Abstract
Background and objective
We recently reported that kidney function declined faster among initiators of sulfonylureas compared to metformin; however, sulfonylurea compared to metformin use was also associated with increases in body mass index (BMI) and systolic blood pressure (SBP). We sought to determine if differences between sulfonylureas and metformin on kidney function decline were mediated by differential effects on BMI, SBP, or glucose control.
Methods
We identified 13238 veterans who initiated sulfonylurea or metformin treatment (2000–2007) with a baseline estimated glomerular filtration rate (eGFR) >60 ml/min, and followed them until a study event occurred, non-persistence on treatment, loss of follow-up or end of the study. The composite outcome was a sustained decline from baseline eGFR of ≥25%, end stage renal disease, or death. We estimated the association of cumulative measurements of potential mediators including BMI, SBP and glycated hemoglobin on the study outcome. We determined if controlling for these time-varying covariates accounted for the differences in outcome between sulfonylurea and metformin initiators.
Results
Compared to sulfonylurea use, metformin use was associated with a lower risk for renal function decline or death [adjusted hazard ratio (aHR) 0.82, 95% confidence interval 0.70, 0.97]. This protective association remained significant [aHR 0.83 (0.70–0.98)] when accounting for the cumulative time varying measurements of the three mediators of interest.
Conclusion
Metformin initiation was associated with a lower risk of kidney function decline or death compared to sulfonylureas which appeared to be independent of changes in BMI, SBP and glycated hemoglobin over time.
Introduction
Diabetes type 2 is the most common cause of chronic kidney disease (CKD) and end stage renal disease (ESRD) in the US and worldwide1. Most studies evaluating the risk of diabetic kidney disease (DKD) have focused on the effects of tight glucose control on urinary albumin excretion2,3 but have not considered the differential effects of oral hypoglycemic medications.
We recently reported that metformin initiation was associated with a lower risk of clinically significant decline in estimated glomerular filtration rate (eGFR) or ESRD compared to sulfonylurea initiators4. Compared to metformin, the adjusted hazard ratio (aHR) of sulfonylurea initiation was 1.20 (95% confidence interval (CI): 1.13, 1.28). However sulfonylurea initiators weighed an average of 3.2 kg more than metformin users after 1 year of use.5,6 We also recently reported that sulfonylurea users had higher systolic blood pressure (SBP) at 12 months, partly due to these differential changes in body mass index (BMI)7. Whether the observed differences in renal function were due to known differences in the effects of these drugs on BMI or SBP or due to the intrinsic effects of the hypoglycemic medications remain unclear5,7.
Addressing this question is highly relevant because a preponderance of data indicate that both obesity8,9 and uncontrolled blood pressure10,11 contribute in a cumulative manner to kidney damage9. Furthermore, metformin, in addition to its hypoglycemic effects, stimulates the adenosine monophosphate-activated protein kinase (AMPK) pathway 12,13, with important anti-inflammatory, anti-oxidant and potentially anti-proteinuric effects which may offer reno-protection14,15.
We sought to determine if the observed beneficial association of metformin with long-term kidney outcomes were mediated in part through metformin’s associated changes in BMI, blood pressure, and glucose control.
METHODS
Study Design, Setting and Data Sources
We conducted a retrospective cohort study of veterans with diabetes seen between October 1, 1999 and June 30, 2008. The primary source of data was the computerized files of the Mid-South VISN 9 Data Warehouse which contain prescriptions data, inpatient and outpatient codes and laboratory results. In addition, for veterans who were also Medicare eligible, Medicare data (through 2004) from the VA Information Resource Center (VIReC) were merged with the analytical database. Health care visits were coded using the International Classification of Diseases, Ninth Revision; Clinical Modification (ICD9-CM).
Study Population
The study population included veterans ≥18 years old, who received regular care in the Veterans Health Administration (VHA) healthcare system VISN 9 and filled an incident oral hypoglycemic drug prescription during the study period. Ten patients with missing date of birth or gender were excluded. The cohort was restricted to patients initiating therapy with oral hypoglycemic drugs, following a “new-user design”7. Incident prescriptions were defined as the first oral hypoglycemic drug prescription filled after at least 365 days of active use of VHA services without prescriptions filled for any hypoglycemic drug (baseline year). We excluded patients with severe medical conditions (congestive heart failure, HIV/AIDS, cancer, end stage renal, liver, or respiratory disease and organ transplantation) during the baseline year. We also excluded patients with a baseline serum creatinine >1.5mg/dL or with an eGFR <60 ml/minute/1.73m2 or with heart failure given that these characteristics are relative contraindications to metformin initiation. 16,17
Follow-up
Patients were followed from the index date (date of incident prescription) until development of the study outcome or a censoring event. Censoring events included leaving the VHA system, defined as 181 days of no contact with the Mid-South VHA system (inpatient, outpatient or pharmacy); the end of the study (June 30, 2008); nonpersistence on the incident hypoglycemic drug, defined as 90 days with no drug in hand, switching or adding a new hypoglycemic drug to the original regimen; and a creatinine value of 1.5 mg/dL or greater, because metformin is often discontinued at this creatinine level while sulfonylureas are not. This approach was chosen to prevent differential censoring and bias. Patients were not allowed to re-enter the cohort if they were censored.
