Abstract
Odorant binding proteins (OBPs) are proposed to be directly required for odorant discrimination and represent potential interesting targets for pest control. In the notoriously agricultural pest Adelphocoris lineolatus, our previous functional investigation of highly expressed antennal OBPs clearly supported this viewpoint, whereas the findings of the current study by characterizing of AlinOBP11 rather indicated that OBP in hemipterous plant bugs might fulfill a different and tantalizing physiological role. The phylogenetic analysis uncovered that AlinOBP11 together with several homologous bug OBP proteins are potential orthologs, implying they could exhibit a conserved function. Next, the results of expression profiles solidly showed that AlinOBP11 was predominantly expressed at adult mouthparts, the most important gustatory organ of Hemiptera mirid bug. Finally, a rigorously selective binding profile was observed in the fluorescence competitive binding assay, in which recombinant AlinOBP11 displayed much stronger binding abilities to non-volatile secondary metabolite compounds than the volatile odorants. These results reflect that AlinOBP11, even its orthologous proteins across bug species, could be associated with a distinctively conserved physiological role such as a crucial carrier for non-volatiles host secondary metabolites in gustatory system.
Keywords: Adelphocoris lineolatus, odorant binding protein, expression profiles, phylogenetic analysis, Fluorescence competitive binding assay
Introduction
Smell is undoubtedly the most important sensory for insects survival and reproduction (Li and Liberles, 2015; Groot et al., 2016). Olfactory system that can sensitively and selectively detect biologically active odorants attracts great attention from researchers who attempt to explore alternative environment-friendly pest management strategy. In insect olfactory signal transduction pathway, several classes of membrane-bound proteins such as odorant receptor (ORs), ionotropic receptors (IRs), and sensory neuron membrane proteins (SNMPs) have been proven to play central roles in facilitating the conversion of the chemical message to an electrical signal, while the carrier proteins like odorant binding proteins (OBPs) or chemosensory proteins (CSPs) are proposed to bind, deliver and even recognize specific pheromones and odorants to their relevant receptors (Jacquin-Joly and Merlin, 2004; Leal, 2013). For decades, various functional studies toward important olfactory protein families such as OBPs or ORs actually lead to a quick discovering of some high-efficiency pest repellents or attractants (Tanaka et al., 2009; Sun Y. F. et al., 2012; Sun L. et al., 2013). For instance, in the alfalfa plant bug, A. lineolatus, behavioral active compounds were successfully screened via ligand binding assay of an antennae highly expressed AlinOBP10 (Sun L. et al., 2013). Synthetic compounds targeting OBP3 or OBP7 which was proven to be responsible for (E)-ß-farnesene perception elicited significantly behavioral responses in aphids (Sun Y. F. et al., 2012).
Insect OBPs which were first identified in antennal sensillum of silk moth, Antheraea polyphemus (Vogt and Riddiford, 1981) belong to the superfamily of small acidic soluble carrier proteins and could be recognized by six highly conserved cysteines (Leal et al., 1999; Sandler et al., 2000; Tegoni et al., 2004; Pelosi et al., 2014). Studies of both immunocytochemical localization and in situ hybridization revealed that OBPs were synthesized by non-neuronal auxiliary cells (trichogen and tormogen cells) and secreted into the sensillum lymph with a very high concentration (up to 10 mM) (Steinbrecht et al., 1995; Hekmat-Scafe et al., 1997; Michael, 2000; De Santis et al., 2006; Sun Y. P. et al., 2013; Sun et al., 2014a). So far, various investigations elucidated that antennal sensillum enriched OBPs indeed played essential roles in recognition of physiologically relevant odorants (Jacquin-Joly et al., 2000; Pophof, 2004; Große-Wilde et al., 2006; He et al., 2010). For example, one subclass of OBP families named pheromone binding proteins, PBPs, was highly abundant in long trichoid sensilla and showed significantly specific binding affinities to insect sex pheromones (Vogt and Riddiford, 1981; Krieger et al., 1996; Leal et al., 1999; Klusák et al., 2003; Pophof, 2004; Große-Wilde et al., 2006; De Santis et al., 2006). Sensilla basiconica expressed OBPs were proposed to be involved in terpenoids or other plant volatiles detection (Feng and Prestwich, 1997). Meanwhile, in two Lepidopteran species, the cotton leafworm Spodoptera littoralis (Poivet et al., 2012) and the diamondback moth Plutella xylostella (Zhu et al., 2016), OBPs were even demonstrated to be associated with the interesting behavior why larvae are attracted by conspecific moth sex pheromone.
However, the functions of insect OBPs may be more complicated and could not be restrict within olfactory cue recognition. In Drosophila melanogaster, most OBPs were detected in both gustatory and olfactory sensilla and some numbers were even expressed exclusively in taste organs (Galindo and Smith, 2001). Jeong et al. (2013) proposed that feeding behavior of D. melanogaster can be suppressed by a gustatory organ expressed OBP49a responding to bitter compounds. Two OBP genes, Obp57d and Obp57e in D. sechellia have been demonstrated to be involved in the evolution of taste perception and host-plant preference (Matsuo et al., 2007). Particularly, expressions of Aedes aegypti OBP22 in antennae and reproductive organs indicated its multiple functions (Li et al., 2008). Likewise, physiological roles of male reproductive organs expressed orthologous OBP10 in two sibling moth species has been proposed to act as a specific carrier for female oviposition deterrents that could help Helicoverpa offspring avoid cannibalism (Sun Y. L. et al., 2012).
The alfalfa plant bug, A. lineolatus, a typical polyphagous insect pest outbreaks frequently in cotton field since the transgenic Bacillus thuringiensis cotton largely cultivation in China (Lu et al., 2010). Worse still, flight behavior enable it to migrate among different host plants (Lu et al., 2009), and many other important crops like alfalfa (Medicago sativa L.), green bean (Phaseolus vulgaris), and tea plant (Camellia sinensis) suffer from its serious destroy (Lu and Wu, 2008). Evidence suggested that this bug heavily relies on chemical cues for host plant location and migration (Lu and Wu, 2008). Thus, studies aiming at the physiological and molecular basis of insect chemosensation may help explore an alternatively effective pest control method.
Previously, 14 OBP transcripts of A. lineolatus were identified (Gu et al., 2011a) and functional studies of several antennae highly expressed OBPs such as AlinOBP1, AlinOBP5, AlinOBP10, and AlinOBP13 indicated their potential olfactory roles (Gu et al., 2011b; Sun L. et al., 2013; Wang et al., 2013; Sun et al., 2014a). Subsequently, Hull et al. (2014) identified 33 putative OBP transcripts in the tarnished plant bug, Lygus lineolaris, and suggested that several OBP genes included LylinOBP19 can be expressed in gustatory organs, implying they may be related to taste compound detected. However, whether OBPs could express at taste organs and fulfill potential gustatory functions in A. lineolatus remains largely unknown. In the current study, we mainly focus our attention on AlinOBP11, a putative orthologous OBP gene of LylinOBP19 in A. lineolatus and our current results of tissue distribution pattern, ligand binding assay, and phylogenetic analysis would provide detail cues for its functional discussion.
