Abstract
Conventional antibody-drug conjugates (ADCs) are heterogeneous mixtures that have poor pharmacokinetic properties and decreased efficacy relative to homogenous ADCs. Furthermore, ADCs that are maleimide-based often have inadequate circulatory stability, which can result in premature drug release with consequent off-target toxicities. Selenocysteine-modified antibodies have been developed that allow site-specific antibody conjugation, yielding homogeneous ADCs. Herein, we survey several electrophilic functional groups that react with selenocystine with high efficiency. Several of these result in conjugates with stabilites that are superior to maleimide conjugates. Among these, the allenamide functional group reacts with notably high efficiency, leads to conjugates with remarkable stability, and shows exquisite selectivity for selenocysteine conjugation.
Keywords: Antibody-drug conjugates, selenocysteine, allenamide, site-specific
Introduction
Antibody-drug conjugates (ADCs) hold remarkable promise as cancer chemotherapeutic agents. The approval of brentuximab vedotin1 and ado-trastuzumab emtansine2 by the FDA for the treatment of relapsed Hodgkin’s lymphoma and HER2+ breast cancer, respectively, has renewed interest in ADCs.3-5 While these two ADCs have been moderately successful, there remains room for improvement. First, the drugs are conjugated using hinge cysteine (Cys) and surface lysine residues, respectively, but owing to the abundance of these residues in antibody molecules, heterogeneous mixtures of ADCs result.6 The heterogeneity convolutes pharmacokinetic studies and diminishes their therapeutic indices.7 Secondly, the ADCs contain a thiol-succinimide unit known to hydrolyze in aqueous media, introducing more heterogeneity.8, 9 In addition, the currently approved conjugates may undergo a retro-Michael elimination resulting in premature release of the cytotoxic drugs; this also produces a maleimide-based drug that can react with plasma thiols. 10-12
To address these issues, several groups are pursuing site-specific antibody conjugation. Published strategies toward the goal of site-specific conjugation fall into four main categories: introducing reactive cysteines7, 13, 14, incorporating unnatural amino acids10, 15, 16, enzymatic engineering of antibodies,17-19 and incorporating natural amino acids with bio-orthogonal reactivities.20-22 Using the fourth strategy, we have incorporated selenocysteine (Sec), the 21st natural amino acid, into antibodies.23, 24 Compared to its cysteine counterpart (pKa = ~ 8), Sec has a lower pKa (~ 5) and enhanced nucleophilicity, which permits selective Sec conjugation in the presence of cysteines and other natural amino acids.25 Based on this property, we have introduced a unique class of selenocysteine (Sec)-modified antibodies (SELENOMABS) that allow site-specific antibody modification leading to homogeneous ADCs.26, 27 Furthermore, we demonstrated that SELENOMAB-oxadiazole conjugates had enhanced stability relative to maleimide counterparts.26 We subsequently incorporated two selenocysteine residues into one antibody28 for increased drug loading and also successfully labeled antibodies with two different reporter groups via engineered Cys and Sec residues.29 In our efforts to expand the utility and applications of Sec-modified proteins in general, we surveyed the conjugation efficiencies of selenocysteine with a variety of electrophilic functional groups and determined the stability of the resulting conjugates. Herein, we identify allenamides as particularly interesting reagents for Sec conjugation as they efficiently react with Sec to produce stable adducts and permit the development of site-specific SELENOMAB conjugates.
Results and Discussion
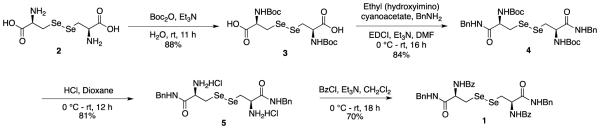
In order to probe the stability profile of Sec adducts, we synthesized protected selenocystine 1 as a model substrate (Scheme 1).
Scheme 1.
Synthesis of bis-N-benzoyl-selenocystine-dibenzamide.