Exposures
The exposure categories were: metformin, sulfonylurea or the combination metformin plus sulfonylurea. Using pharmacy information, we calculated “days supply in hand”. Given that patients may “stockpile” medications, we estimated how many pills a patient possessed on each day of follow-up. Days supply in hand was reset to 0 with a change in oral hypoglycemic drug dose. Current use was defined as the person-time from the index date through the end of the days of drug supply, allowing for gaps of less than 90 days4.
Outcomes and Measurements
All estimated glomerular filtration rates (eGFRs) were estimated using the Modification of Diet in Renal Disease (MDRD) four-component equation. High eGFR values were truncated at 150ml/min/1.73m2. Serum creatinine values <0.4 mg/dl were considered implausible and excluded. The primary outcome was a composite of a GFR event, reaching ESRD, or all cause mortality8–10. A GFR event was defined as a persistent 25% or greater decline from the baseline eGFR. This threshold is clinically significant and similar to the one chosen by other studies that included this higher range of eGFR values (incident or early CKD) 8, 9, 11. ESRD was defined as reaching one of the following: an eGFR <15 ml/min/1.73m2 or the first inpatient or outpatient code for dialysis or related procedures or renal transplantation (see supplemental information). We required that ESRD or GFR events be confirmed between 3–12 months after the first diagnosis of a GFR event or ESRD to prevent capturing reversible acute kidney injury episodes. All cause mortality was determined by a date of death in the VA Vital Status Master file. Information from multiple sources including Medicare, VHA utilization, Social Security and VHA compensation and pension benefits is used to determine this date and has been shown to be highly accurate when compared to the National Death Index.12 The secondary outcome was a composite of GFR event or ESRD.
Covariates
Important co-morbidities were identified using ICD9-CM coded healthcare encounters or prescriptions for specific medications in the baseline year. The study covariates included: age, sex, race (white, black, unknown), marital status, systolic and diastolic blood pressure closest to cohort entry, pre-existing diagnosis of hypertension defined as having filled a prescription medication for an antihypertensive or an ICD-9 code for hypertension (401.xx–405.99), history of atherosclerotic disease (yes, no) (supplemental table 3), BMI, glycated hemoglobin (HbA1c), use of medications known to affect creatinine values (angiotensin converting enzyme inhibitors or angiotensin receptor blocker, loop and thiazide diuretics), proteinuria (tested for [yes, no] and present [yes, no] if urine dipstick was ≥ +1 or albumin creatinine ratio (ACR) was ≥ 30 mg/g,18 year of incident prescription, and measures of healthcare utilization (number of outpatient visits [including primary care and subspecialty care], hospitalization during the baseline period [yes, no], unique number of prescription medications on the index date). All baseline covariates represent the closest value to cohort entry during the baseline year.
Measurements of BMI, SBP and HbA1c present during follow-up were used for the time varying covariate models to determine if changes in these covariates over time mediated the relationship between the drug exposures and the primary renal composite outcome (see statistical analysis section). The time varying covariates were updated monthly, drawing from the measures in the month and one year preceding the start of that month. When more than one value was present, the date closest to the start of the month was used. If no value was present within the year then the covariate was considered missing and multiply imputed. We conducted multiple imputations using the Markov-chain Monte Carlo method and a non-informative Jeffrey’s prior (SAS software, version 9.2, SAS Institute, Cary, North Carolina)19. All covariates, survival time, and a censoring indicator were included in 20 imputation models and used to compute final estimates.
Statistical Analysis
Systolic blood pressure11,20,21,22,23,24, BMI8,9,25–29 and HbA1c30 are established risk factors for the incidence and progression of CKD 31 In our current study, we aim to determine if metformin-associated changes in BMI, SBP and HbA1c account for the observed slower renal function decline relative to sulfonylureas.
We performed a mediation analysis using a cumulative effect time-varying covariate model with adjustment for the three variables of interest.32,33 Mediation analyses are conducted to indirectly assess the effect of an exposure on some outcome through a proposed mediator.32,33 The proposed mediator is considered a mediator if the exposure significantly predicts the outcome, the exposure significantly predicts the mediator; and the mediator significantly predicts the outcome while controlling for the exposure. In a national VHA cohort, we previously demonstrated that metformin compared to sulfonylureas was associated with a slower decline in kidney function 4. We also reported that initiation of metformin compared to sulfonylureas was associated with lower blood pressure and BMI5,7 at 1 year of follow-up, using a local VHA cohort.
