Abstract
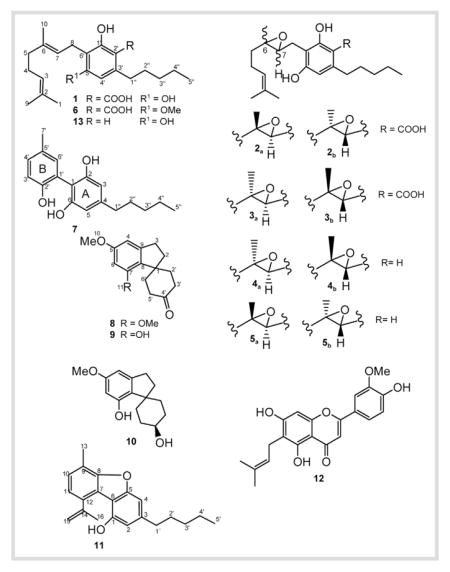
Phytochemical investigation of a high potency variety of Cannabis sativa L. resulted in the isolation of six new metabolites, (±)-6,7-trans-epoxycannabigerolic acid (2), (±)-6,7-cis-epoxycannabigerolic acid (3), (±)-6,7-cis-epoxycannabigerol (4), (±)-6,7-trans-epoxycannabigerol (5), 5′-methyl-4-pentylbiphenyl-2,2′,6-triol (7), and 7-methoxycannabispirone (8), along with seven known compounds namely, cannabigerolic acid (1), 5′-methoxycannabigerolic acid (6), cannabispirone (9), β-cannabispiranol (10), dehydrocannabifuran (11), cannflavin B (12) and cannabigerol (13). The antimicrobial as well as the antileishmanial activities were investigated.
Keywords: Cannabis sativa, Cannabaceae, epoxy cannabigerolic acid, cannabispirone, antimicrobial activity, antileishmanial activity
Introduction
Cannabis sativa L. (Cannabaceae), one of the oldest plants known in medicine, is the most widely used illicit drug in the world today. A total of almost 500 natural constituents have been isolated and/or identified from Cannabis [1], with Δ9-THC as the main biologically active component [2]. The availability of high potency marijuana on the illicit market with unprecedented Δ9-THC concentrations (> 20% by dry weight) [3] has renewed our interest in the discovery of new constituents from cannabis. We herein report the isolation and structure elucidation of six new metabolites (2, 3, 4, 5, 7 and 8) and seven known compounds (1, 6 and 9–13). This is the first report of the full NMR data for 1, 6 and 11. The antimicrobial and antileishmanial activities of the isolates are also reported.
Materials and Methods
General experimental procedures
1H-NMR (400 MHz), 13C-NMR (100 MHz) and 2D-NMR spectra were recorded using the residual solvent signal as an internal standard on a Varian AS 400. IR spectra were measured on a Bruker Tensor 27. UV spectra were obtained on a Varian Cary 50 Bio UV-Visible spectrophotometer. Optical rotation was measured on an Autoplot IV automatic polarimeter. High resolution mass spectra were measured using a Bruker BioApex. HPLC was performed on a Waters Delta Prep 4000 Preparative Chromatography System connected to a Waters 486 Tunable Absorbance detector (206 nm) using a Phenomenex Luna C18 column (250×21.2 mm, 5 μm, 100 Å). Flash silica gel (J.T. Baker, 40–63 μm, 60 Å), C18 silica gel (Fluka, 40–63 μm, 60 Å) and Sephadex LH 20 (Fluka) were used for column chromatography. GC-MS analyses were carried out on a ThermoQuest Trace 2000 GC, equipped with a single split/splitless capillary injector, a ThermoQuest AS2000 autosampler and a Phenomenex ZB-5 column (30 m×0.25 mm×0.25 μm), interfaced to a ThermoQuest-Finnigan Trace MS quadrupole ion trap detector. The injector temperature was 250 °C and 1 μL injections were performed in the splitless mode, with the splitless time set at 60 s, the split flow set at 50 mL/min and the septum purge valve set to close 60 s after the injection occurred. The oven temperature was raised from 70 to 270 °C (hold 20 min) at a rate of 5 °C/min, for a total run time of 60 min; the transfer line temperature was 250 °C. Helium was used as the carrier gas at a constant pressure of 20 psi. The mass spectrometer was operated in the electron impact mode (EI+) and scanned from 40 to 800 amu at 1 scan/s, with an ionizing voltage of 70 eV and an emission current of 350μA. Data was recorded using an IBM Netfinity 3000 workstation with Microsoft Windows NT 4.0 operating system and Xcalibur (Version 1.2) data acquisition and analysis software.