Commercially available L-selenocystine 2 was protected as bis tert-butyl carbamate 3, which was coupled with benzyl amine to give bis-Boc-selenocystine-dibenzylamide 4. The Boc protecting groups were removed with HCl in dioxane and the resulting hydrochloride salt 5 was coupled with benzoyl chloride to give model substrate 1. We next examined conditions that would enable diselenide 1 to react with phenyloxadiazolyl sulfone (ODA) 6 and other electrophilic reagents 7-10 (Scheme 2). The optimal conditions deriving from this survey involved first reducing a solution of diselenide in EtOH with NaBH4 at 0 °C, before adding pH 5.2 NaOAc buffer and substrate. The reaction with 6 was complete after 2 h at 0 °C as determined by 1H NMR analysis of the crude product. Using these conditions, we surveyed the reaction of diselenide 1 with the other electrophilic groups summarized in Fig. S1 (Supporting Information) that have been used in cysteine conjugation.30-32 Reactive probes that showed highest conversion are 6, 7, 9, and 10 (Scheme 2).
Scheme 2.
Conjugation of selenocysteine to select reactive probes.
It is interesting to note that benzothiazolyl sulfone (BTA) 7 reacted with comparable efficiency to phenyloxadiazolyl sulfone (ODA) 6 with diselenide, unlike with cysteine as described by Toda et al.30 This is presumably due to the increased nucleophilicity of Sec compared to Cys.
Next, we investigated the stability of the selenocysteine adducts 11-15 (Scheme 2) under several conditions (Fig. 1). Sec-MAL 13 was included for comparison. Under mildly acidic conditions, Sec-ODA 11, Sec-BTA 12 and Sec-allene (Sec-ALL) 15 (Scheme 2) adducts were stable with minor degradation (Fig. 1, blue bars). However, Sec-MAL 13 and Sec-iodoacemide (Sec-IAM) 14 adducts degraded significantly at pH 5.2 under these conditions. Analogous trends were observed under basic and oxidizing conditions where ≥ 60% of Sec-ODA 11, Sec-BTA 12, and Sec-ALL 15 remained after 72 h while less than 60% Sec-MAL 13 and Sec-IAM 14 remained under these conditions (Fig. 1, red and green bars). In fact, Sec-MAL 13 and Sec-IAM 14 were completely degraded under basic conditions and in the presence of hydrogen peroxide, respectively. The main species observed from the treatment of Sec-MAL 13 with K2CO3 were hydrolyzed benzyl maleimide 16 (Scheme 3), diselenide 1, and N-benzoyl dehydroalanine benzyl amide 17. These compounds are consistent with the observed modes of chemical instability for maleimide-based conjugates (i.e. retro-Michael elimination and hydrolysis in aqueous media).10-12
Fig. 1.
Stability evaluation for selenocysteine conjugates. Blue: 1 mL THF, NaOAc buffer pH 5.2 (3:1), rt, 3 days. Red: K2CO3 (4 equiv.), 1 mL THF, H2O (3:1), rt, 3 days. Green: H2O2 (5 mM), 1 mL THF, pH 7 buffer (3:1), rt, 3 days. Purple: Glutathione (20 μM), 1 mL THF, PBS buffer pH 7.4 (3:1), 37 °C, 3 days. % Remaining shown as mean ± standard deviation from three independent experiments.
Scheme 3.
Stability of Sec-MAL 13 and Sec-IAM 14 in K2CO3 and H2O2, respectively. % Remaining determined by LC-MS.
Subsequently, we studied the stability of our conjugates under physiologically relevant conditions. The presence of thiol containing compounds in plasma has been implicated in in vivo degradation of maleimide-cysteine conjugates.12 The source of this instability includes a retro-Michael reaction of the conjugate resulting in a reactive maleimide group. Human serum albumin and glutathione are capable of reacting with reactive maleimides formed from this process.10-12 It also seemed conceivable that adducts such as Sec-ODA 11 and Sec-BTA 12 could be unstable due to competing nucleophilic aromatic substitution reactions. As such, we wanted to examine the stability of our selenocysteine adducts in the presence of glutathione. We incubated Sec-adducts with reduced glutathione using the upper limit of extracellular concentration (20 μM),33 in PBS buffer, at 37 °C, for 3 days and the results are shown in Fig. 1 (purple bars). Again, Sec-ODA 11, Sec-BTA 12 and Sec-ALL 15 were the most stable with ≥ 70% remaining while Sec-MAL 13 (32% remaining) and Sec-IAM 14 (60% remaining) were considerably less stable under these conditions.