Therefore, we assessed whether the association of metformin with slower kidney function decline compared to sulfonylureas was robust to the inclusion of three potential mediators of kidney disease. BMI, was categorized as obese (BMI 30–39kg/m2) and morbidly obese (BMI≥40kg/m2) according to the World Health Organization.34 Hypertension was characterized as stage I (SBP 140–159mmHg) and stage II (SBP ≥160mmHg)35 according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Uncontrolled diabetes was characterized by a glycated hemoglobin (HbA1c) 7–9, and ≥9 similar to the thresholds described in the UKPDS 36. Our approach is based on the pathophysiology of these three major risk factors for CKD, in which time spent uncontrolled can result in kidney damage that is slow to reverse or irreversible. We modeled the association of changes in these three risk factors as the number of months of follow-up from treatment initiation spent in each risk factor category.
We assessed time to the primary renal composite outcome: eGFR event, ESRD or death, or the secondary outcome: eGFR event or ESRD, for patients who remained persistent on their initial study regimen with gaps up to 90 days. Kaplan Meier univariate estimates were calculated and the log rank test was used to compare these estimates using sulfonylurea as the reference group for all comparisons. Cox proportional hazards regression models were used to analyze the association between study regimen and time-to the renal composite adjusting for the covariates of interest. Continuous covariates, including the time varying months of elevated BMI, SBP, and HbA1c, were modeled with third-degree polynomials to allow for nonlinear associations. The proportional hazards assumption for the drug groups was checked graphically and by testing for interactions with drug group and time.
Subgroup and sensitivity Analyses
Stratified analyses were conducted based on presence of proteinuria. Furthermore, to address concerns about the potential influence of unmeasured confounders, we quantified the strength of the association of a hypothetical unmeasured binary confounder that would be required to eliminate a statistically significant association37. We assumed a confounder–outcome association similar to that which we observed among measured covariates (hazard ratio, 1.25) and considered a range of different confounder prevalences between sulfonylurea and metformin users; we also considered a stronger confounder–outcome association (hazard ratio, 2.0) (supplemental tables 4 & 5). Statistical analyses were conducted using R Statistical Program (R Foundation, available at: http://www.r-project.org.) and SAS for Windows 9.2. (SAS Institute, Cary, NC). The study protocol was reviewed and approved by the VA IRB and the Vanderbilt University IRB.
Results
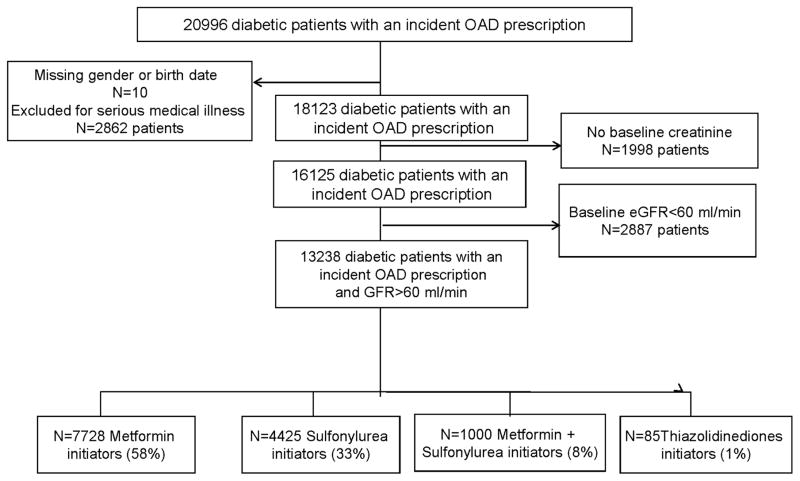
Of 20,996 type 2 diabetic veterans identified as incident users of oral hypoglycemic drugs (Figure 1), 13.6% were excluded for severe medical conditions, 9.5% for lack of baseline creatinine, 13.7% with GFR<60 ml/min listed in Table 1. There were 13238 patients who had an eGFR>60 ml/min/1.73m2 and were included in our analyses.
Figure 1.
Diabetic Patients with type 2 DM within the VISN 9, number of incident prescriptions, number that enter in the analysis
Table 1.