Materials and methods
Insect rearing and tissue collection
A. lineolatus adults were collected from alfalfa fields at the Langfang Experimental Station of Chinese Academy of Agricultural Sciences, Hebei Province, China. The laboratory colony was established in plastic containers (20 × 13 × 8 cm), which were maintained at 29 ± 1°C, 60 ± 5% relative humidity, and 14 h light:10 h dark cycle. The adults and newly emerged nymphs were reared on green beans and 10% honey. Different tissues from A. lineolatus adults of both sexes including antennae, mouthparts, heads (without antennae and mouthparts), thoraxes, abdomens, legs, and wings were collected for qRT-PCR. Each tissue was collected from three biological pools and all the specimens were immediately stored in −80°C for further process.
RNA isolation and cDNA synthesis
Total RNA of each sample was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA), and the first-strand cDNA was synthesized by FastQuant RT-kit with gDNA Eraser (TianGen, Beijing, China) according to the manufacturer's instructions.
qRT-PCR
qRT-PCR assay regarding different developmental stages and tissues were carried out using an ABI 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Two house-keeping genes Alinβ-actin (GenBank No.GQ477013) and AlinElongation factor (GenBank No.AEY99651) were used as endogenous controls to normalize the target gene expression and correct for sample-to-sample variation. Taqman primers of Alinβ-actin and AlinOBP11 cited Gu et al. (2011a) and primers of AlinElongation factor were designed using Primer Express 3.0 (Applied Biosystems) and listed in Table S1. For the qRT-PCR reaction, the cDNA was diluted to concentration of 200 ng /μL. Each reaction was performed in a 25 μL mixture of 12.5 μL of Premix Ex Taq (TaKaRa), 1 μL of each primer (10 mM), 0.5 μL probe (10 mM), 0.5 μL of Rox Reference Dye II, 1 μL of sample cDNA (200 ng), and 8.5 μL of sterilized H2O. Negative controls were non-template reactions (H2O instead of cDNA). The reaction cycling parameters were as follows: 95°C for 10 s, 40 cycles at 95°C for 20 s, 60°C for 34 s. For the data reproducibility, qRT-PCR reaction for each sample was performed in three technical replicates and three biological replicates. Since our preliminary experiment demonstrated that the amplification efficiency between targeted genes and reference gene was similar (data not shown), the comparative 2−ΔΔCT method was used to calculate the relative quantification between tissues (Livak and Schmittgen, 2001).
The comparative analyses of target gene among different tissues and developmental stages were determined using a one-way nested analysis of variance (ANOVA), followed by Tukey's honestly significance difference (HSD) test using the software SPSS Statistics 18.0 (SPSS Inc., Chicago, IL, USA).
Phylogenetic construction and selective pressure analysis
The 92 OBP sequences of five mirid bug species (GenBank accession numbers and references can be seen in Table S2) were used to infer the evolutionary history with the software MEGA 6.0 with a p-distance model and a pairwise deletion of gaps (Tamura et al., 2013). The bootstrap support of tree branches was assessed by re-sampling amino acid positions 1000 times. Estimation of the non synonymous (dN) to synonymous (dS) substitution rate (ω) was performed by the maximum likelihood method (Anisimova et al., 2001) using the Codeml program in the PAML 4.6 package (Yang, 1997).
Western blot assay
The polyclonal antiserum against the recombinant AlinOBP11 was produced by injecting robust adult rabbits subcutaneously and intramuscularly with the highly purified recombinant protein. Recombinant protein was emulsified with an equal volume of Freund's complete adjuvant (Sigma, St. Louis, MO, USA) for the first time injection (500 μg) and then with incomplete adjuvant for the three additional injections (300 mg each time). The interval between each injection was approximately half a month, and blood was collected 7 days after the last injection and centrifuged at 6000 rpm for 20 min. The serum was purified based on a MAb Trap kit (GE Healthcare) following the manufacturer's instructions. The rabbits were maintained in large cages at room temperature, and all of the operations were performed according to ethical guidelines to minimize the pain and discomfort of the animals.
Crude extracts from different tissues of female and male adult bugs included the antennae, mouthparts, legs, wings, and bodies (without aforesaid parts) were separated on 15% SDS-PAGE, respectively. Samples were transferred to a polyvinylidene fluoride membrane (PVDF, Millipore, Carrigtwohill, Ireland) at the condition of 200 mA for 50 min, and then membrane was blocked using 5% dry skimmed milk (BD Biosciences, San Jose, CA, USA) in phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBST) for 2 h at room temperature. After washing three times with PBST (10 min each time), the blocked membrane was incubated with purified rabbit anti-AlinOBP11antiserum (dilution 1:2000) for 1 h. Three times washing with PBST again, the membrane was incubated with anti-rabbit IgG horseradish peroxidase (HRP) conjugate and HRP-streptavidin complex (Promega, Madison, WI, USA) at a dilution of 1:10000 for 1 h. The membrane was then incubated with the western blot substrates of the enhanced chemiluminescence western blot kit (CoWinbiotech, China), and the bands were visualized by exposing to X-OMATBT films (Kodak, New York, USA).
Fluorescence competitive binding assay
The recombinant protein expression and purification was performed according to our previous protocols (Sun L. et al., 2013; Sun et al., 2014a). Briefly, the plasmid containing AlinOBP11 gene was constructed and transformed into Escherichia coli BL21 (DE3) competent cells for recombinant protein expression, and the protein was largely induced with 1 mM isopropyl ß-D-1-thiogalactopyranoside (IPTG) at 37°C for 3–6 h. The purification was performed using two rounds of Ni ion affinity chromatography (GE-Healthcare), and the His-tag was removed with recombinant enterokinase (Novagen). The highly purified proteins were desalted through extensive dialysis, and then the size and purity of the recombinant proteins were verified by 15% SDS-PAGE.
For the ligand binding assays, 45 compounds include 41 volatiles and four non-volatiles were selected based on previously reported isolation from A. lineolatus host plants (Meisner et al., 1977; Halloin, 1982; Aldrich, 1988; Loughrin et al., 1995; Röse and Tumlinson, 2004; Millar, 2005). The binding assay was performed on an F-380 fluorescence spectrophotometer (Tianjin, China) at room temperature (25°C) with a 1-cm light path quartz cuvette and 10-nm slits for both excitation and emission. The excitation wavelength was 337 nm, and the emission spectrum was recorded between 390 and 460 nm. Firstly, the constant of AlinOBP11 with the fluorescent probe N-phenyl-1-naphthylamine (1-NPN) was measured, a final concentration of 2 μM protein solution in 50 mM Tris-HCl (pH 7.4) was titrated with aliquots of 1 mM 1-NPN dissolved in methanol to final concentrations ranging from 1 to 16 μM. Then the affinities of other ligands were tested through competitive binding assays using 1-NPN as the fluorescent reporter at a concentration of 2 μM, and the concentration of each competitor ranged from 2 to 30 μM. The fluorescence intensities at the maximum fluorescence emission between 390 and 460 nm were plotted against the free ligand concentration to determine the binding constants. The bound chemical was evaluated based on its fluorescence intensity with the assumption that the protein was 100% active with a stoichiometry of 1:1 (protein: ligand) saturation. The binding curves were linearized using a Scatchard plot, and the dissociation constants of the competitors were calculated from the corresponding IC50 values based on the following equation: Ki= [IC50] / (1+ [1-NPN]/K1−NPN), where [1-NPN] is the free concentration of 1-NPN and K1−NPN is the dissociation constant of the complex protein/1-NPN.