In addition to stability in the presence of glutathione, we also assessed the stability of Sec-adducts in human plasma at 37 °C. A comparison between the stability of a SELENOMAB-maleimide conjugate and a SELENOMAB-oxadiazole conjugate has been discussed elsewhere, where the latter was shown to be significantly more stable than the former.26 As such, the Sec-MAL 13 conjugate was not included in this study. Of the remaining adducts, Sec-ODA 11 was the least stable (t1/2 < 24 h) (Fig. 2, blue line). In a manner analogous to the cysteine adducts, Sec-BTA 12 was more stable in plasma than Sec-ODA 11 with a half-life of 33 h. Sec-IAM 14 was next most stable in plasma (t1/2 = 77 h, calculated with Excel) and Sec-ALL 15 was the most stable of these adducts (t1/2 = 139 h).
Fig. 2.
Stability evaluation of Sec-conjugates in human plasma. 50 μL of 4 mM substrate in DMSO was added to human plasma (950 μL) and incubated at 37 °C for 72 h. Aliquots were analyzed by LC-MS with dibenzyl benzamide as the internal standard.
Due to the excellent robustness of Sec-ALL 15 from this stability profile, the allene carboxamide functional group may potentially be regarded as a compelling reactive handle for selenocysteine-based bioconjugation. While a fluorescein-oxadiazole sulfone probe (FL-ODA) 18, based on a phenyl oxadiazole coupling partner (Scheme 4) has been shown to selectively conjugate selenocysteine in antibody applications, the selectivity for allene conjugation with Sec is as of yet unknown.26, 29 However, the allene electrophiles have been shown to conjugate cysteine with high efficiency.32 In order to determine the selectivity of allene conjugation and establish whether allenes might be useful for SELENOMAB conjugation, we synthesized an allene-based fluorescein reactive probe 19 (Scheme 4, Fl-ALL). The synthesis commenced with the coupling of 3-butynoic acid (20) with tBoc-1,4-diaminobutane giving allene 21. Trifluoroacetic acid-mediated removal of the Boc protecting group followed by coupling with 5-carboxyfluorescein provided the requisite allene probe 19.
Scheme 4.
Synthesis of allene-based fluorescent probe 19.
To test the conjugation selectivity of fluorescein-allene probe 19, selenocysteine-modified trastuzumab scFv-Fc-Sec (SELENOMAB) was reduced with DTT (0.1 mM) in pH 5.2 NaOAc buffer and the reduced antibody was allowed to react with Fl-ALL 19 for 2 h at room temperature (Fig. 3a). In parallel, trastuzumab scFv-Fc lacking selenocysteine (scFv-Fc) was also incubated with Fl-ALL 19. For each reaction, the reaction mixture was purified using size-exclusion chromatography and analyzed by SDS-PAGE. The result showed that Fl-ALL 19 was selective for selenocysteine and did not react with any other functional groups on the antibody (Fig. 3b). Furthermore, reduced SDS-PAGE highlighted the stability of SELENOMAB-fluorescein conjugate 23 (SFC-ALL, Fig. 3a) under reducing conditions (50 mM DTT) (data not shown). Interestingly, oxadiazole-based SELENOMAB-fluorescein conjugate 24 (SFC-ODA, Scheme S1, Supporting Information) is degraded in reduced SDS-PAGE (data not shown).
Fig. 3.
(a) Conjugation of SELENOMAB or scFv-Fc to fluorescein-allene probe 19 (Fl-ALL). (b) Gel image of scFv-Fc and SELENOMAB reaction with Fl-ALL 19.
We next evaluated whether our SELENOMAB-fluorescein conjugates retained antigen binding activity. We investigated the ability of our SELENOMAB-fluorescein conjugates to bind to HER2 receptors on the surface of SK-BR-3 breast cancer cells using flow cytometry. As shown in Fig. 4, SK-BR-3 cells incubated with allene-based 23 (SFC-ALL) and those incubated with oxadiazole-based 24 (SFC-ODA) SELENOMAB-fluorescein conjugates had a shift in fluorescence relative to background (cells incubated in PBS buffer). Also, the SELENOMAB-fluorescein conjugates bound only to HER2 receptors since HER2-negative breast cancer MDA-MB-468 cells showed no difference in fluorescence between those incubated in PBS and SELENOMAB-fluorescein conjugates (Fig. 4).
Fig. 4.