Baseline characteristics of patients
| Characteristics | Metformin N=7728 |
Sulfonylurea N=4425 |
Metformin + Sulfonylurea N=1000 |
|---|---|---|---|
| Age, years median (IQR)* | 59 (54, 67) | 60 (54, 71) | 58 (53,65) |
| Male % | 95 | 97 | 96 |
| Race, % | |||
| White | 77 | 77 | 69 |
| Black | 12 | 17 | 19 |
| Other/Unknown | 11 | 8 | 13 |
| Estimated glomerular filtration rate (eGFR) ml/min, median (IQR) | 81 (72, 93) | 80 (71, 93) | 82 (73, 97) |
| Creatinine (mg/dL), median (IQR) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| Urine protein measure available at baseline, % | 64 | 67 | 68 |
| Proteinuria, % | 13 | 17 | 17 |
| Systolic blood pressure (mmHg), median (IQR) | 134 (124, 145) | 137 (126, 149) | 137(125,148) |
| Diastolic blood pressure (mmHg), median (IQR) | 78 (70, 84) | 78 (70, 85) | 77 (70, 85) |
| History of hypertension, % | 74 | 70 | 68 |
| History of Cardiovascular disease, % | 219 | 23 | 17 |
| HbA1c, median (IQR) | 7.1 (6.5, 7.9) | 7.3 [6.6,8.4] | 7.9 (6.8,10) |
| Body mass index (kg/m2), median (IQR) | 32 (29, 36) | 30 (27, 34) | 31(28, 36) |
| Baseline use of medications | |||
| ACEI or ARBs, % † | 53 | 50 | 52 |
| Diuretics, % | 41 | 37 | 31 |
| Statins, % | 54 | 45 | 42 |
| Number of outpatient medications, median (IQR) | 5 (2, 8) | 4 (2, 7) | 2 (0, 5) |
| Number of outpatient visits, median (IQR) | 5 (3, 8) | 5 (3, 8) | 2 (0,5) |
| Marital status (% married) | 64 | 58 | 60 |
| Hospitalized % | 11 | 16 | 14 |
IQR interquartile range
ACEI angiotensin converting enzyme inhibitors, ARB angiotensin receptor blocker,
Patient baseline characteristics are shown in Table 1. The 13238 incident patients included metformin (58%), sulfonylureas (33%), combination of both (8%) and thiazolidinediones (1%). The latter were excluded due to the small number (n=85). Mean age was 59 years (interquartile range [IQR] 54, 68), 95% were males, and 14% were African Americans. The median baseline eGFR was 81 ml/min/1.73 m2 (IQR 72, 93), median SBP and DBP were 134 mmHg (IQR 124, 145), and 78 mmHg (IQR 70, 84) respectively. These characteristics were similar across all drug groups.
Sixty six percent of the study sample (n=8672) had a urine protein measurement within the baseline period, with 21% testing positive for proteinuria. The median creatinine for all individuals and for those with a documented urine protein test was similar: median 1.0 (IQR 0.9, 1.1) for both groups (p=0.8). Baseline proteinuria was present in 19.2% (95%CI 18.0%, 20.4%) of metformin users, 24.4% (95%CI 22.7%, 26.2%) of sulfonylurea users (p <0.001 versus metformin), and 23.3% (95%CI 19.8%, 27.1%) of combination users (p = ns versus metformin or sulfonylurea).
Primary and secondary outcomes
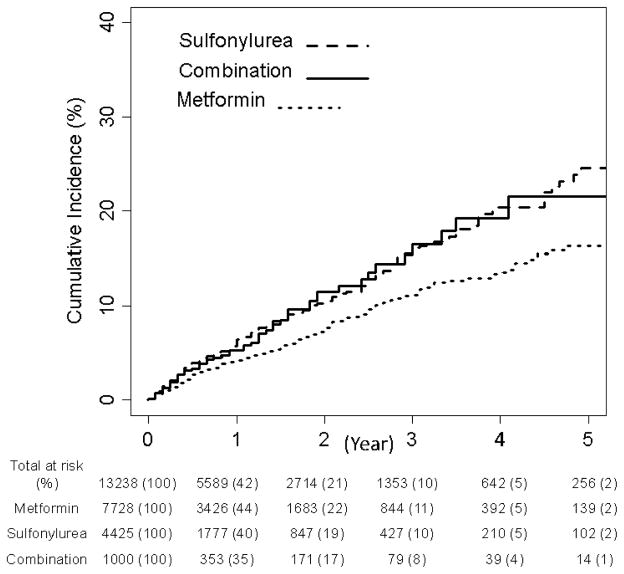
Table 2 shows results for the entire study sample and restricted to those with urine protein measurement. Among the total study population (n=13,238), metformin users had a lower risk of reaching the primary renal composite of an eGFR event, ESRD or death, aHR 0.82 (95% CI 0.70, 0.97) compared with sulfonylurea users. Use of metformin + sulfonylurea was not associated with a statistically significant risk of the primary outcome, compared with sulfonylurea users alone. The risk of developing the secondary outcome (eGFR event or ESRD) for metformin relative to sulfonylurea users was numerically lower but not statistically different. Unadjusted cumulative incidence curves are shown in Figure 2.
Table 2.