Plant material
C. sativa plants were grown from high potency Mexican seeds (variety code CHPF-01). The seeds and plants were authenticated by Dr. Suman Chandra, The University of Mississippi, and the specimen (S1310V1) was deposited at the Coy Waller Complex, The University of Mississippi. Whole buds of mature female plants were harvested, air-dried, packed in barrels (# 1196) and stored at low temperature (−24 °C). The THC, CBG and CBD contents in the plant material determined by GC/FID analysis were 9.89 %, 0.42% and 0.25 %, respectively.
Extraction and isolation
The plant material (9.0 kg) was sequentially extracted with hexanes (2×60 L), CH2Cl2 (2×24 L), EtOAc (2×20 L), EtOH (2×20 L), EtOH/H2O (36 L, 1: 1) and H2O (40 L) at room temperature. The extracts were evaporated under reduced pressure at 40 °C to afford hexanes (1.48 kg), CH2Cl2 (0.15 kg), EtOAc (0.13 kg), EtOH (0.09 kg), EtOH/H2O (0.77 kg) and H2O (0.54 kg) extracts for a total extract of 3.16 kg (35.1%, w/w). A portion of the hexanes extract (40 g) was chromatographed on flash silica gel (1.2 kg, 10×50 cm) eluting with n-hexane. Fractions with Rf close to that of Δ9-THC according to silica gel TLC (n-hexane/EtOAc, 9: 1) were combined and purified by flash silica chromatography and Sephadex LH-20 (n-hexane as eluent), followed by final purification by preparative C18 HPLC (MeCN, 25 mL/min) to afford 4 (3.3 mg, rt = 9.5 min), 5 (9.0 mg, rt = 9.0 min), 11 (2.6 mg, rt = 5.3 min.) and 13 (45 mg, rt = 2.5 min). Portions of the CH2Cl2, EtOAc and EtOH extracts were combined (232.0 g) since they showed similar TLC profiles (EtOAc/n-hexane, 4: 6), and the resulting extract was subjected to VLC over silica gel (6 kg, 15×90 cm) eluting with EtOAc/n-hexane [0:100, 10: 90, 20: 80, 30: 70, 40: 60, 50: 50, 75: 25, 100:0 (2 L of each mixture)] followed by EtOH (4 L), yielding 9 fractions (I – IX). Fraction III (1.70 g) was chromatographed on silica gel (50 g, 2.0×50 cm, n-hexane/EtOAc, 100:0 to 85:15), yielding 36 fractions (III1–36, 100 mL each). Fraction III10–14 (35 mg) was purified by C18 HPLC (MeCN/H2O, 95:5, 25 mL/min) to afford 6 (3.0 mg, rt = 7.4 min) and 8 (7.4 mg, rt = 8.8 min). Fraction III15–30 (526 mg) afforded 1 (382 mg) upon precipitation from n-hexane/EtOAc. Fraction III32–36 (130 mg) was purified by C18 HPLC (MeOH/H2O, 65: 35, 25 mL/min) to give 2 (13.4 mg, rt = 9.4), 3 (4.0 mg, rt = 10.8 min) and 7 (2.4 mg, rt = 5.1 min). Fraction VII (6.70 g) was subjected to silica gel chromatography (400 g, 7.0×80 cm, n-hexane/EtOAc, 80:20) yielding 13 fractions (VII1–13, 250 mL each). Fraction VII9–10 (41 mg) was purified by C18 HPLC (MeOH/H2O, 75: 25) to afford 9 (5.3 mg, rt = 15.3 min) and 10 (5.0 mg, rt = 4.0 min). Fraction VII11 (2.78 g) was purified by Sephadex LH-20 (50 g, 2.0 × 50 cm, MeOH) followed by C18 flash chromatography (MeOH/H2O, 8: 2) to afford 12 (264.1 mg).
Isolates
Cannabigerolic acid (1): White amorphous powder; UV (EtOH): λmax = 255, 299 nm; IR (neat): νmax = 3390, 1650 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (negative ion mode): m/z = 359.2214 [M − H]− (calcd. for C22H31O4:359.2222). (±)-6,7-trans-Epoxycannabigerolic acid (2): Yellow oil; UV (MeOH): λmax = 215, 260, 300 (sh) nm; : 0 (c 1.2, MeOH); IR (neat): νmax = 3402, 1650 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (positive ion mode): m/z = 399.2156 [M + Na]+ (calcd. for C22H32O5Na: 399.2147).
Table 1.