Fluorescein conjugated SELENOMABs retain HER2-binding activity. SELENOMAB was incubated with HER2 expressing SK-BR-3 cells and bound antibody was detected by flow cytometry. No bound antibody was detected using HER2− MDA-MB-468 cells. SFC-ALL: allene-based SELENOMAB-fluorescein conjugate; SFC-ODA: oxadiazolyl sulfone-based SELENOMAB-fluorescein conjugate.
Finally, we wanted to examine the stability of SELENOMAB-fluorescein conjugates in plasma. The conjugates were incubated in human plasma at 37 °C for 3 days. Aliquots were collected at 0, 4, 8, 12, 24, 48, and 72 h and analyzed by SDS-PAGE. While the sulfone based conjugate 24 (SFC-ODA) was moderately stable with a half-life of about 12 h, the allene based conjugate 23 (SFC-ALL) was very stable with a half-life of over 28 days (Calculated with Excel using current data) (Fig. 5).
Fig. 5.
Allene and sulfone-based SELENOMAB-fluorescein conjugate stability in human plasma. SFC-ALL and SFC-ODA: allene-based and oxadiazolyl sulfone-based SELENOMAB-fluorescein conjugate, respectively.
Conclusion
We demonstrated that heteroaryl sulfones, iodoacetamide, and allenamides are suitable for conjugation to selenocysteine with high conjugation efficiency. The heteroaryl sulfone and allenamide based conjugates were stable under a wide variety of conditions including physiologically relevant ones. The allenamide-based fluorescent probe was conjugated to SELENOMAB with remarkable chemo- and site selectivity. Furthermore, the resulting SELENOMAB-fluorescein conjugate was impressively stable in plasma. The allene functionality provides a way to generate stable, site-specific SELENOMAB conjugates with drugs, fluorophores, and contrast agents. Also, it offers another functional group that together with heteroaryl sulfones can be used for dual labeling of ADCs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (U01 CA174844).
Footnotes
NOTES
The authors declare no competing financial interests.
References
- 1.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD. Nat. Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 2.Phillips GDL, Li GM, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RVJ, Lutz RJ, Wong WLT, Jacobson FS, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 3.Senter PD, Sievers EL. Nat. Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 4.Lambert JM, Chari RVJ. J. Med. Chem. 2014;57:6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 5.Younes A, Yasothan U, Kirkpatrick P. Nat. Rev. Drug Discov. 2012;11:19–20. doi: 10.1038/nrd3629. [DOI] [PubMed] [Google Scholar]
- 6.Hamblett KJ, Senter PD, Chace DF, Sun MMC, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA. Clin. Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 7.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, Lu YM, Meng YG, Ng C, Yang JH, Lee CC, Duenas E, Gorrell J, Katta V, Kim A, McDorman K, Flagella K, Venook R, Ross S, Spencer SD, Wong WL, Lowman HB, Vandlen R, Sliwkowski MX, Scheller RH, Polakis P, Mallet W. Nat. Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine SD, Reid R, Robinson L, Ashley GW, Santi DV. Bioconjugate Chem. 2015;26:145–152. doi: 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- 9.Tumey LN, Charati M, He T, Sousa E, Ma DS, Han XG, Clark T, Casavant J, Loganzo F, Barletta F, Lucas J, Graziani EI. Bioconjugate Chem. 2014;25:1871–1880. doi: 10.1021/bc500357n. [DOI] [PubMed] [Google Scholar]
- 10.Jackson D, Atkinson J, Guevara CI, Zhang CY, Kery V, Moon SJ, Virata C, Yang P, Lowe C, Pinkstaff J, Cho H, Knudsen N, Manibusan A, Tian F, Sun Y, Lu YC, Sellers A, Jia XC, Joseph I, Anand B, Morrison K, Pereira DS, Stover D. Plos One. 2014;9 doi: 10.1371/journal.pone.0083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen BQ, Xu KY, Liu LN, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, Li DW, Tibbitts J, Baudys J, Saadi OM, Scales SJ, McDonald PJ, Hass PE, Eigenbrot C, Nguyen T, Solis WA, Fuji RN, Flagella KM, Patel D, Spencer SD, Khawlil LA, Ebens A, Wong WL, Vandlen R, Kaur S, Sliwkowski MX, Scheller RH, Polakis P, Junutula JR. Nat. Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 12.Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, Senter PD. Bioconjugate Chem. 2008;19:759–765. doi: 10.1021/bc7004329. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey SC, Burke PJ, Lyon RP, Meyer DW, Sussman D, Anderson M, Hunter JH, Leiske CI, Miyamoto JB, Nicholas ND, Okeley NM, Sanderson RJ, Stone IJ, Zeng WP, Gregson SJ, Masterson L, Tiberghien AC, Howard PW, Thurston DE, Law CL, Senter PD. Bioconjugate Chem. 2013;24:1256–1263. doi: 10.1021/bc400217g. [DOI] [PubMed] [Google Scholar]