Results
Phylogenetic tree construction and selective pressure analysis
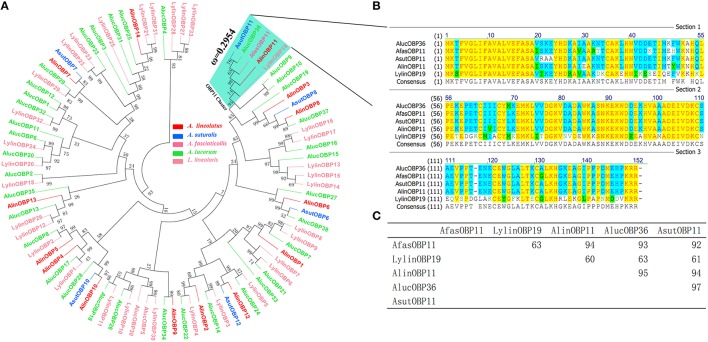
A phylogenetic tree of 92 OBPs was constructed using the neighbor-joining method to analyze evolutional relationships between AlinOBP11 and other OBPs of different mirid species. Figure 1 revealed a divergent OBP repertoire. AlinOBP11 and four other OBPs i.e., AlucOBP36, AfasOBP11, AsutOBP11, and LylinOBP19 from each bug species clustered into one same clade with bootstrap support value up to 74 (Figure 1A). Sequence alignment analysis showed AlinOBP11 has 94, 95, 94, and 60% identity to AsutOBP11, AlucOBP36, AfasOBP11, and LylinOBP19, respectively (Figures 1B,C). These results reflect AlinOBP11 and these four OBPs form a clear orthologous group across bug species.
Figure 1.
Sequence alignment and phylogeny of AlinOBP11 with other OBPs identified in five bug species. (A) Neighbor-joining phylogenetic tree was constructed used MEGA 6.0 with a p-distance model and a pairwise deletion of gaps; The non-synonymous (dN) to synonymous (dS) substitution rate (ω) of OBP11 orthologs was labeled beside its cluster. (B) Sequence alignment was performed using the program ClustalX 2.1 with default gap penalty parameters of gap opening 10 and extension 0.2, and was edited using the GeneDoc 2.7.0 software. (C) The percent identity matrix of OBP11 orthologs is calculated using Vector NTI 10.0.
To evaluate potential selective pressure acting on this OBP11 orthologous cluster, we calculated the ratio of non synonymous to synonymous substitutions (dN/dS or ω) of this cluster with branch models using PAML and compared the log likelihoods (lnL) for the one ratio model M0 (assuming one ω ratio for all branches) and the free ratio model M1 (assuming one ω ratio for each branch) in likelihood ratio tests. The results uncovered that the one ratio model (M0) could not be rejected (p>0.01) and all branches shared a normalized ω ratio of 0.2954 (Figure 1A), implying that purifying selection was acting on this cluster and AlinOBP11 would share a relatively conserved physiological function with its orthologous genes (Qiao et al., 2009; Zhou et al., 2010; Vandermoten et al., 2011).
Specific tissue and developmental expression profiles of AlinOBP11
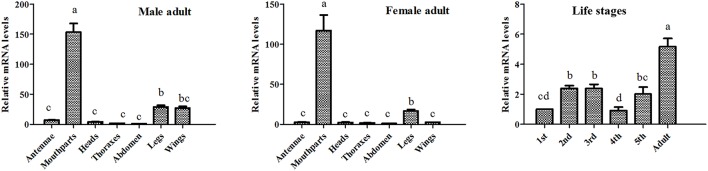
The results of our western blot assay showed that clear protein bands could be found at mouthparts, legs, antennae as well as other tissues, which seems that AlinOBP11 can be ubiquitously expressed at adult tissues of both sexes (Figure 2). To compare the expression levels of AlinOBP11 among different tissues, we then conducted the qRT-PCR assay. Interestingly, unlike previously reported uniformly antennae predominant expressed AlinOBPs (Gu et al., 2011a; Sun L. et al., 2013; Sun et al., 2014a), our current results revealed that AlinOBP11 was strongly expressed at mouthparts, and slightly expressed at legs, antennae, and other tissues (Figure 3). Meanwhile, AlinOBP11 transcript abundance varied among different developmental instars and significantly higher expression level was observed in adult bugs (Figure 3).
Figure 2.
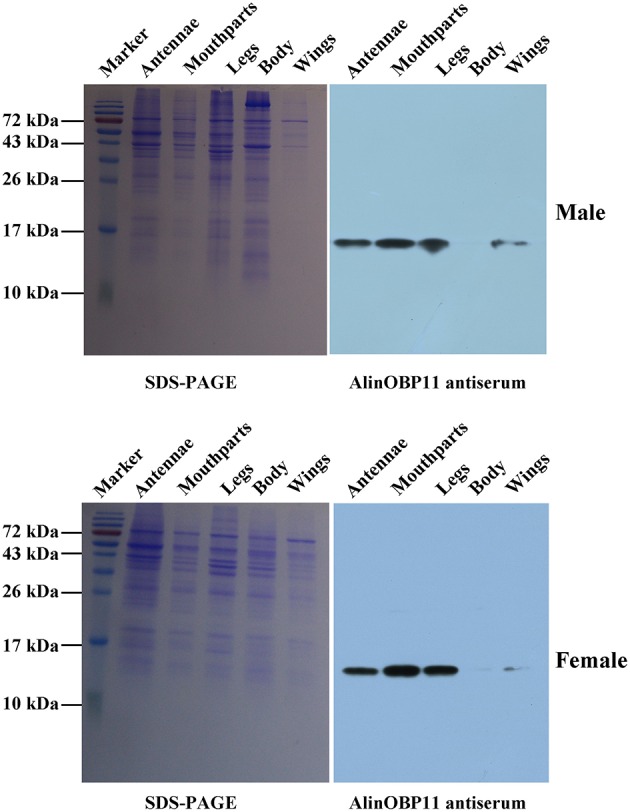
SDS-PAGE and AlinOBP11 expression profiles among different adult tissues of both sexes assessed by western blot analysis. The results showed that AlinOBP11 was detected at both male and female adult mouthparts, legs, antennae, and weakly or even undetectably at other tissues.
Figure 3.