Risk of renal composite outcome (GFR event, end stage renal disease [ESRD] or Death) among initiators of metformin compared with sulfonylureas. For all analyses patients are censored after reaching a serum creatinine of 1.5mg/dL. Adjusted Hazard ratios and 95% confidence intervals are reported.
| Persistent exposure required (Full cohort)* | Persistent exposure required (urine protein subgroup)† | |
|---|---|---|
| Patients | 13238 | 8672 |
| Events (composite endpoint) | 759 | 515 |
| GFR events | 700 | 472 |
| ESRD | 1 | 1 |
| Death | 58 | 42 |
| Time at Risk (years) | 15,793 | 10123 |
|
| ||
| Adjusted hazard of composite outcome (GFR event, ESRD, or death) | ||
|
| ||
| Sulfonylurea | ref. | ref |
| Metformin | 0.82 (0.70, 0.97) | 0.77 (0.63, 0.95) |
| Metformin + Sulfonylurea | 1.05 (0.79, 1.40) | 1.17 (0.83, 1.64) |
|
| ||
| Adjusted hazard of GFR event or ESRD | ||
|
| ||
| Sulfonylurea | ref. | ref |
| Metformin | 0.85 (0.72, 1.01) | 0.78 (0.64, 0.97) |
| Metformin + Sulfonylurea | 1.01 (0.75, 1.37) | 1.11 (0.78, 1.59) |
Persistent exposure required: considers patients persistent on their incident regimen until they have a gap in use of medications that reaches 90 days, or have added or switched to a different hypoglycemic agent, have a study outcome, have left the VA, reached the end of the study or reached a creatinine of 1.5 mg/dL or higher.
Persistent exposure required among subgroup with baseline urine protein testing. This analysis is among the subgroup of patients who had testing for urine protein within the baseline period (N=8672).
Models were adjusted for: age, sex, race, baseline creatinine (fifth degree polynomial), baseline blood pressure, history of hypertension, history of cardiovascular disease, baseline HbA1c, baseline BMI (third degree polynomial), the use of ACEI or ARBs, diuretics, baseline number of medications (third degree polynomial), year of cohort entry, number of outpatient visits, history of hospitalization at baseline and marital status. Among patients with baseline urine protein testing (N=8672), model adjusts for proteinuria.
Figure 2.
Unadjusted Cumulative Proportion of Patients Reaching the Composite Outcome of Persistent Clinically Significant Decline of Baseline eGFR, ESRD or Death by OAD group.
Mediation analyses
We investigated whether the association between metformin and the composite outcome were mediated through the time-varying changes in BMI, SBP or HbA1c. Table 3 demonstrates that inclusion of potential mediating variables yielded similar results for the association of metformin on the primary outcome [aHR of 0.82 (95%CI 0.63, 0.96)].
Table 3.
Cumulative association of time varying covariates measurements on the composite primary outcome GFR event, End stage renal disease (ESRD), or Death and secondary outcome
| Variables | Adjusted hazard of composite outcome (GFR event, ESRD, or death)* | |
|---|---|---|
|
| ||
| HR | 95%CI | |
| Sulfonylurea | ref | |
| Metformin | 0.83 | (0.70, 0.98) |
| Metformin+Sulfonylurea | 1.08 | (0.81, 1.44) |
| Systolic Blood Pressure >140 mmHg | 1.05 | (0.97, 1.14) |
| Systolic Blood Pressure >160 mmHg | 1.26 | (1.05, 1.52) |
| Body mass index 30–39 kg/m2 | 0.98 | (0.93, 1.03) |
| Body mass index >=40 kg/m2 | 0.87 | (0.70, 1.09) |
| Glycated hemoglobin 7%–8.9% | 1.02 | (0.94, 1.11) |
| Glycated hemoglobin >=9% | 0.76 | (0.55, 1.05) |
|
| ||
| Adjusted hazard of GFR event or ESRD* | ||
|
| ||
| Sulfonylurea | ref | |
| Metformin | 0.86 | (0.72, 1.02) |
| Metformin+Sulfonylurea | 1.04 | (0.77, 1.40) |
| Systolic Blood Pressure >140 mmHg | 1.04 | (0.96, 1.14) |
| Systolic Blood Pressure >160 mmHg | 1.26 | (1.04, 1.53) |
| Body mass index 30–39 kg/m2 | 0.98 | (0.93, 1.04) |
| Body mass index >=40 kg/m2 | 0.88 | (0.70, 1.10) |
| Glycated hemoglobin 7%–8.9% | 1.01 | (0.93, 1.10) |
| Glycated hemoglobin >=9% | 0.75 | (0.54, 1.04) |
Cox proportional hazards model adjusts for the time varying cumulative association of each additional 3 months of uncontrolled glycated hemoglobin*, systolic blood pressure* and body mass index* along with and baseline covariates. Hazard ratios reflect the relative increase in risk of an additional 3 months spent with moderately or severely uncontrolled value; ranges are specified in the table. For example, a patient, who was at the moment identical to another patient except that they had 3 more months of severely uncontrolled SBP, would have a 26% greater hazard of experiencing the primary outcome (HR 1.26). The following baseline covariates were included: age, sex, race, history of hypertension, history of cardiovascular disease, the use of ACEI or ARBs, diuretics, year of cohort entry, history of hospitalization at baseline, marital status, protein tested, proteinuria, baseline creatinine**, baseline blood pressure*, baseline HbA1c*, baseline BMI*, baseline number of medications*, number of outpatient visits*. Continuous variables were fit as *third or **fifth degree polynomials to allow for nonlinear associations. Linear effects are shown unless specified otherwise.