1H-NMR data for 1–7 and 13 (400 MHz, δ in ppm, J in Hz)
| Position | 1a | 2a | 1b | 3b | 4b | 5b | 6b | 13b | 7b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.59 s | 1.53 s | 1.52 s | 1.57 s | 1.57 s | 1.57 s | 1.55 s | 1.60 s | – |
| 3 | 5.06 t (6.4) | 5.06 t (6.4) | 5.03 t (6.4) | 5.08 t (6.4) | 5.08 t (6.4) | 5.07 t (6.4) | 5.06 (m) | 5.07 m | 6.45 s |
| 4 | 2.12 m | 2.10 m | 1.91 t (6.8) | 2.13 m | 2.13 m | 2.11 m | 2.03 m | 2.09 m | |
| 5 | 2.22 m | 1.49 m | 2.10 t (7.2) | 1.53 m | 1.53 m | 1.53 m | 1.94 m | 2.09 m | 6.45 s |
| 6 | – | – | – | – | – | – | – | – | – |
| 7 | 5.28 t (6.8) | 3.62 t (5.2) | 5.17 t (6.8) | 3.88 t (5.2) | 3.88 t (5.2) | 3.87 t (5.2) | 5.18 t (6.6) | 5.29 m | – |
| 8 | 3.43 d (6.8) | 2.68 dd (5.2, 16.4) 2.33 dd (8.4, 16.8) |
3.19 d (6.8) | 2.45 m | 2.45 m | 2.43 m | 3.32 d (7.6) | 3.41 d (7.0) | – |
| 9 | 1.67 s | 1.61 s | 1.59 s | 1.66 s | 1.66 s | 1.65 s | 1.63 s | 1.69 s | – |
| 10 | 1.82 s | 1.04 s | 1.71 s | 1.32 s | 1.32 s | 1.32 s | 1.76 s | 1.82 s | – |
| 2′ | – | – | – | – | 6.20 s | 6.20 s | – | 6.26 s | – |
| 3′ | – | – | – | – | – | – | – | – | 6.99 d (8.2) |
| 4′ | 6.28 s | 6.18 s | 6.25 s | 6.30 s | 6.30 s | 6.28 s | 6.31 s | 6.26 s | 7.15 dd (2.0, 8.2) |
| 6′ | – | – | – | – | – | – | – | – | 7.03 d (2.0) |
| 7′ | – | – | – | – | – | – | – | – | 2.30 s |
| 1″ | 2.88 t (7.2) | 2.86 t (7.2) | 2.80 t (7.2) | 2.90 t (7.2) | 2.90 t (7.2) | 2.84 t (7.2) | 2.88 t (7.6) | 2.45 t (7.5) | 2.52 t (5.6) |
| 2″ | 1.60 m | 1.45 m | 1.60 m | 1.58 m | 1.58 m | 1.57 m | 1.58 m | 1.56 q(7.8) | 1.59 m |
| 3″ | 1.35 m | 1.23 m | 1.28 m | 1.26 m | 1.26 m | 1.26 m | 1.35 m | 1.33 m | 1.33 m |
| 4″ | 1.35 m | 1.23 m | 1.28 m | 1.26 m | 1.26 m | 1.26 m | 1.35 m | 1.33 m | 1.33 m |
| 5″ | 0.91 t (7.2) | 0.83 t (6.4) | 0.85 t (6.8) | 0.88 t (6.4) | 0.88 t (6.4) | 0.88 t (6.4) | 0.89 t (6.4) | 0.90 t (6.9) | 0.89 t (6.4) |
| OCH3 | – | – | – | – | – | – | 3.86 s | – | – |
Assignments confirmed by DEPT-135, gHMQC, gCOSY, and gHMBC experiments.
In DMSO-d6.
In CDCl3.
Table 2.
