb may activate D4 receptors and thereby suppress VTA activity via the LHb-RMTg-VTA circuit. Of course this still leaves unanswered the question about the endogenous ligand that normally binds to the DA D2 receptors within the LHb. Cocaine has been reported to depolarize and accelerate the spontaneous firing of LHb neurons in acute brain slices, an effect mimicked by D2 agonists, and reversed by application of D2 receptor antagonists (Zuo et al., 2013), a result consistent with the behavioral findings of the current study. Additionally, there are other sources of DA inputs to the LHb, including the periaqueductal grey, posterior hypothalamus and substantia nigra pars compacta (Gruber et al., 2007; Li et al, 1993) and electrical stimulation of the latter structure has been shown to alter neuronal activity within the LHb (Shen et al., 2012). In conclusion, while the source of the endogenous input responsible for activating the LHb D2 receptor remains unknown, we have shown that stimulation and inhibition of that receptor has reliable and predictable effects on the anxiogenic response to self-administered cocaine.
HIGHLIGHTS.
Rats are conflicted about approaching a goal box associated with IV cocaine
D2 receptor antagonism within the LHb reduced cocaine‐induced conflict behavior
D2 receptor activation within the LHb enhanced cocaine‐induced conflict behavior
Modulation of D2 receptor activity within the LHb alters to the anxiogenic response to cocaine
Acknowledgements
This work was supported by the National Institute on Drug Abuse-National Institutes of Health (Grant DA033370 awarded to A.E.). We thank Michael Brito, Maia Kurland, and Anjali Dixit for valuable technical support for this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Institute for Brain Science © Allen Mouse Brain Atlas [Internet] 2015 Available from: http://mouse.brain-map.org.
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphé nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Research. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Tien AY, Petronis KR. Epidemiologic evidence on cocaine use and panic attacks. American Journal of Epidemiology. 1989;129:543–549. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Kalivas PW. Brain norepinephrine rediscovered in addiction research. Biological Psychiatry. 2008;63:1005–1006. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. The Journal of Comparative Neurology. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neuroscience and Biobehavioral Reviews. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neuroscience and Biobehavioral Reviews. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;15:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nature Neuroscience. 2011;14:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JR, Jansen HT, Sorg BA. Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug and Alcohol Dependence. 2014;134:387–390. doi: 10.1016/j.drugalcdep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. Journal of Neuroscience. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. Clarendon Press; Oxford: 1989. [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Research Monograph. 1988;81:74–80. [PubMed] [Google Scholar]
- Creese I, Sibley DR, Hamblin MW, Leff SE. The classification of dopamine receptors: relationship to radioligand binding. Annual Review of Neuroscience. 1983;6:43–71. doi: 10.1146/annurev.ne.06.030183.000355. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, Williams EA, Zerbe GO, Ingersoll NC. Cocaine, social behavior, and alcohol-solution drinking in monkeys. Drug and Alcohol Dependence. 1992;29:205–223. doi: 10.1016/0376-8716(92)90094-s. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Levitin M, MacConell LA, Lee LK, Ettenberg A. Opposite effects of prefrontal cortex and nucleus accumbens infusions of flupenthixol on stimulant-induced locomotion and brain stimulation reward. Brain Research. 1992;576:104–110. doi: 10.1016/0006-8993(92)90614-f. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neuroscience and Biobehavioral Reviews. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacology, Biochemistry and Behavior. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Formenko V, Kaganovsky K, Shelton K, Wenzel JM. On the positive and negative affective responses to cocaine and their relation to drug self-administration in rats. Psychopharmacology. 2015;232:2363–2375. doi: 10.1007/s00213-015-3873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. An animal model for investigating the anxiogenic properties of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of cocaine and heroin reinforced rats. Pharmacology Biochemistry and Behavior. 1992;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphé nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacology Biochemistry and Behavior. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacology, Biochemistry, and Behavior. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Archives of General Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R, Yadid G. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao DM, Hoffman D, Benabid AL. Simultaneous recording of spontaneous activities and nociceptive responses from neurons in the pars compacta of substantia nigra and in the lateral habenula. European Journal of Neuroscience. 1996;8:1474–1478. doi: 10.1111/j.1460-9568.1996.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cerebral Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in a runaway. Pharmacology Biochemistry and Behavior. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. Concurrent positive and negative goal box events produce runway behaviors comparable to those of cocaine reinforced rats. Pharmacology Biochemistry and Behavior. 1997;57:145–150. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- George FR, Goldberg SR. Genetic approaches to the analysis of addiction processes. Trends in Pharmacological Sciences. 1989;10:78–83. doi: 10.1016/0165-6147(89)90083-7. [DOI] [PubMed] [Google Scholar]
- Good CH, Wang H, Chen YH, Mejias-Aponte CA, Hoffman AF, Lupica CR. Dopamine D4 receptor excitation of lateral habenula neurons via multiple cellular mechanisms. Journal of Neuroscience. 2013;33:16853–16864. doi: 10.1523/JNEUROSCI.1844-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z. Origin and distribution of noradrenergic innervation in the habenula: a neurochemical study. Brain Research. 1983;275:299–304. doi: 10.1016/0006-8993(83)90990-3. [DOI] [PubMed] [Google Scholar]
- Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rats. Neuroscience Letters. 2007;427:165–170. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Frontiers in Behavioral Neuroscience. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behavioral Neuroscience. 1989;103:345–55. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One. 2010;5:9. doi: 10.1371/journal.pone.0012980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. The Journal of Comparative Neurology. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. Journal of Neuroscience. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the central tegmental area in the rat. Journal of Comparative Neurology. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behavioral Pharmacology. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowski AB, Veh RW, Weiss T. Dopaminergic activation excites rat lateral habenular neurons in vivo. Neuroscience. 2009;161:1154–1165. doi: 10.1016/j.neuroscience.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. European Journal of Neuroscience. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. doi: 10.1038/nature0545. [DOI] [PubMed] [Google Scholar]
- Li S, Shi Y, Kirouac GJ. They hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Frontiers in Neuroanatomy. 2014;8:136. doi: 10.3389/fnana.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Wang M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Structure and Function. 2013;218:1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Shinonaga Y, Mizuno N. The sites of origin of dopaminergic afferent fibers to the lateral habenular nucleus in the rat. Journal of Comparative Neurology. 1993;333:118–133. doi: 10.1002/cne.903330110. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. Journal of Neuroscience. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neuroscience Letters. 2015;608:34–39. doi: 10.1016/j.neulet.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, Lookingland KJ, Bédard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson’s disease. Journal of Pharmacology and Experimental Therapeutics. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00860. Article 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behavioural Brain Research. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Morency MA, Beninger RJ. Dopaminergic substrates of cocaine-induced place conditioning. Brain Research. 1986;399:33–41. doi: 10.1016/0006-8993(86)90598-6. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, Ben-Shahar O, Ettenberg A. External incentives and internal states guide goal-directed behavior via the differential recruitment of the nucleus accumbens and the medial prefrontal cortex. Neuroscience. 2010;170:468–477. doi: 10.1016/j.neuroscience.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, DiCamillo AM, Haun F, Murray M. Lesion of the habenular efferent pathway produces anxiety and locomotor hyperactivity in rats: a comparison of the effects of neonatal and adult lesions. Behavioral Brain Research. 1996;81:43–52. doi: 10.1016/s0166-4328(96)00041-1. [DOI] [PubMed] [Google Scholar]
- Murrin LC. Characteristics of 3H-cis-flupenthixol binding in rat striatum. Life Sciences. 1983;33:2179–2186. doi: 10.1016/0024-3205(83)90289-8. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A,, Audinot-Bouchez V, Gobert A, Millan MJ. Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. European Journal of Pharmacology. 1997;319:379–383. doi: 10.1016/s0014-2999(96)00985-5. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic; New York, NY: 2005. [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–73. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Experimental and Clinical Psychopharmacology. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195:696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Resnick EB. Cocaine abuse and its treatment. Psychiatric Clinics of North America. 1984;7:713–728. [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacology, Biochemistry and Behavior. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacology, Biochemistry, and Behavior. 1977;6:615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacology Biochemistry and Behavior. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, Morales M. Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. Journal of Neuroscience. 2015;35:3460–3469. doi: 10.1523/JNEUROSCI.4525-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. Journal for the Study of Alcohol and Drugs. 2007;68:641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Shen X, Ruan X, Zhao H. Stimulation of midbrain dopaminergic structures modifies firing rates of rat lateral habenula neurons. PLoS One. 2012:7. doi: 10.1371/journal.pone.0034323. Article 34323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O, Björklund A. Origin, course and termination of mesohabenular dopamine pathway in the rat. Brain Research. 1984;307:99–108. doi: 10.1016/0006-8993(84)90465-7. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Structure and Function. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behavioral Brain Research. 1987;26:57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature Neuroscience. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Campbell KJ, Moriizumi T, Hattori T. On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neuroscience Letters. 1990;115:33–36. doi: 10.1016/0304-3940(90)90513-9. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, et al. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology. 2011;214:863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez KM, Molfes e D., Salas R. The role of the habenula in drug addiction. Frontiers in Human Neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00174. Article 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Gehlert DR, Fillous FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. Journal of Chemical Neuroanatomy. 1989;2:119–137. [PubMed] [Google Scholar]
- Weber M, Chang WL, Breier MR, Yang A, Millan MJ, Swerdlow NR. The effects of the dopamine D2 agonist sumanirole on pre-pulse inhibition in rats. European Neuropsychopharmacology. 2010;20:421–425. doi: 10.1016/j.euroneuro.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey A, Sunhara RK, Niznik HB, O’Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proceedings of the National Academy of Sciences (USA) 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug and Alcohol Dependence. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Determinants of cocaine self-administration by laboratory-animals. Ciba Found Symp. 1992;166:149–161. doi: 10.1002/9780470514245.ch9. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration – neurochemical and behavioral studies. Pharmacology Biochemistry and Behavior. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye J-H. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–189. doi: 10.1016/j.neuropharm.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]