- 14.Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, Bhakta S, Nguyen T, Dugger DL, Li GM, Mai E, Phillips DL, Hiraragi H, Fuji RN, Tibbitts J, Vandlen R, Spencer SD, Scheller RH, Polakis P, Sliwkowski MX. Clin. Cancer Res. 2010;16:4769–4778. doi: 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- 15.Tian F, Lu YC, Manibusan A, Sellers A, Tran H, Sun Y, Phuong T, Barnett R, Hehli B, Song F, DeGuzman MJ, Ensari S, Pinkstaff JK, Sullivan LM, Biroc SL, Cho H, Schultz PG, DiJoseph J, Dougher M, Ma DS, Dushin R, Leal M, Tchistiakova L, Feyfant E, Gerber HP, Sapra P. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1766–1771. doi: 10.1073/pnas.1321237111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman ES, Heibeck TH, Gill A, Li XF, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, Yam AY, Wang WD, Steiner AR, Bajad SU, Penta K, Yang WJ, Hallam TJ, Thanos CD, Sato AK. Bioconjugate Chem. 2014;25:351–361. doi: 10.1021/bc400490z. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Shui WQ, Carlson BL, Hu N, Rabuka D, Lee J, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3000–3005. doi: 10.1073/pnas.0807820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, Zhou DH, Chandrasekaran R, Samain C, Loo C, Rossi A, Rickert M, Krimm S, Wong T, Chin SM, Yu J, Dilley J, Chaparro-Riggers J, Filzen GF, O'Donnell CJ, Wang F, Myers JS, Pons J, Shelton DL, Rajpal A. Chem. Biol. 2013;20:161–167. doi: 10.1016/j.chembiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Beerli RR, Hell T, Merkel AS, Grawunder U. Plos One. 2015;10:e0131177. doi: 10.1371/journal.pone.0131177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Mabs-Austin. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCombs JR, Owen SC. Aaps J. 2015;17:339–351. doi: 10.1208/s12248-014-9710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal P, Bertozzi CR. Bioconjugate Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofer T, Thomas JD, Burke TR, Rader C. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12451–12456. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofer T, Skeffington LR, Chapman CM, Rader C. Biochemistry. 2009;48:12047–12057. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byun BJ, Kang YK. Biopolymers. 2011;95:345–353. doi: 10.1002/bip.21581. [DOI] [PubMed] [Google Scholar]
- 26.Patterson JT, Asano S, Li XL, Rader C, Barbas CF. Bioconjugate Chem. 2014;25:1402–1407. doi: 10.1021/bc500276m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vire B, Skarzynski M, Thomas JD, Nelson CG, David A, Aue G, Burke TR, Rader C, Wiestner A. Cancer Res. 2014;74:7510–7520. doi: 10.1158/0008-5472.CAN-14-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XL, Yang JH, Rader C. Methods. 2014;65:133–138. doi: 10.1016/j.ymeth.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XL, Patterson JT, Sarkar M, Pedzisa L, Kodadek T, Roush WR, Rader C. Bioconjugate Chem. 2015;26:2243–2248. doi: 10.1021/acs.bioconjchem.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toda N, Asano S, Barbas CF. Angew. Chem. Int. Ed. 2013;52:12592–12596. doi: 10.1002/anie.201306241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cal PMSD, Bernardes GJL, Gois PMP. Angew. Chem. Int. Ed. 2014;53:10585–10587. doi: 10.1002/anie.201405702. [DOI] [PubMed] [Google Scholar]
- 32.Abbas A, Xing BG, Loh TP. Angew. Chem. Int. Ed. 2014;53:7491–7494. doi: 10.1002/anie.201403121. [DOI] [PubMed] [Google Scholar]
- 33.Wu GY, Fang YZ, Yang S, Lupton JR, Turner ND. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.