13C-NMR data for 1–7 and 13 (100 MHz, δ in ppm)
| Position | 1a | 2a | 1b | 3b | 4b | 5b | 6b | 13b | 7b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.9 | 18.1 | 17.9 | 17.7 | 17.8 | 17.8 | 17.9 | 17.6 | 117.3 |
| 2 | 132.1 | 131.4 | 131.0 | 132.8 | 132.8 | 132.2 | 131.3 | 132.0 | 154.1 |
| 3 | 124.1 | 125.2 | 124.6 | 123.4 | 123.4 | 124.2 | 124.7 | 123.8 | 108.4 |
| 4 | 26.8 | 21.6 | 26.7 | 21.9 | 21.9 | 21.9 | 26.1 | 26.4 | 146.6 |
| 5 | 40.0 | 38.8 | 40.0 | 37.1 | 36.9 | 36.9 | 40.0 | 39.7 | 108.4 |
| 6 | 138.7 | 78.9 | 133.9 | 81.9 | 81.4 | 78.4 | 135.3 | 138.0 | 154.1 |
| 7 | 121.7 | 66.9 | 123.1 | 67.2 | 67.3 | 68.3 | 122.4 | 121.8 | – |
| 8 | 22.3 | 26.7 | 22.4 | 26.0 | 26.0 | 26.0 | 22.1 | 22.5 | – |
| 9 | 25.9 | 26.2 | 25.9 | 25.8 | 25.8 | 25.9 | 25.9 | 25.6 | – |
| 10 | 16.4 | 18.1 | 16.3 | 17.7 | 17.8 | 17.8 | 16.3 | 16.1 | – |
| 1′ | 163.9 | 151.4 | 163.3 | 153.0 | 152.8 | 154.5 | 163.0 | 154.8 | 152.5 |
| 2′ | 103.4 | 106.1 | 103.6 | 105.0 | 110.6 | 109.6 | 104.0 | 108.4 | 117.3 |
| 3′ | 147.7 | 138.8 | 145.1 | 145.1 | 143.3 | 143.3 | 147.5 | 142.7 | 132.0 |
| 4′ | 111.5 | 107.1 | 110.3 | 108.4 | 108.4 | 107.5 | 106.2 | 108.4 | 131.6 |
| 5′ | 160.7 | 156.0 | 160.0 | 153.8 | 152.8 | 153.6 | 162.3 | 154.8 | 132.1 |
| 6′ | 112.1 | 116.1 | 112.6 | 110.9 | 110.6 | 110.6 | 115.2 | 110.7 | 116.4 |
| 7′ | – | – | – | – | – | – | – | – | 20.7 |
| 1″ | 36.8 | 36.4 | 36.2 | 35.2 | 36.8 | 36.9 | 37.4 | 35.5 | 36.1 |
| 2″ | 31.7 | 31.1 | 31.6 | 31.2 | 31.3 | 31.0 | 31.9 | 30.8 | 30.8 |
| 3″ | 32.2 | 31.8 | 32.0 | 32.1 | 31.8 | 31.7 | 32.3 | 31.5 | 31.7 |
| 4″ | 22.8 | 22.6 | 22.0 | 22.7 | 22.7 | 22.7 | 22.7 | 22.2 | 22.8 |
| 5″ | 14.3 | 14.5 | 14.3 | 14.2 | 14.3 | 14.2 | 14.3 | 14.0 | 14.2 |
| COOH | 176.6 | 170.0 | 174.3 | 176.4 | – | – | 176.6 | – | – |
| OMe | – | – | – | – | – | – | 55.7 | – | – |
Assignments confirmed by DEPT-135, gHMQC, gCOSY and gHMBC experiments.
In DMSO-d6.
In CDCl3.
(±)-6,7-cis-Epoxycannabigerolic acid (3): Yellow oil; UV (MeOH): λmax = 215, 260, 300 (sh) nm; : 0 (c 1.2, MeOH); IR (neat): νmax = 3402, 1650 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (positive ion mode): m/z = 399.2194 [M + Na]+ (calcd. for C22H32O5Na: 399.2147) and HR-ESI-MS (negative ion mode): m/z = 375.2116 [M − H]− (calcd. for C22H31O5: 375.2172).
(±)-6,7-cis-Epoxycannabigerol (4): Yellow oil; UV (MeOH): λmax = 215, 260, 300 (sh) nm; : 0 (c 1.2, MeOH); IR (neat): νmax = 3402, 1610 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (positive ion mode): m/z = 333.2493 [M + H]+ (calcd. for C21H33O3:333.2431).
(±)-6,7-trans-Epoxycannabigerol (5): Yellow oil; UV (MeOH): λmax = 215, 260, 300 (sh) nm; : 0 (c 1.2, MeOH); IR (neat): νmax = 3402, 1610 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (positive ion mode): m/z = 333.2486 [M + H]+ (calcd. for C21H33O3:333.2431).
5′-Methoxycannabigerolic acid (6): Yellow oil; UV (EtOH): λmax = 221, 262, 300 nm; IR (neat): νmax = 3400, 1650 cm−1; 1H-and 13C-NMR, see Table 1 and Table 2; HR-ESI-MS (positive ion mode): m/z = 375.2540 [M + H]+ (calcd. for C23H35O4: 375.2535).
5′-Methyl-4-pentylbiphenyl-2,2′,6-triol (7): Yellow oil; UV (EtOH): λmax = 210, 282 nm; IR (neat): νmax = 3390, 2910, 1615 cm−1; 1H- and 13C-NMR, see Table 1 and Table 2 HR-ESI-MS (positive ion mode): m/z = 287.1612 [M+H]+ (calcd. for C18H23O3:287.1647). 7-Methoxycannabispirone (8): White needles; m.p.123–124 °C; UV (EtOH): λmax = 224, 275 nm; IR (neat): νmax=3370, 1710, 1600 cm−1; 1H- and 13C-NMR, see Table 3; HR-ESI-MS (positive ion mode): m/z=261.1434 [M+H]+ (calcd. for C16H21O3:261.1491).