When evaluating each of the three potential mediators in a model which investigated the cumulative associations of uncontrolled risk factors on the primary outcome, SBP (estimated as at least 3 months of uncontrolled SBP) was independently associated with the outcome [SBP140–160 mmHg aHR 1.05 (0.97, 1.14); SBP ≥160 mmHg was aHR 1.26 (1.05, 1.52)]. HbA1c and BMI were not independently associated with the outcome indicating they were not mediators. The results were similar when considering the secondary outcome of renal events (eGFR event or ESRD).
Subgroup Analyses
Among patients with baseline urine protein measurements (n=8672), metformin users had a lower risk of both the primary renal outcome aHR 0.77 (0.63, 0.95) and the secondary outcome aHR 0.78 (0.64, 0.97) compared to sulfonylurea users (Table 2). There were no significant difference observed between the combination users and sulfonylurea for either analysis.
Our finding of protective hazard for the composite outcome among metformin users could have resulted from an unmeasured confounder that had a greater prevalence among the metformin users compared with sulfonylurea users. Assuming a degree of association similar to that observed among measured covariates, we calculated that an unmeasured binary confounder would need to be at least 20% more prevalent among metformin users than sulfonylurea users to explain our main findings (Supplemental Tables 4 & 5).
DISCUSSION
In this cohort of veterans with type 2 diabetes, kidney function declined faster among initiators of sulfonylureas compared to metformin. We assessed whether these findings could be attributable to differential control of important risk factors during follow-up. In our mediation analysis, the risk of an eGFR event (a persistent clinically significant decline of 25% of baseline eGFR), ESRD or death for metformin compared to sulfonylurea initiators appeared to be independent of time-varying values for BMI, SBP and HbA1c during follow-up.
Although the mechanisms for the benefit of metformin compared to sulfonylureas on kidney function remain unclear, metformin has several recently recognized renoprotective effects. First, recent studies have shown that metformin has important antioxidant features.38–41 Recent phase II trials in the treatment of advanced CKD, using a new antioxidant inflammation modulator “Bardoxolone Methyl”, demonstrated significant improvements in kidney function over 52 weeks 42. Patients with CKD even without diabetes suffer from a plethora of metabolic abnormalities which are highly interrelated including insulin resistance27,43, oxidative stress44, and chronic inflammation45–50. The insulin sensitizing and antioxidant properties of metformin are potential and relevant pathways that might delay CKD progression, independent of glucose control.
Second, metformin is an activator of adenosine monophosphate-activated protein kinase (AMPK) and it increases adiponectin levels13,51. Adiponectin has a protective effect on podocyte function through AMPK activation15,52. Animal studies have reported that low intracellular AMPK initiates early kidney damage in mice fed with a high fat diet and that the administration of AMPK activators prevents proteinuria and glomerular hyperfiltration in animal models14,15,53. Additionally plasma adiponectin concentrations have been inversely related to urinary albumin excretion in obese African Americans, a group prone to obesity and chronic kidney disease. 15
Third, diabetic patients with kidney disease are at high risk for acute kidney injury (AKI). AKI is one of the main contributors to CKD progression and reaching ESRD54,55. Morales et al. recently reported that metformin prevented gentamicin-induced nephropathy by normalizing oxidative stress and restoring mitochondrial functional integrity56. In this sense any intervention that prevents or reduces the severity of AKI has the potential to slow CKD progression.
In our cohort, both high SBP at baseline as well as persistent poor blood pressure control, categorized as stage I hypertension SBP 140–160 mmHg and stage II hypertension ≥160 mmHg, increased the risk of an eGFR event, ESRD or death. In this regard our study is consistent with a large number of experimental and observational data indicating that hypertension is a risk factor for progression of diabetic kidney disease31. It is likely that multiple measures of SBP during follow-up reflect SBP control over the prior years and as well as during follow-up. However, differential SBP control during follow-up did not appear to account for the different outcomes observed between metformin and sulfonylurea initiators. In regard to glycemic control, our study did not show an association between glycemic control and GFR decline. Although the long term follow-up of the UKPDS showed a 67% risk reduction for a doubling of creatinine at 9 years57, the vast majority of the randomized controlled trials have failed to show an effect of glycemic control on GFR decline in type 2 diabetes58 most likely because long-term follow up is required to observe these effects. Our study, which focused on initiators of hypoglycemic drugs and required persistence on the initial regimens, potentially reduced the follow-up time for analyses and limited our ability to adequately address the association with glycemic control. Our findings did not demonstrate an association between renal outcomes and a BMI ≥ 30 kg/m2. The reasons for this discrepancy with other studies are not clear.9 One explanation is that the risk seen with increasing BMI may be similar to glycemic control and require longer follow-up time.