The relative transcript levels of AlinOBP11 at different developmental stages and adult tissues of both sexes analyzed by qRT-PCR. All data were normalized to endogenous house-keeping genes Alinβ-actin (GenBank No. GQ477013) and relative fold changes are normalized to transcript level of the first instar nymph or abdomen. The error bars represents the standard errors, and the different letters a, b, c, and d indicate significant differences (p < 0.05) among different samples. The similar results were obtained with AlinElongation factor (GenBank No. AEY99651) as internal control (Figure S2).
In vitro expression and purification of AlinOBP11
The recombinant AlinOBP11 was successfully expressed using a bacterial system. Induced targeted recombinant appeared at both supernatant and insoluble inclusion bodies and the former was selected to be purified using two rounds of Ni ion affinity chromatography (GE Healthcare, Little Chalfont, UK). The finally purified AlinOBP11 recombinant protein on the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis displayed a single band (Figure S1).
Ligand-binding of recombinant AlinOBP11
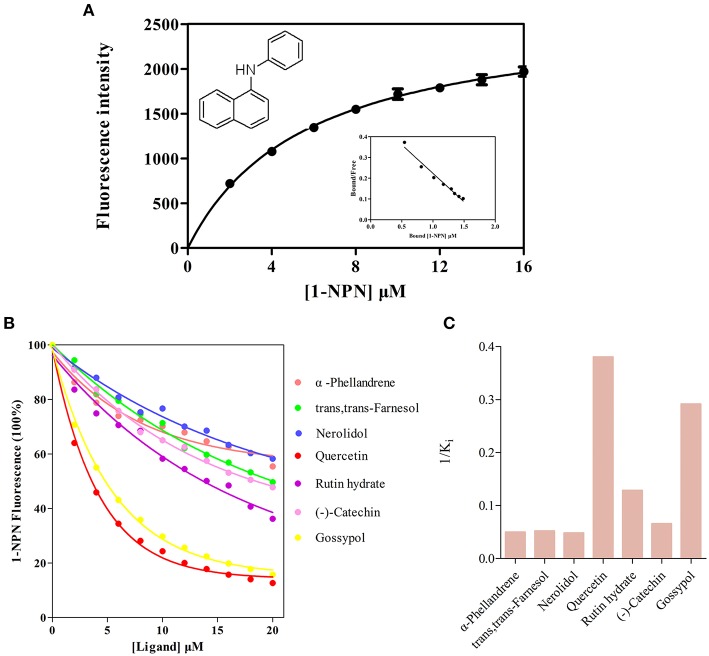
Before the ligand-binding analysis, we measured the binding affinities of fluorescence probe 1-NPN with purified AlinOBP11. The results showed AlinOBP11 could solidly bind to 1-NPN with binding affinity of 5.86 ± 0.47 μM (Figure 4A). Consequently, the binding properties of AlinOBP11 to compounds with different functional groups were analyzed and the results suggested it had a relatively narrow binding profile. Notably, all the tested non-volatile compounds showed strong binding abilities to AlinOBP11, and quercetin was the best ligand (Ki = 2.63 ± 0.23 μM), followed by gossypol (Ki = 3.43 ± 0.32 μM), rutin hydrate (Ki = 7.78 ± 1.23 μM), and (−)-catechin (Ki = 15.26 ± 0.70 μM). Additionally, the tested host volatiles such as aliphatic alcohols, aldehydes, ketones, esters, aromatics could hardly bind to recombinant AlinOBP11, except of three terpenoids α-phellandrene, nerolidol, and trans, trans-farnesol, which can bind to AlinOBP11 and their binding constant Ki was 20.07 ± 0.41, 20.76 ± 0.55, and 19.26 ± 1.78 μM, respectively (Figures 4B,C; Table 1).
Figure 4.
Fluorescence competitive binding assay. (A) Binding curve and relative Scatchard plot of 1-NPN to AlinOBP11. The dissociation constant of the AlinOBP11/1-NPN complex was calculated as 5.86 ± 0.47 μM. (B) Competitive binding curves of selected host plant compounds to AlinOBP11. (C) The reverse values of the dissociation constants (Ki) measured with putative ligands of AlinOBP11. A mixture of the recombinant AlinOBP11 protein and N-phenyl-1-naphthylamine (1-NPN) in 50 mM Tris-Hcl buffer (pH 7.4) both at the concentration of 2 μM was titrated with 1 mM solutions of each competing ligand to the final concentration range of 2 to 30 μM. Fluorescence intensities are reported as percent of the values in the absence of competitor. Data are represented as means of three independent experiments.
Table 1.
Binding affinities of all of the selected compounds to the recombinant AlinOBP11 protein.
| Ligand | CAS Number | AlinOBP11 | |
|---|---|---|---|
| IC50 (μM) | Ki (μM) | ||
| GENERAL ODORANTS | |||
| 2-Hexanol | 626-93-7 | u.d. | u.d. |
| Pentanol | 71-41-0 | u.d. | u.d. |
| Valeraldehyde | 110-62-3 | u.d. | u.d. |
| Hexanal | 66-25-1 | u.d. | u.d. |
| Heptanal | 111-71-7 | u.d. | u.d. |
| Octanal | 124-13-0 | u.d. | u.d. |
| Nonanal | 124-19-6 | u.d. | u.d. |
| 2-Hexanone | 591-78-6 | u.d. | u.d. |
| 2-Heptanone | 110-43-0 | u.d. | u.d. |
| 2-Octanone | 111-13-7 | u.d. | u.d. |
| 3-Hexanone | 589-38-8 | u.d. | u.d. |
| 6-Methyl-5-hepten-2-one | 110-93-0 | u.d. | u.d. |
| Amyl acetate | 628-637-7 | u.d. | u.d. |
| Nonyl acetate | 1143-13-5 | u.d. | u.d. |
| Undecane | 1120-21-4 | u.d. | u.d. |
| Indole | 120-72-9 | u.d. | u.d. |
| Benzaldehyde | 100-52-7 | u.d. | u.d. |
| 3,4-Dimethyl-benzaldehyde | 5973-71-7 | u.d. | u.d. |
| Acetophenone | 98-86-2 | u.d. | u.d. |
| Methyl salicylate | 119-36-8 | u.d. | u.d. |
| GREEN LEAF VOLATILES | |||
| 1-Hexanol | 111-27-3 | u.d. | u.d. |
| cis-3-Hexen-1-ol | 928-96-1 | u.d. | u.d. |
| trans-2-Hexenal | 6278-26-3 | u.d. | u.d. |
| cis-3-hexenyl acetate | 3681-71-8 | u.d. | u.d. |
| TERPENOIDS | |||
| E-β-Ocimene | 3016-19-1 | u.d. | u.d. |
| Limonene | 5989-27-5 | u.d. | u.d. |
| α-Phellandrene | 99-83-2 | 26.16 ± 0.52 | 20.07 ± 0.41 |
| β-Pinene | 18172-67-3 | u.d. | u.d. |
| (+)-α-Pinene | 7785-70-8 | u.d. | u.d. |
| β-Ionone | 79-77-6 | u.d. | u.d. |
| Myrcene | 123-35-3 | u.d. | u.d. |
| Nerolidol | 7212-44-4 | 27.23 ± 0.71 | 20.76 ± 0.55 |
| β-Caryophyllene | 87-44-5 | u.d. | u.d. |
| α-Humulene | 6753-98-6 | u.d. | u.d. |
| trans-β-Farnesene | 18794-84-8 | u.d. | u.d. |
| trans,trans-Farnesol | 106-28-5 | 25.21 ± 2.36 | 19.26 ± 1.78 |
| PUTATIVE SEX PHEROMONES | |||
| Hexyl butyrate | 2639-63-6 | u.d. | u.d. |
| Hexyl hexanoate | 6378-65-0 | u.d. | u.d. |
| Butyl butyrate | 109-21-7 | u.d. | u.d. |
| Ethyl butyrate | 105-54-4 | u.d. | u.d. |
| trans-2-hexenyl butyrate | 53398-83-7 | u.d. | u.d. |
| HOST PLANT SECONDARY METABOLITES | |||
| (−)-Catechin | 18829-704 | 19.26 ± 0.93 | 15.26 ± 0.70 |
| Rutin hydrate | 207671-50-9 | 9.89 ± 1.61 | 7.78 ± 1.23 |
| Quercetin | 117-39-5 | 3.36 ± 0.30 | 2.63 ± 0.23 |
| Gossypol | 303-45-7 | 4.45 ± 0.34 | 3.43 ± 0.32 |
U.d. means that the IC50 value exceeds 30 μM and thus that the binding affinities (Ki) of the candidate competitive ligand were not calculated in this study.
