Abstract
Electrophysiological techniques and Xenopus oocytes were used to study the expression of neurotransmitter receptors encoded by mRNAs isolated from three human glioma cell lines. Oocytes injected with mRNAs from two glioblastoma cell lines did not show electrical responses to the various neurotransmitters tested. In contrast, oocytes injected with mRNA from an astrocytoma cell line (R-111) acquired acetylcholine and glutamate receptors as well as a small number of N-methyl-D-aspartate (NMDA) receptors. Acetylcholine elicited oscillatory Cl- currents that were abolished by muscarinic antagonists. The muscarinic receptors are coupled to the inositol phosphate-Ca2+ receptor-channel coupling system. Glutamate and its analogs kainate, quisqualate, and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid induced smooth currents. The non-NMDA responses were potently blocked by 6,7-dinitroquinoxaline-2,3 dione. Our results show that human astrocytoma cells contain mRNAs coding for functional acetylcholine and glutamate receptors that have properties similar to those of neurons. In contrast, human glioblastoma cells lacked those mRNAs. These differences might be useful for the development of new diagnostic and therapeutic procedures.
Full text
PDF

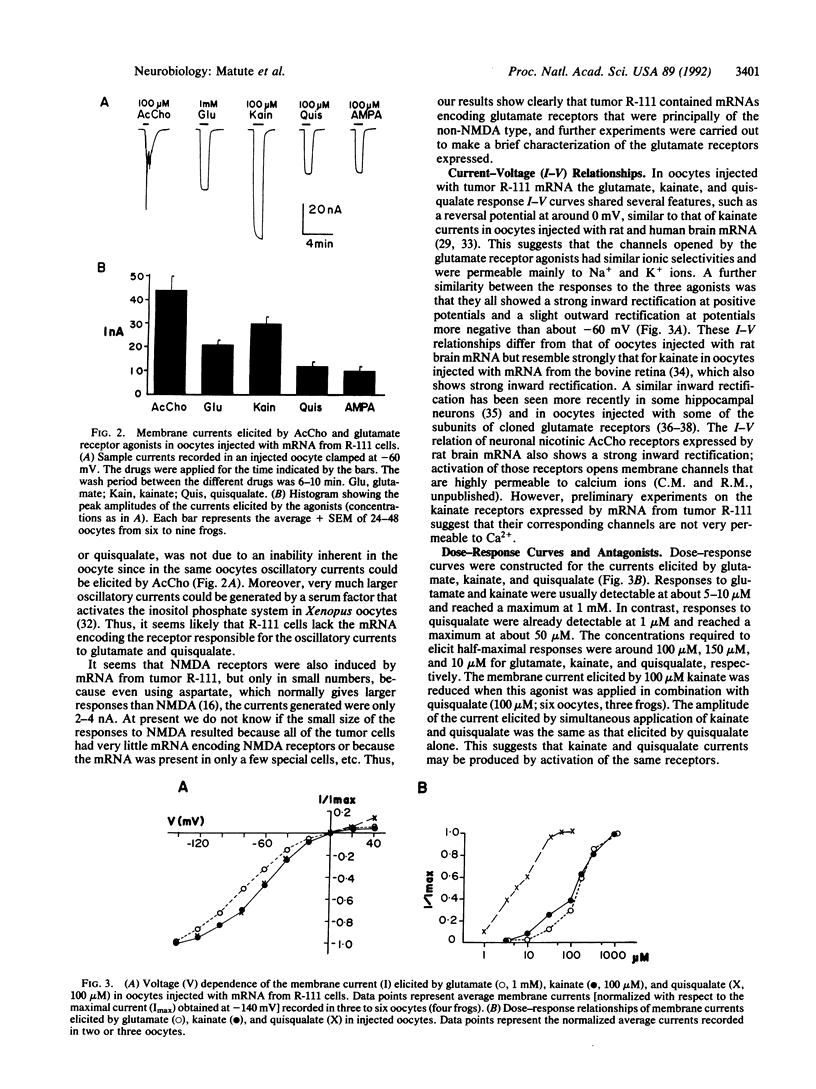
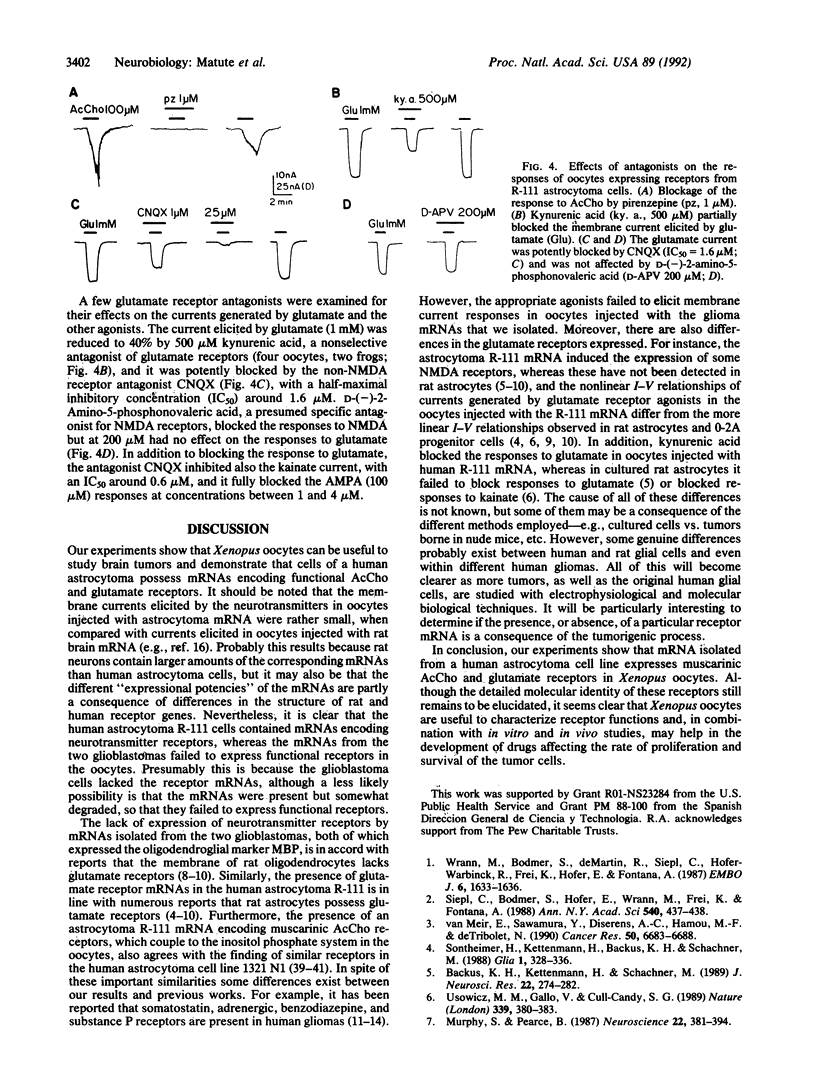

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backus K. H., Kettenmann H., Schachner M. Pharmacological characterization of the glutamate receptor in cultured astrocytes. J Neurosci Res. 1989 Mar;22(3):274–282. doi: 10.1002/jnr.490220307. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990 Apr;4(4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Black P. M. Brain tumor. Part 2. N Engl J Med. 1991 May 30;324(22):1555–1564. doi: 10.1056/NEJM199105303242205. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Broaddus W. C., Bennett J. P., Jr Peripheral-type benzodiazepine receptors in human glioblastomas: pharmacologic characterization and photoaffinity labeling of ligand recognition site. Brain Res. 1990 Jun 4;518(1-2):199–208. doi: 10.1016/0006-8993(90)90973-f. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Goldstein D., Masters S. B. The putative M1 muscarinic receptor does not regulate phosphoinositide hydrolysis. Studies with pirenzepine and McN-A343 in chick heart and astrocytoma cells. Mol Pharmacol. 1985 May;27(5):525–531. [PubMed] [Google Scholar]