The strength of this comparative effectiveness study is that it includes the two most widely used drugs for the initial treatment of diabetes and evaluates their association with kidney function decline in a real clinical practice setting. Limitations include first, the predominantly male population of veterans. Second, despite our extensive efforts to control confounding, we cannot rule out some residual confounding. Nevertheless, we estimated that an unmeasured confounder or an underreported confounder, such as proteinuria, will be required to have a very large prevalence imbalance among exposure groups to explain our findings (Supplemental table 1 & 2) Third, given the known limitations of using creatinine alone for estimating kidney function59, we used eGFR (by MDRD four component equation) as the measurement of kidney function, which is known to be less accurate for values greater than 60 ml/min. However, our approach of requiring a 25% drop in baseline eGFR, confirmed by a second value at 3–12 months represents a clinically relevant decline in renal function. Fourth, we had no non-pharmacological comparison. However, prior studies have shown the benefits of hypoglycemic agents including sulfonylureas, in reducing microvascular complications compared to non-pharmacological strategies. Hence, in this study we cannot determine whether sulfonylureas have a detrimental association on renal outcomes or if metformin has beneficial effects. 36 In addition, we utilized refill data as a proxy for medication taking. While prescription fills have been shown to be a good proxy for medication use, exposure misclassification may have occurred. This exposure misclassification was likely non-differential making it harder to ascertain real medication effects. Finally, there was the potential for confounding by indication (patients with kidney disease preferentially started on sulfonylureas). To minimize this potential we allowed into the study only patients with normal kidney function.
In summary, our data suggest that the reduction in the risk of kidney function decline, ESRD or death in metformin initiators compared with sulfonylurea initiators is largely independent of the metformin-associated changes in BMI, SBP and HgbA1c. The anti-inflammatory and antioxidant effects of metformin or the effect on the AMPK pathway may be responsible for these between drug differences. Overall, our findings support the current consensus statement by the American Diabetes Association and the European Association recommending metformin as first line therapy. Furthermore this study lends support to the recent changes in guidelines outside the U.S. In the U.K the National Institute for Health and Clinical Excellence lowered the threshold for stopping metformin to an eGFR below 30 ml/min.60 Similar recommendations exist for the Canadian Diabetes Association practice guidelines and the Australian Diabetes Society practice guidelines.61,62
Supplementary Material
Acknowledgments
NIH Funding: I01 CX000570
This project was funded under Contract No. 290-05-0042 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Dr Hung is supported in full by the CDA 2-031-09S from the Clinical Science Research and Development and Dr. Roumie (04-342-2) was supported by a VA Career Development Awards from HSR&D and VA CSRD investigator initiated grant I01CX000570-01.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24:2047–55. doi: 10.1097/01.hjh.0000244955.39491.88. [DOI] [PubMed] [Google Scholar]
- 3.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 4.Hung AM, Roumie CL, Greevy RA, et al. Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney Int. 2012;81:698–706. doi: 10.1038/ki.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huizinga MM, Roumie CL, Greevy RA, et al. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a veterans administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19:1108–12. doi: 10.1002/pds.2035. [DOI] [PubMed] [Google Scholar]
- 6.Bennett WL, Maruthur NM, Singh S, et al. Comparative Effectiveness and Safety of Medications for Type 2 Diabetes: An Update Including New Drugs and 2-Drug Combinations. Ann Intern Med. 2011;154:602–13. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roumie CL, Liu X, Choma NN, et al. Initiation of sulfonylureas versus metformin is associated with higher blood pressure at one year. Pharmacoepidemiol Drug Saf. 2012 doi: 10.1002/pds.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22:1846–55. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–8. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fogo AB. Hypertensive risk factors in kidney disease in African Americans. Kidney Int Suppl. 2003:S17–21. doi: 10.1046/j.1523-1755.63.s83.5.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine reviews. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 13.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MJ, Feliers D, Mariappan MM, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–27. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 15.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. The Journal of clinical investigation. 2008;118:1645–56. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eurich DT, Tsuyuki RT, Majumdar SR, et al. Metformin treatment in diabetes and heart failure: when academic equipoise meets clinical reality. Trials. 2009;10:12. doi: 10.1186/1745-6215-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulkin TV, Bosman D, Krentz AJ. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care. 1997;20:925–8. doi: 10.2337/diacare.20.6.925. [DOI] [PubMed] [Google Scholar]
- 18.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y. Concepts and New Development (Version 90) Rockville, MD: 2011. Multiple Imputation for Missing Data. [Google Scholar]
- 20.Wright JT, Jr, Agodoa L, Contreras G, et al. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2002;162:1636–43. doi: 10.1001/archinte.162.14.1636. [DOI] [PubMed] [Google Scholar]