Discussion
Insect OBPs may serve as important molecular target for designing and screening new effectively behavioral blocking agents used in the application of eco-friendly pest management strategies as they are considered to be strongly expressed in antennal sensillum lymph and are involved in olfactory cues discrimination, binding and transduction (Qiao et al., 2009; He et al., 2011; Sun Y. F. et al., 2012; Pelosi et al., 2013, 2014; Sun L. et al., 2013; Sun et al., 2014a). However, a plenty of studies suggested that OBPs' expression patterns are not restricted in olfactory organs and thus their physiological functions would be more complex and diversified (Park et al., 2000; Foret and Maleszka, 2006; Li et al., 2008; Sun Y. F. et al., 2012; Yuan et al., 2015). To confirm whether OBPs in the Hemiptera mirid bug species could fulfill putative gustatory function, in the present study we especially focus on a putative non-olfactory organ biased OBP gene, the OBP11 in A. lineolatus.
Previously, Gu et al. identified 14 putative OBP genes from the antennal cDNA library of A. lineolatus and suggested AlinOBP11 was strongly expressed at adult legs of both sexes (Gu et al., 2011a). Subsequently, a large number of potential OBP genes were identified in the tarnished plant bug, L. lineolaris and the green plant bug, Apolygus lucorum via transcriptome strategy, and more OBP transcripts were found to be expressed at gustatory organs such as legs and mouthparts (Hull et al., 2014; Yuan et al., 2015). Therefore, we firstly re-confirmed the tissue expression profiles of AlinOBP11 after taking mouthparts into account, the most important gustatory organs of Hemiptera species. The results of our western blot analysis revealed that clear single bands could be seen at mouthparts, legs as well as antennae of both male and female adult bugs (Figure 2). Interestingly, we found that relative mRNA level of AlinOBP11 was extraordinarily higher at adult mouthparts of both sexes than that of previous reported legs and other tissues (Figure 3). In addition, higher expression level was also observed in adult bugs than different instars of nymph (Figure 3). If we considered the tissue distribution patterns of all the 14 identified OBP genes, according to the inference that mRNA expression is indicative of physiological function of its encoded protein, a putative functional subdivision of different OBP genes in the same species of A. lineolatus would occur and the mouthparts-biased AlinOBP11 could be separated from other OBPs such as AlinOBP1, 10, 13 which have been demonstrated strongly expressed at antennae sensillum and fulfilled vital roles in bug olfactory cue perception (Gu et al., 2011b; Sun L. et al., 2013; Sun et al., 2014a). Indeed, unlike the antennae which are equipped with various olfactory sensilla (Chinta et al., 1997; Sun et al., 2014b), mouthparts of Hemiptera bug species consist of piercing-sucking stylets and labium, the former is used to eject saliva for food ingestion and is considered to be directly related to oviposition behavior (Romani et al., 2005), while the latter has 11–12 uniporous gustatory sensilla which are responsible for assessing the suitability of food substrates (Ave et al., 1978; Hatfield and Frazier, 1980). Our current results of tissue expression pattern merely confirmed that AlinOBP11 was preferentially expressed at A. lineolatus adult mouthparts. Although it is not known whether AlinOBP11 was expressed in stylets or the gustatory sensillum of labium, we can conceivably speculate that this provocatively specific expression profile would benefit the alfalfa plant bugs, to a great extent, in many importantly behavioral performances such as egg laying, host plants selection, and even of toxic substances avoidance.
Our fluorescence competition assay provides further insight into understanding of physiological roles of AlinOBP11. The results clearly showed that recombinant AlinOBP11 protein displayed preferential binding abilities to tested non-volatile host plant secondary metabolites than all the volatile compounds (Figures 4B,C; Table 1). These results correspond well to its specific tissue distribution within mouthparts which could give a functional implication that AlinOBP11 could function as carrier in gustatory system for non-volatile compounds detection when plant bugs begin to search suitable food substrates by using the mouthparts to rub or tap on plant surfaces or insert plant tissues. Additionally, plant secondary compounds play key roles in the long-term evolution of plant-herbivore interactions (Elsayed, 2011; Mithöfer and Boland, 2012), and the content level variation of quercetin, gossypol, and rutin hydrate, three AlinOBP11 best ligands, over the course of host plant maturation have been demonstrated to be involved in herbivore defense. In particular, gossypol and rutin hydrate were proposed to increase the resistance of cotton plants in response to mirid bug feeding, while the content of quercetin in cotton tended to perform a negatively correlation between their interactions (Lin et al., 2011). Thus, A. lineolatus might employ the mouthparts-biased expressed AlinOBP11 to perceive and discriminate these functional different non-volatile secondary metabolites; however, this speculation still needs to be supported by more evidences.