Table 3.
1H-NMR and 13C-NMR data for 8, 9 and 11 (CDCl3, δ in ppm, J in Hz)
| Position | 8 | 9 | 11 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | – | 48.2 | – | 47.8 | – | 154.3 |
|
| ||||||
| 2 | 2.22 t (7.2) | 35.5 | 2.21 t (7.2) | 35.6 | 7.06 bs | 110.5 |
|
| ||||||
| 3 | 2.93 t (7.2) | 31.2 | 2.93 t (7.2) | 31.2 | – | 134.8 |
|
| ||||||
| 4 | 6.37 bs | 101.0 | 6.35 bs | 102.0 | 6.66 bs | 103.5 |
|
| ||||||
| 5 | – | 160.8 | – | 160.5 | – | 145.0 |
|
| ||||||
| 6 | 6.28 bs | 97.4 | 6.15 bs | 101.0 | – | 114.5 |
|
| ||||||
| 7 | – | 157.5 | – | 153.6 | – | 138.7 |
|
| ||||||
| 8 | – | 125.8 | – | 126.9 | – | 155.6 |
|
| ||||||
| 9 | – | 145.7 | – | 146.5 | – | 117.1 |
|
| ||||||
| 10 | 3.75 s | 55.6 | 3.72 s | 55.6 | 7.04 d (8.0) | 127.0 |
|
| ||||||
| 11 | 3.78 s | 55.1 | – | – | 7.13 d (8.0) | 121.2 |
|
| ||||||
| 12 | – | – | – | – | – | 128.3 |
|
| ||||||
| 13 | – | – | – | – | 2.59 s | 15.2 |
|
| ||||||
| 14 | – | – | – | – | – | 150.2 |
|
| ||||||
| 15 | – | – | – | – | 5.23 bs 5.64 bs |
110.7 |
|
| ||||||
| 16 | – | – | – | – | 2.31 s | 22.7 |
|
| ||||||
| 1′ | – | – | – | – | 2.73 (7.2) | 36.3 |
|
| ||||||
| 2′ | 2.40 m 2.53 dt (6.4, 13.2) |
39.2 | 2.42 m 2.53 dt (6.4, 13.2) |
39.2 | 1.55 m | 29.9 |
|
| ||||||
| 3′ | 1.82 dd (2.8, 6.4) 2.63 dt (4.6, 13.2) |
34.5 | 1.82 dd (2.8, 6.4) 2.67 dt (4.6, 13.2) |
34.5 | 1.25 m | 31.5 |
|
| ||||||
| 4′ | – | 213.3 | – | 214.5 | 1.25 m | 22.7 |
|
| ||||||
| 5′ | 1.81 dd (2.8, 6.4) 2.63 dt (4.6, 13.2) |
34.5 | 1.82 dd (2.8, 6.4) 2.67 dt (4.6, 13.2) |
34.5 | 0.88 t (6.8) | 14.2 |
|
| ||||||
| 6′ | 2.40 m 2.53 dt (6.4, 13.2) |
39.2 | 2.42 m 2.53 dt (6.4, 13.2) |
39.2 | – | – |
Assignments confirmed by DEPT-135, gHMQC, gCOSY and gHMBC experiments.
Biological activity
The isolated compounds were tested in vitro against a culture of Leishmania donovani (L. Rivas, Centro de Investigaciones Biologocas CSIC. Madrid, Spain) promastigotes, using pentamidine (Sigma) and amphotericin B (Sigma) as positive controls (IC50=0.15 and 0.9 ng/mL, resectively) [4]. Their antimicrobial activity against Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Cryptococcus neoformans, Mycobacterium intracellulare and Aspergillus fumigates (all from ATCC) [5] as well as the cytotoxicity [6] against Vero cells (African green monkey kidney fibroblast; ATCC) were also tested.
GC-MS trimethylsilyl derivatization
Dried samples (ca. 100μg) were mixed with pyridine (5 μL, silylation grade, Pierce) and BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide] (100μL, 98+%, Acros Organics), followed by heating at 75 °C for 1 h. After cooling to room temperature, CH2Cl2 (0.9 mL) was added to the reaction mixture and the solution analyzed by GC-MS.