- 21.Kramer H, Berns JS, Nally J, Choi MJ, Rocco MV. A decade after the KDOQI CKD guidelines: impact on NKF-KDOQI. Am J Kidney Dis. 2012;60:694–6. doi: 10.1053/j.ajkd.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Flack JM, Peters R, Shafi T, Alrefai H, Nasser SA, Crook E. Prevention of hypertension and its complications: theoretical basis and guidelines for treatment. Journal of the American Society of Nephrology: JASN. 2003;14:S92–8. doi: 10.1097/01.asn.0000070142.14843.8e. [DOI] [PubMed] [Google Scholar]
- 23.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. Journal of the American Society of Nephrology: JASN. 2003;14:2934–41. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 24.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–65. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 25.de Boer IH, Katz R, Fried LF, et al. Obesity and change in estimated GFR among older adults. Am J Kidney Dis. 2009;54:1043–51. doi: 10.1053/j.ajkd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. Journal of the American Society of Nephrology: JASN. 2010;21:406–12. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nerpin E, Riserus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care. 2008;31:1550–5. doi: 10.2337/dc08-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int. 2009;76:145–8. doi: 10.1038/ki.2009.137. [DOI] [PubMed] [Google Scholar]
- 29.de Boer IH, Sibley SD, Kestenbaum B, et al. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. Journal of the American Society of Nephrology: JASN. 2007;18:235–43. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer IH, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–76. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.K/DOQI. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 32.Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18:16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–6. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancia G, Grassi G. Joint National Committee VII and European Society of Hypertension/European Society of Cardiology guidelines for evaluating and treating hypertension: a two-way road? Journal of the American Society of Nephrology: JASN. 2005;16(Suppl 1):S74–7. doi: 10.1681/asn.2004110963. [DOI] [PubMed] [Google Scholar]
- 36.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 37.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16:17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]
- 38.Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48:353–7. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- 39.Rosen P, Wiernsperger NF. Metformin delays the manifestation of diabetes and vascular dysfunction in Goto-Kakizaki rats by reduction of mitochondrial oxidative stress. Diabetes Metab Res Rev. 2006;22:323–30. doi: 10.1002/dmrr.623. [DOI] [PubMed] [Google Scholar]
- 40.Massy ZA, Stenvinkel P, Drueke TB. The role of oxidative stress in chronic kidney disease. Semin Dial. 2009;22:405–8. doi: 10.1111/j.1525-139X.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 41.Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009:S3–11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- 42.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45:275–80. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 45.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–92. [PubMed] [Google Scholar]
- 46.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–91. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 47.Hung AM, Crawford DC, Griffin MR, et al. CRP polymorphisms and progression of chronic kidney disease in African Americans. Clin J Am Soc Nephrol. 2010;5:24–33. doi: 10.2215/CJN.01900309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shlipak MG, Katz R, Cushman M, et al. Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 49.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–45. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 50.Hung A, Pupim L, Yu C, et al. Determinants of C-reactive protein in chronic hemodialysis patients: relevance of dialysis catheter utilization. Hemodial Int. 2008;12:236–43. doi: 10.1111/j.1542-4758.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 51.Sanz P. AMP-activated protein kinase: structure and regulation. Current protein & peptide science. 2008;9:478–92. doi: 10.2174/138920308785915254. [DOI] [PubMed] [Google Scholar]
- 52.Yano Y, Hoshide S, Ishikawa J, et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J Clin Hypertens (Greenwich) 2007;9:775–82. doi: 10.1111/j.1524-6175.2007.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohashi K, Iwatani H, Kihara S, et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1910–7. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 54.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–8. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4:520–2. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales AI, Detaille D, Prieto M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–9. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 57.KDOQI. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850–86. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Golbahar J, Das NM, Al-Ayadhi MA, Gumaa K. Leptin-to-Adiponectin, Adiponectin-to-Leptin Ratios, and Insulin Are Specific and Sensitive Markers Associated with Polycystic Ovary Syndrome: A Case-Control Study from Bahrain. Metab Syndr Relat Disord. 2012;10:98–102. doi: 10.1089/met.2011.0075. [DOI] [PubMed] [Google Scholar]
- 59.Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–9. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 60.NICE. Clinical Guideline CG87 (May 2009) 2009. Type 2 diabetes – newer agents (a partial update of CG66) [Google Scholar]
- 61.Association CD. Clinical practice guidelines. 2008 http://wwwdiabetesca/files/cpg2008/cpg-2008pdf.
- 62.guidelines ADSp. National evidence based guidelines for blood glucose control in type 2 diabetes. 2009 http://wwwnhmrcgovau/_files_nhmrc/file/publications/synopses/di19-diabetes-blood-glucose-controlpdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.