Gene duplication was pointed to be the main mechanism underlying the fast expansion and functional evolution of chemosensory genes (Zhou et al., 2010; Zhang and Löfstedt, 2013); nevertheless, physiological functions of putative orthologs also attract great interest. In aphid species, the distribution of orthologous OBP genes may reflect their life styles and host relationships. As an example, homologous OBP3 proteins of different aphid species were proved to be associated with recognition of alarm pheromone (E)-ß-farnesene (Qiao et al., 2009; Vandermoten et al., 2011; Sun Y. F. et al., 2012). We re-constructed the phylogenetic trees used reported OBPs of several bug species (Figure 1) and the results clearly suggest AlinOBP11 and AsutOBP11, AlucOBP36, AfasOBP11, and LylinOBP19 fall into the same clade and support they are potential orthologs across bug species which was consistent with Hull's assumption (Hull et al., 2014). Selective pressure assess by calculation of dN/dS or ω = 0.295 (Figure 1) also indicates that genes in this cluster are under purifying selection and would perform conserved functions (Qiao et al., 2009; Zhou et al., 2010; Vandermoten et al., 2011). Meanwhile, we found the AlinOBP11 was predominately expressed at mouthparts similar to tissue expression profiles of previous reported LylinOBP19 (Hull et al., 2014) and our further studies of AfasOBP11 and AsutOBP11 (data not shown here). However, Hua et al. (2012) suggested that AlucOBP36 (named as AlucOBP3 in their study) was antennae-biased expressed. This could be explained by different genetic relationships and evolutionary processes of these bugs. A. lineolatus, A. suturalis, and A. fasciaticollis belong to the same genus Adelphocoris, while A. lucorum belongs to the other genus Apolygus. Notably, the in vitro functional studies of antennae expressed AlucOBP36 resembled our results of AlinOBP11, which also showed better binding abilities to non-volatile host plant secondary compounds of rutin hydrate, but not to quercetin and gossypol (Hua et al., 2012), and this could be attributed to the mutations of several amino acids in these two proteins' binding pockets.
In conclusion, this study characterizes a mouthparts enriched OBP11 protein in A. lineolatus which preferentially binds to non-volatile plant secondary compounds; to our current knowledge, AlinOBP11 represents the first physiological function of mouthparts highly expressed OBP in Hemiptera species. As putative orthologous genes probably exhibited conserved physiological function, orthologous OBP11 could be involved in mirid bug feeding behaviors and serve as potential molecular targets for the development of eco-friendly pest management strategies against mirid bugs' outbreaks.
Author contributions
LS and YZ conceived and designed the experimental plan. LS, XM, and YX preformed the experiments. LS, YW, DZ, YZ, XY, QX, and YG analyzed the data. LS and DZ drafted the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by China National “973” Basic Research Program (2012CB114104), the National Natural Science Foundation of China (31272048, 31321004, 31471778, and 31501652) and Research Foundation of State Key Laboratory for Biology of Plant Diseases and Insect Pests (SKLOF201514).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2016.00201
SDS-PAGE analyses of AlinOBP11 expression and purification. Protein markers are shown in the left side; −, crude bacterial extract before induction with IPTG; + crude bacterial extracts after induction with IPTG; Sup, supernatant of disrupted PET/AlinOBP11; Pel, inclusion body of disrupted PET/AlinOBP11; P/His-tag, purified AlinOBP11 protein with His-tag; P, finally purified AlinOBP11 protein obtained after two rounds of purification.
The relative transcript levels of AlinOBP11 at different developmental stages and adult tissues of both sexes evaluated by qRT-PCR with AlinElongation factor (GenBank No.AEY99651) as internal control. The results clearly showed AlinOBP11 was strongly expressed at adult mouthparts.
The primers used in this article.
The protein names, GenBank accession numbers, and references of OBPs used in the phylogenetic analysis.
References
- Aldrich J. R. (1988). Chemical ecology of the Heteroptera. Annu. Rev. Entomol. 33, 211–238. 10.1146/annurev.en.33.010188.001235 [DOI] [Google Scholar]
- Anisimova M., Bielawski J. P., Yang Z. H. (2001). Accuracy and power of the likelihood ratio test in detecting adaptive molecular evolution. Mol. Biol. Evol. 18, 1585–1592. 10.1093/oxfordjournals.molbev.a003945 [DOI] [PubMed] [Google Scholar]
- Ave D., Frazier J. L., Hatfield L. D. (1978). Contact chemoreception in the tarnished plant bug Lygus lineolaris. Entomol. Exp. Appl. 24, 217–227. 10.1111/j.1570-7458.1978.tb02776.x [DOI] [Google Scholar]
- Chinta S., Dickens J. C., Baker G. T. (1997). Morphology and distribution of antennal sensilla of the tarnished plant bug, Lygus lineolaris (Palisot de beauvois) (Hemiptera: Miridae). Inter. J. Insect Morphol. Embryol. 26, 21–26. 10.1016/S0020-7322(96)00022-0 [DOI] [Google Scholar]
- De Santis F., Francois M.-C., Merlin C., Pelletier J., Maibeche-Coisne M., Conti E., et al. (2006). Molecular cloning and in situ expression patterns of two new pheromone-binding proteins from the corn stemborer Sesamia nonagrioides. J. Chem. Ecol. 32, 1703–1717. 10.1007/s10886-006-9103-2 [DOI] [PubMed] [Google Scholar]
- Elsayed G. (2011). Plant secondary substances and insects behaviour. Arch. Phytopathol. Plant Prot. 44, 1534–1549. 10.1080/03235408.2010.507957 [DOI] [Google Scholar]
- Feng L., Prestwich G. D. (1997). Expression and characterization of a Lepidopteran general odorant binding protein. Insect Biochem. Mol. Biol. 27, 405–412. 10.1016/S0965-1748(97)00012-X [DOI] [PubMed] [Google Scholar]
- Foret S., Maleszka R. (2006). Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 16, 1404–1413. 10.1101/gr.5075706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo K., Smith D. P. (2001). A large family of divergent drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics 159, 1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot A. T., Dekker T., Heckel D. G. (2016). The genetic basis of pheromone evolution in moths. Annu. Rev. Entomol. 61, 99–117. 10.1146/annurev-ento-010715-023638 [DOI] [PubMed] [Google Scholar]
- Große-Wilde E., Svatoš A., Krieger J. (2006). A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem. Senses 31, 547–555. 10.1093/chemse/bjj059 [DOI] [PubMed] [Google Scholar]