Results and Discussion
Compound 1 was isolated as a white amorphous powder. Its HR-ESI-MS displayed a pseudomolecular ion at m/z = 359.2214 [M − H]−, indicating the molecular formula C22H32O4. The IR spectrum of 1 showed the presence of hydroxy groups (3390 cm−1) and a chelated carboxyl group (1650 cm−1). The 1H-NMR spectrum of 1 in DMSO-d6 (Table 1) showed three methyl singlets at δH = 1.59 (H-1), 1.67 (H-9) and 1.82 (H-10), three methylenes at δH = 2.12 (m, H-4), 2.22 (m, H-5) and 3.43 (d, H-8) and two olefinic protons at δH = 5.06 (t, H-3) and 5.28 (t, H-7), attributed to a geranyl substituent. It also displayed an aromatic proton at δH = 6.28 (s, H-4′) corresponding to δC = 111.5 (C-4′) in the HMQC spectrum. The 13C-NMR, DEPT-135 and HMQC spectra showed 22 resonances, including 4 methyl, 7 methylene, 3 methine and 8 quaternary carbons. Two of these resonances were assigned to aromatic carbons bearing hydroxy groups at δC = 160.7 (C-5′) and 163.9 (C-1′), while a carboxyl group at δC = 176.6 (COOH) was attached to C-2′ (δC = 103.4). The structure was further confirmed by GC-MS: 1 spontaneously decarboxylated on injection to give cannabigerol (13) ([M]+ = 316). The trimethylsilyl derivative of 1 ([M]+ = 576) confirmed the HR-ESI-MS result and the presence of two phenolic and one carboxyl groups. The 1H-NMR and IR data of 1 were similar to those previously reported for cannabigerolic acid [7], however, this is the first report of the 13C-NMR, DEPT, 2D-NMR and HR-ESI-MS data for 1.
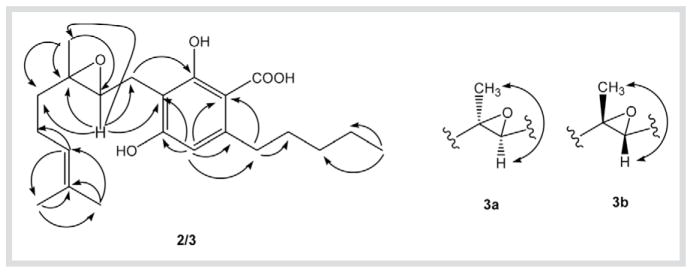
Compound 2 was isolated as a yellow, optically inactive oil. Its molecular formula, C22H32O5, was derived from HR-ESI-MS (m/z = 399.2156 [M + Na]+, 775.4364 [2M+ Na]+). The IR spectrum of 2 diplayed hydroxy groups at 3402 cm−1 and a chelated carboxyl group at 1650 cm−1. The 1H- and 13C-NMR, DEPT and HMQC data of 2 in DMSO-d6 (Table 1 and Table 2) were similar to those of 1 except for the presence of a 6,7-epoxy group [δH = 3.62 (t, J = 5.2 Hz, H-7); δC = 66.9 (C-7), 78.9 (C-6)] instead of the 6,7-double bond [δH = 5.28 (t, J = 6.8 Hz, H-7); δC = 121.7 (C-7), 138.7 (C-6)]. The 6,7-epoxy position was determined by the HMBC correlations of H-7 with C-5, C-6, C-10, C-8 and C-6′, and H3–10 with C-5, C-6 and C-7 (Fig. 1). The structure was further confirmed by GC-MS: the trimethylsilylderivative of 2 ([M]+=592) confirmed the HR-ESI-MS result and the presence of two phenolic and one carboxyl groups. The lack of a NOESY correlation between H-7 and H3–10 indicated a 6,7-trans-configuration, while lack of any optical rotation points to a racemic mixture of enantiomers (2a and 2b). Thus, 2 is (±)-6,7-trans-epoxycannabigerolic acid. Compound 3 was isolated as an optically inactive yellow oil. The HR-ESI-MS of 3 afforded an [M + Na]+ ion at m/z = 399.2194 implying a molecular formula C22H32O5. The 1H-, 13C- and 2D-NMR data of 3 (Table 1 and Table 2) are similar to those of 2 except for the downfield shift of C-6 (+ 3 ppm) [8] and the ROESY correlation between H-7 and H3–10 (Fig. 1), these findings indicated the 6,7-cis configuration (3a and 3b). Therefore, 3 is (±)- 6,7-cis-epoxycannabigerolic acid.
Fig. 1.
HMBC correlations of 2 and 3 (→) and key ROESY correlations of 3 (↔).
Compound 4 was obtained as an optically inactive oil, with a molecular formula C21H32O3 based on HR-ESI-MS (m/z = 333.2493 [M + H]+). GC-MS analysis (rt = 38.84 min) displayed a base peak at m/z = 193. The IR, 1H- and 13C-NMR data of 4 in CDCl3 were similar to those of cannabigerol (13) [9] (Table 1 and Table 2) except for the presence of a 6,7-epoxy group [δH = 3.88 (t, J = 5.2 Hz, H-7); δC = 67.3 (C-7), 81.4 (C-6)], while the ROESY correlation between H-7 and H3–10, established 4 as (±)-6,7-cis-epoxycannabigerol.