- Burger P. C., Vogel F. S., Green S. B., Strike T. A. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985 Sep 1;56(5):1106–1111. doi: 10.1002/1097-0142(19850901)56:5<1106::aid-cncr2820560525>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Carpenter M. K., Parker I., Miledi R. Expression of GABA and glycine receptors by messenger RNAs from the developing rat cerebral cortex. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):159–170. doi: 10.1098/rspb.1988.0042. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Evans T., Smith M. M., Tanner L. I., Harden T. K. Muscarinic cholinergic receptors of two cell lines that regulate cyclic AMP metabolism by different molecular mechanisms. Mol Pharmacol. 1984 Nov;26(3):395–404. [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Hirono C., Ito I., Yamagishi S., Sugiyama H. Characterization of glutamate receptors induced in Xenopus oocytes after injection of rat brain mRNA. Neurosci Res. 1988 Dec;6(2):106–114. doi: 10.1016/0168-0102(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunysz E. A., Michel A. D., Whiting R. L., Woods K. The human astrocytoma cell line 1321 N1 contains M2-glandular type muscarinic receptors linked to phosphoinositide turnover. Br J Pharmacol. 1989 Feb;96(2):271–278. doi: 10.1111/j.1476-5381.1989.tb11813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B., Curutchet P., Stinnakre J., Bregestovski P., Rossier J., Prado de Carvalho L. Electrophysiological and pharmacological properties of GluR1, a subunit of a glutamate receptor-channel expressed in Xenopus oocytes. Neurosci Lett. 1991 Feb 11;123(1):69–72. doi: 10.1016/0304-3940(91)90160-u. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Kum W., Cockram C. S., Teoh R., Young J. D. Functional substance P receptors on a human astrocytoma cell line (U-373 MG). Brain Res. 1989 May 29;488(1-2):328–331. doi: 10.1016/0006-8993(89)90724-5. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Intracellular Ca2+-dependent and Ca2+-independent responses of rat brain serotonin receptors transplanted to Xenopus oocytes. Neurosci Res. 1985 Aug;2(6):491–496. doi: 10.1016/0168-0102(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Messenger RNA from bovine retina induces kainate and glycine receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):99–106. doi: 10.1098/rspb.1985.0052. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Lang W., Maurer R., Koper J. W., Lamberts S. W. Distribution and biochemical characterization of somatostatin receptors in tumors of the human central nervous system. Cancer Res. 1987 Nov 1;47(21):5758–5764. [PubMed] [Google Scholar]
- Siepl C., Bodmer S., Hofer E., Wrann M., Frei K., Fontana A. Glioblastoma-cell-derived T-cell suppressor factor (G-TsF). Sequence analysis and biologic mechanism of G-TsF. Ann N Y Acad Sci. 1988;540:437–439. doi: 10.1111/j.1749-6632.1988.tb27126.x. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Kettenmann H., Backus K. H., Schachner M. Glutamate opens Na+/K+ channels in cultured astrocytes. Glia. 1988;1(5):328–336. doi: 10.1002/glia.440010505. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. E., Szücs M., Mamone J. Y., Bem W. T., Rush M. D., Johnson F. E., Coscia C. J. Sigma and opioid receptors in human brain tumors. Life Sci. 1990;46(18):1279–1286. doi: 10.1016/0024-3205(90)90360-4. [DOI] [PubMed] [Google Scholar]
- Tigyi G., Dyer D., Matute C., Miledi R. A serum factor that activates the phosphatidylinositol phosphate signaling system in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1521–1525. doi: 10.1073/pnas.87.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J., Sherman P., Yokota S., Moore T., Basta P. Neurotransmitter modulation of the human class II gene DR alpha on multiforme glioblastoma cell lines. A molecular analysis. Ann N Y Acad Sci. 1988;540:477–478. doi: 10.1111/j.1749-6632.1988.tb27141.x. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Van Meir E., Sawamura Y., Diserens A. C., Hamou M. F., de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990 Oct 15;50(20):6683–6688. [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Wrann M., Bodmer S., de Martin R., Siepl C., Hofer-Warbinek R., Frei K., Hofer E., Fontana A. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987 Jun;6(6):1633–1636. doi: 10.1002/j.1460-2075.1987.tb02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie D. J., Mathie A., Symonds C. J., Cull-Candy S. G. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. doi: 10.1113/jphysiol.1991.sp018383. [DOI] [PMC free article] [PubMed] [Google Scholar]