- Gu S. H., Wang S. P., Zhang X. Y., Wu K. M., Guo Y. Y., Zhou J. J., et al. (2011a). Identification and tissue distribution of odorant binding protein genes in the lucerne plant bug Adelphocoris lineolatus (Goeze). Insect Biochem. Mol. Biol. 41, 254–263. 10.1016/j.ibmb.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Gu S.-H., Wang W.-X., Wang G.-R., Zhang X.-Y., Guo Y.-Y., Zhang Z. D., et al. (2011b). Functional characterization and immunolocalization of odorant binding protein 1 in the lucerne plant bug, Adelphocoris lineolatus (Goeze). Arch. Insect Biochem. Physiol. 77, 81–99. 10.1002/arch.20427 [DOI] [PubMed] [Google Scholar]
- Halloin J. M. (1982). Localization and changes in catechin and tannins during development and ripening of cottonseed. New Phytol. 90, 651–657. 10.1111/j.1469-8137.1982.tb03274.x [DOI] [Google Scholar]
- Hatfield L. D., Frazier J. L. (1980). Ultrastructure of the labial tip sensilla of the tarnished plant bug, Lygus lineolaris (P. de Beauvois) (Hemiptera: Miridae). Inter. J. Insect Morphol. Embryol. 9, 59–66. 10.1016/0020-7322(80)90036-7 [DOI] [Google Scholar]
- He P., Zhang J., Liu N. Y., Zhang Y. N., Yang K., Dong S. L. (2011). Distinct expression profiles and different functions of odorant binding proteins in Nilaparvata lugens Stål. PLoS ONE 6:e28921. 10.1371/journal.pone.0028921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Tzotzos G., Woodcock C., Pickett J. A., Hooper T., Field L. M., et al. (2010). Binding of the general odorant binding protein of Bombyx mori BmorGOBP2 to the moth sex pheromone components. J. Chem. Ecol. 36, 1293–1305. 10.1007/s10886-010-9870-7 [DOI] [PubMed] [Google Scholar]
- Hekmat-Scafe D. S., Steinbrecht R. A., Carlson J. R. (1997). Coexpression of two odorant-binding protein homologs in Drosophila: implications for olfactory coding. J. Neurosci. 17, 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J. F., Zhang S., Cui J. J., Wang D. J., Wang C. Y., Luo J. Y., et al. (2012). Identification and binding characterization of three odorant binding proteins and one chemosensory protein from Apolygus lucorum (Meyer-Dür). J. Chem. Ecol. 38, 1163–1170. 10.1007/s10886-012-0178-7 [DOI] [PubMed] [Google Scholar]
- Hull J. J., Perera O. P., Snodgrass G. L. (2014). Cloning and expression profiling of odorant-binding proteins in the tarnished plant bug, Lygus lineolaris. Insect Mol. Biol. 23, 78–97. 10.1111/imb.12064 [DOI] [PubMed] [Google Scholar]
- Jacquin-Joly E., Bohbot J., Francois M. C., Cain A. H., Nagnan-Le Meillour P. (2000). Characterization of the general odorant-binding protein 2 in the molecular coding of odorants in Mamestra brassicae. Eur. J. Biochem. 267, 6708–6714. 10.1046/j.1432-1327.2000.01772.x [DOI] [PubMed] [Google Scholar]
- Jacquin-Joly E., Merlin C. (2004). Insect olfactory receptors: contributions of molecular biology to chemical ecology. J. Chem. Ecol. 30, 2359–2397. 10.1007/s10886-004-7941-3 [DOI] [PubMed] [Google Scholar]
- Jeong Y. T., Shim J., Oh S. R., Yoon H. I., Kim C. H., Moon S. J., et al. (2013). An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron 79, 725–737. 10.1016/j.neuron.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusák V., Havlas Z., RulıìŠek L. R., Vondrášek J., Svatoš A. (2003). Sexual attraction in the silkworm moth: nature of binding of bombykol in pheromone binding protein—an ab initio study. Chem. Biol. 10, 331–340. 10.1016/S1074-5521(03)00074-7 [DOI] [PubMed] [Google Scholar]
- Krieger J., Von Nickisch-Rosenegk E., Mameli M., Pelosi P., Breer H. (1996). Binding proteins from the antennae of Bombyx mori. Insect Biochem. Mol. Biol. 26, 297–307. 10.1016/0965-1748(95)00096-8 [DOI] [PubMed] [Google Scholar]
- Leal W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- Leal W. S., Nikonova L., Peng G. (1999). Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 464, 85–90. 10.1016/S0014-5793(99)01683-X [DOI] [PubMed] [Google Scholar]
- Li Q., Liberles S. D. (2015). Aversion and attraction through olfaction. Curr. Biol. 25, R120–R129. 10.1016/j.cub.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Picimbon J. F., Ji S., Kan Y., Chuanling Q., Zhou J. J., et al. (2008). Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem. Biophy. Res. Commun. 372, 464–468. 10.1016/j.bbrc.2008.05.064 [DOI] [PubMed] [Google Scholar]
- Lin F., Wu D., Lu Y., Zhang Y., Wang M., Wu K. (2011). The relationship between the main secondary metabolites and the resistance of cotton to Apolygus lucorum. Acta Phytophy. Sin. 38, 202–208. [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loughrin J., Manukian A., Heath R., Tumlinson J. (1995). Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 21, 1217–1227. 10.1007/BF02228321 [DOI] [PubMed] [Google Scholar]
- Lu Y. H., Wu K. M. (2008). Biology and Control of Cotton Mirids. Beijing: Golden Shield Press. [Google Scholar]
- Lu Y. H., Wu K. M., Jiang Y. Y., Xia B., Li P., Feng H. Q., et al. (2010). Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154. 10.1126/science.1187881 [DOI] [PubMed] [Google Scholar]
- Lu Y., Wu K., Wyckhuys K., Guo Y. (2009). Comparative flight performance of three important pest Adelphocoris species of Bt cotton in China. B. Entomol. Res. 99, 543–550. 10.1017/S000748530800655X [DOI] [PubMed] [Google Scholar]
- Matsuo T., Sugaya S., Yasukawa J., Aigaki T., Fuyama Y. (2007). Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 5:e118. 10.1371/journal.pbio.0050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner J., Zur M., Kabonci E., Ascher K. (1977). Influence of gossypol content of leaves of different cotton strains on the development of Spodoptera littoralis larvae. J. Econom. Entomol. 70, 714–716. 10.1093/jee/70.6.714 [DOI] [Google Scholar]
- Michael L. (2000). Immunolocalization of general odorant-binding protein in antennal sensilla of moth caterpillars. Arthropod Struct. Dev. 29, 57–73. 10.1016/S1467-8039(00)00013-X [DOI] [PubMed] [Google Scholar]
- Millar J. G. (2005). Pheromones of true bugs. Top Curr. Chem. 240, 37–84. 10.1007/b98315 [DOI] [Google Scholar]
- Mithöfer A., Boland W. (2012). Plant defense against herbivores: chemical aspects. Annu. Rev. Plant Biol. 63, 431–450. 10.1146/annurev-arplant-042110-103854 [DOI] [PubMed] [Google Scholar]
- Park S. K., Shanbhag S. R., Wang Q., Hasan G., Steinbrecht R. A., Pikielny C. W. (2000). Expression patterns of two putative odorant-binding proteins in the olfactory organs of Drosophila melanogaster have different implications for their functions. Cell Tissue Res. 300, 181–192. 10.1007/s004410050059 [DOI] [PubMed] [Google Scholar]
- Pelosi P., Iovinella I., Felicioli A., Dani F. R. (2014). Soluble proteins of chemical communication: an overview across arthropods. Front. Physiol. 5:320. 10.3389/fphys.2014.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P., Mastrogiacomo R., Iovinella I., Tuccori E., Persaud K. (2013). Structure and biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 98, 61–70. 10.1007/s00253-013-5383-y [DOI] [PubMed] [Google Scholar]