Compound 5 was also isolated as an optically inactive oil and its molecular formula was determined as C21H32O3 by HR-ESI-MS and 13C-NMR spectroscopy. GC-MS analysis of 5 (rt = 38.68 min) revealed a base peak at m/z = 193, while the NMR data were almost identical to those of 4 except for an upfield shift of C-6 (Table 2) and the absence of ROESY correlation between H-7 and H3–10. Based on the above, 5 was elucidated as (±)-6,7-trans-epoxycannabigerol.
Compound 6 was isolated as a yellow oil. On the basis of its HR-ESI-MS at m/z = 375.2540 [M + H]+ and 13C-NMR spectroscopic data, the molecular formula was established as C23H34O4. The structure was determined by comparing its 1H- and 13C-NMR data in CDCl3 (Table 1 and Table 2)with 1. Compound 6 contained an additional methoxy group [δH = 3.86 (s, OMe); δC = 55.7 (OMe)] instead of a hydroxy group. The location of the methoxy group was determined to be at C-5′ from HMBC correlations (OMe/C-5′; OMe/C-6′; H-4′/OMe), establishing 6 as 5′-methoxycannabigerolic acid. Although 6 is a known cannabis constituent [10], this is the first report of its full NMR assignments.
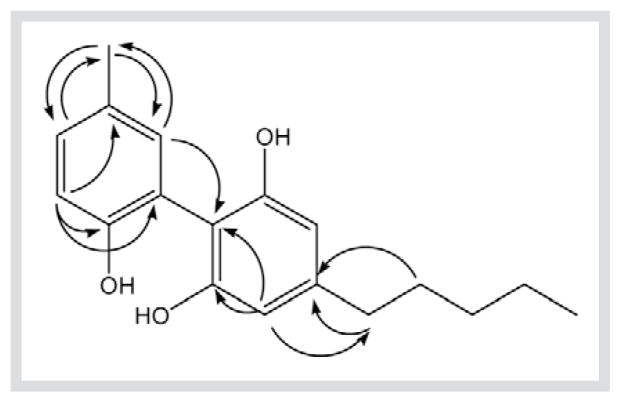
Compound 7 was isolated as a yellow oil. It gave a molecular formula of C18H22O3 based on HR-ESI-MS (m/z= 287.1612 [M+H]+, 285.1581 [M − H]−), GC-MS ([M]+ 286) and 13C-NMR data. The IR absorption bands at 3390, 1615, 1242 and 1035 cm−1 indicated the presence of hydroxy and benzene ring functionalities. The 1H-NMR (Table 1) and COSY spectra of 7 indicated two aromatic ring systems, 1,2,4,6-tetrasubstituted ring A with two magnetically equivalent protons [δH = 6.45 (2H, s, H-3 and H-5)] and an ABX spin system for ring B [δH = 6.99 (1H, d, J = 8.2 Hz, H-3′), 7.15 (1H, dd, J = 2.0, 8.2 Hz, H-4′), 7.03 (1H, d, J = 2.0 Hz, H-6′)]. The 1H-NMR also showed the presence of one aromatic methyl singlet at δH = 2.30 (H-7′) and an n-pentyl moiety. The 13C-NMR (Table 2), DEPT-135 and HMQC spectra revealed the presence of 18 carbon resonances, including 2 methyl, 4 methylene, 5 sp2 methine, 3 oxyaryl and 4 quaternary carbons. The 1H- and 13C-NMR data of ring A are suggestive of a phenyl substituted olivetol (biphenyl) [11], and together with HMBC correlations (Fig. 2) [H3 –7′/C-4′, C-6′; H-3′/C-2′, C-1′] indicates that 7 is 5′-methyl-4-pentylbiphenyl-2,2′,6-triol.
Fig. 2.
Key HMBC correlations of 6.
Compound 8 was obtained as white needles. HR-ESI-MS at m/z = 261.1434 [M + H]+ and 13C-NMR established the molecular formula as C16H20O3. GC-MS analysis of 8 showed a base peak at m/z = 203 and two other characteristic ions at m/z = 189 and 175, indicating that 8 is a spiroindane derivative [12]. 1H- and 13C-NMR data of 8 were similar to those reported for cannabispirone 9 (Table 2) [13], [14], except for a methoxy instead of hydroxy group at C-7, establishing 8 as 7-methoxycannabispirone. The structure was confirmed by DEPT-135, COSY, HMQC and HMBC analysis. Although 8 is a known synthetic product [14], [15], this is the first report of its isolation from a natural source.
Compounds 9–13 were identified as cannabispirone [12], β-cannabispiranol [15], dehydrocannabifuran [16], [17], cannflavin B [9] and cannabigerol [9] by comparing their spectroscopic data with reported values. However, this is the first report for the 13C-NMR assignments for dehydrocannabifuran (11) (Table 3).
Compound 13 exhibited selective antimicrobial activity against Mycobacterium intracellulare with an IC50 value of 15.0 μg/mL. Compounds 1 and 12 displayed moderate antileishmanial activity with IC50 values of 12.0 and 5.0 μg/mL, respectively. All isolates lacked cytotoxicity against Vero cells (African green monkey kidney fibroblast).
Acknowledgments
This work is supported by the Center of Research Excellence in Natural Products Neuroscience, The University of Mississippi, contract # 1P20RR021929-01, and by the National Institute on Drug Abuse, contract # N01DA-5-7746. We are grateful to Dr. Bharathi Avula for assistance with the HR-ESI-MS, and to Dr. Melissa Jacob, Ms. Marsha Wright and Dr. Babu Tekwani for conducting the antimicrobial and antileishmanial testing.
Abbreviations
- CBG
cannabigerol
- CBD
cannabidiol
- FID
flame ionization detector
- Δ9-THC
Δ9-tetrahydrocannabinol
- VLC
vacuum liquid chromatography
References
- 1.El-Sohly MA, Slade D. Chemical constituents of Marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–14. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]
- 3.El-Sohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF. Potancy trends of Δ9-THC and other cannabinoids in confiscated Marijuana from 1980–1997. J Forensic Sci. 2000;45:24–30. [PubMed] [Google Scholar]
- 4.Bharate SB, Khan SI, Yunus NA, Chaulhe SK, Jacob MR, Tekwani B, et al. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Radwan MM, Manly SP, Ross SA. Two new sulfated sterols from the marine sponge Lendenfeldia dendyi. Nat Prod Comm. 2007;2:901–4. [Google Scholar]
- 6.Yang CR, Zhang Y, Jacob MR, Khan SI, Zhang YJ, Li XC. Antifungal activity of C-27 steroidal saponins. Antimicrob Agents Chemother. 2006;50:1710–4. doi: 10.1128/AAC.50.5.1710-1714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mechoulam R, Gaoni Y. The isolation and structure of cannabinolic, cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21:1223–9. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- 8.Hevesi L, Nagy JB, Derouane EG. H and C studies of alkenes, epoxides and cyclic thionocarbonates. Org Magn Reson. 1977;10:14–9. [Google Scholar]
- 9.Choi YH, Hazekamp A, Peltenburg-Looman AG, Frederich M, Erkelens C, Lefeber AM, et al. NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of cannabis sativa. Phytochem Anal. 2004;15:345–54. doi: 10.1002/pca.787. [DOI] [PubMed] [Google Scholar]
- 10.Shoyama Y, Yamauchi T, Nishioka I. Cannabis V, cannabigerolic acid monomethyl ether and cannabinolic acid. Chem Pharm Bull. 1970;18:1327–32. [Google Scholar]
- 11.McClanahan R, Robertson LW. Microbial transformation of olivetol by Fusarium roseum. J Nat Prod. 1985;48:660–3. [Google Scholar]
- 12.El-Feraly FS, El-sherei MM, Muhtadi FJ. Spiro-indans from Cannabis sativa. Phytochemistry. 1986;25:1992–4. [Google Scholar]
- 13.Bercht CA, Dongen JP, Heerma W, Lousberg RC, Kuppers FJ. Cannabispirone and cannabispirenone, two naturally occurring spiro-compounds. Tetrahedron. 1976;32:2939–43. [Google Scholar]
- 14.El-Feraly FS, Chan YM. Total synthesis of cannabispiran and (±)-dehydrocannabispiran. J Nat Prod. 1981;44:557–61. [Google Scholar]
- 15.Boeren EG, El-Sohly MA, Turner CE, Salemink CA. β-Cannabispiranol: a non-cannabinoid phenol from Cannabis sativa L. Experientia. 1977;33:848. doi: 10.1007/BF01951236. [DOI] [PubMed] [Google Scholar]
- 16.Papadakis DP, Salemink CA. Isolation and identification of new cannabinoids in cannabis smoke. Tetrahedron. 1983;39:2223–5. [Google Scholar]
- 17.Jorapur VS, Duffley RP, Radzan RK. A biogenetic-type synthesis of cannabifuran and dehydrocannabifuran. Synth Commun. 1984;14:203–7. [Google Scholar]