- Poivet E., Rharrabe K., Monsempes C., Glaser N., Rochat D., Renou M., et al. (2012). The use of the sex pheromone as an evolutionary solution to food source selection in caterpillars. Nat. Commun. 3, 1047. 10.1038/ncomms2050 [DOI] [PubMed] [Google Scholar]
- Pophof B. (2004). Pheromone-binding proteins contribute to the activation of olfactory receptor neurons in the silkmoths Antheraea polyphemus and Bombyx mori. Chem. Senses 29, 117–125. 10.1093/chemse/bjh012 [DOI] [PubMed] [Google Scholar]
- Qiao H., Tuccori E., He X., Gazzano A., Field L., Zhou J. J., et al. (2009). Discrimination of alarm pheromone (E)-β-farnesene by aphid odorant-binding proteins. Insect Biochem. Molecul. Biol. 39, 414–419. 10.1016/j.ibmb.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Romani R., Salerno G., Frati F., Conti E., Isidoro N., Bin F. (2005). Oviposition behaviour in Lygus rugulipennis: a morpho-functional study. Entomol. Experiment. Appl. 115, 17–25. 10.1111/j.1570-7458.2005.00268.x [DOI] [Google Scholar]
- Röse U. R., Tumlinson J. (2004). Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta 218, 824–832. 10.1007/s00425-003-1162-9 [DOI] [PubMed] [Google Scholar]
- Sandler B. H., Nikonova L., Leal W. S., Clardy J. (2000). Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 7, 143–151. 10.1016/S1074-5521(00)00078-8 [DOI] [PubMed] [Google Scholar]
- Steinbrecht R., Laue M., Ziegelberger G. (1995). Immunolocalization of pheromone-binding protein and general odorant-binding protein in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell Tissue Res. 282, 203–217. 10.1007/BF00319112 [DOI] [Google Scholar]
- Sun L., Gu S. H., Xiao H. J., Zhou J. J., Guo Y. Y., Liu Z. W., et al. (2013). The preferential binding of a sensory organ specific odorant binding protein of the alfalfa plant bug Adelphocoris lineolatus AlinOBP10 to biologically active host plant volatiles. J. Chem. Ecol. 39, 1221–1231. 10.1007/s10886-013-0333-9 [DOI] [PubMed] [Google Scholar]
- Sun L., Xiao H. J., Gu S. H., Guo Y. Y., Liu Z. W., Zhang Y. J. (2014b). Perception of potential sex pheromones and host-associated volatiles in the cotton plant bug, Adelphocoris fasciaticollis (Hemiptera: Miridae): morphology and electrophysiology. Appl. Entomol. Zool. 49, 43–57. 10.1007/s13355-013-0223-1 [DOI] [Google Scholar]
- Sun L., Xiao H. J., Gu S. H., Zhou J. J., Guo Y. Y., Liu Z. W., et al. (2014a). The antenna-specific odorant-binding protein AlinOBP13 of the alfalfa plant bug Adelphocoris lineolatus is expressed specifically in basiconic sensilla and has high binding affinity to terpenoids. Insect Mol. Biol. 23, 417–434. 10.1111/imb.12089 [DOI] [PubMed] [Google Scholar]
- Sun Y. F., De Biasio F., Qiao H. L., Iovinella I., Yang S. X., Ling Y., et al. (2012). Two odorant-binding proteins mediate the behavioural response of aphids to the alarm pheromone E-ß-farnesene and structural analogues. PLoS ONE 7:e32759. 10.1371/journal.pone.0032759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. L., Huang L. Q., Pelosi P., Wang C. Z. (2012). Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PLoS ONE 7:e30040. 10.1371/journal.pone.0030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. P., Zhao L. J., Sun L., Zhang S. G., Ban L. P. (2013). Immunolocalization of odorant-binding proteins on antennal chemosensilla of the peach aphid Myzus persicae (Sulzer). Chem. Senses 38, 129–136. 10.1093/chemse/bjs093 [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Uda Y., Ono Y., Nakagawa T., Suwa M., Yamaoka R., et al. (2009). Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr. Biol. 19, 881–890. 10.1016/j.cub.2009.04.035 [DOI] [PubMed] [Google Scholar]
- Tegoni M., Campanacci V., Cambillau C. (2004). Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem. Sci. 29, 257–264. 10.1016/j.tibs.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Vandermoten S., Francis F., Haubruge E., Leal W. S. (2011). Conserved odorant-binding proteins from aphids and eavesdropping predators. PLoS ONE 6:e23608. 10.1371/journal.pone.0023608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt R. G., Riddiford L. M. (1981). Pheromone binding and inactivation by moth antennae. Nature 293, 161–163. 10.1038/293161a0 [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gu S. H., Han L., Guo Y. Y., Zhou J. J., Zhang Y. J. (2013). Specific involvement of two amino acid residues in cis-nerolidol binding to odorant-binding protein 5 AlinOBP5 in the alfalfa plant bug, Adelphocoris lineolatus (Goeze). Insect Mol. Biol. 22, 172–182. 10.1111/imb.12012 [DOI] [PubMed] [Google Scholar]
- Yang Z. H. (1997). PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13, 555–556. 10.1093/bioinformatics/13.5.555 [DOI] [PubMed] [Google Scholar]
- Yuan H. B., Ding Y. X., Gu S. H., Sun L., Zhu X. Q., Liu H. W., et al. (2015). Molecular characterization and expression profiling of odorant-binding proteins in Apolygus lucorum. PLoS ONE 10:e140562. 10.1371/journal.pone.0140562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. D., Löfstedt C. (2013). Functional evolution of a multigene family: orthologous and paralogous pheromone receptor genes in the turnip moth, Agrotis segetum. PLoS ONE 8:e77345. 10.1371/journal.pone.0077345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. J., Vieira F. G., He X. L., Smadja C., Liu R., Rozas J., et al. (2010). Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum. Insect Mol. Biol. 19(Suppl. 2), 113–122. 10.1111/j.1365-2583.2009.00919.x [DOI] [PubMed] [Google Scholar]
- Zhu J., Ban L., Song L. M., Liu Y., Pelosi P., Wang G. (2016). General odorant-binding proteins and sex pheromone guide larvae of Plutella xylostella to better food. Insect Biochem. Mol. Biol. 72, 10–19. 10.1016/j.ibmb.2016.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE analyses of AlinOBP11 expression and purification. Protein markers are shown in the left side; −, crude bacterial extract before induction with IPTG; + crude bacterial extracts after induction with IPTG; Sup, supernatant of disrupted PET/AlinOBP11; Pel, inclusion body of disrupted PET/AlinOBP11; P/His-tag, purified AlinOBP11 protein with His-tag; P, finally purified AlinOBP11 protein obtained after two rounds of purification.
The relative transcript levels of AlinOBP11 at different developmental stages and adult tissues of both sexes evaluated by qRT-PCR with AlinElongation factor (GenBank No.AEY99651) as internal control. The results clearly showed AlinOBP11 was strongly expressed at adult mouthparts.
The primers used in this article.
The protein names, GenBank accession numbers, and references of OBPs used in the phylogenetic analysis.