Abstract
Various anthropometric measures, including height, have been associated with atrial fibrillation (AF). This raises questions about the appropriateness of using ratio measures such as body mass index (BMI), which contains height squared in its denominator, in the evaluation of AF risk. Among older adults, the optimal anthropometric approach to risk stratification of AF remains uncertain. Anthropometric and bioelectrical impedance measures were obtained from 4,276 participants (mean age = 72.4 years) free of cardiovascular disease in the Cardiovascular Health Study. During follow-up (1989–2008), 1,050 cases of AF occurred. BMI showed a U-shaped association, whereas height, weight, waist circumference, hip circumference, fat mass, and fat-free mass were linearly related to incident AF. The strongest adjusted association occurred for height (per each 1-standard-deviation increment, hazard ratio = 1.38, 95% confidence interval: 1.25, 1.51), which exceeded all other measures, including weight (hazard ratio = 1.21, 95% confidence interval: 1.13, 1.29). Combined assessment of log-transformed weight and height showed regression coefficients that departed from the 1 to −2 ratio inherent in BMI, indicating a loss of predictive information. Risk estimates for AF tended to be stronger for hip circumference than for waist circumference and for fat-free mass than for fat mass, which was explained largely by height. These findings highlight the prominent role of body size and the inadequacy of BMI as determinants of AF in older adults.
Keywords: aging, atrial fibrillation, body size, obesity
Atrial fibrillation (AF), the most common sustained cardiac dysrhythmia (1), is a growing public health concern among aging populations worldwide (2). In addition to being an independent risk factor for stroke (3), AF has been linked to higher risks of heart failure (4), cognitive decline (5), and death (6). Together with aging trends, the global prevalence of obesity has registered a marked increase (7). It has been shown in multiple prior studies that excess adiposity, as defined by elevated body mass index (BMI) (8–11) or waist circumference (WC) (12), increases the risk of AF. Although the mechanisms linking obesity to AF are not well defined, proposed mediators include hypertension, diabetes, chronic inflammation, and obstructive sleep apnea, along with left ventricular diastolic dysfunction and left atrial enlargement (13–17). Given the intersection of aging, obesity, and AF, a key question is the extent to which body composition in older adults might determine the higher incidence of AF later in life. Because aging is associated with an increase in adipose mass and its central redistribution (18), along with a decrease in skeletal muscle mass (19), the same relationships between adiposity and AF that have been documented in younger individuals may not apply equally in older people. Moreover, beyond adiposity measures, taller heights (20–22) and larger hip circumferences (HCs) (21, 23) have been shown to be significantly associated with the risk of AF. These findings suggest that BMI, which includes height squared in its denominator, may not be the best anthropometric variable for assessing AF risk. They also raise questions about the suitability of waist-to-hip ratio (WHR) for capturing AF risk. In this regard, both fat mass and fat-free mass were recently associated with a higher risk of AF in a middle-aged cohort (21), but the corresponding associations of adipose and lean body mass in older adults have not been examined. We therefore investigated the relationships of various measures of body size and composition with incident AF in the Cardiovascular Health Study (CHS), a large cohort study of older adults with a high number of AF events.
METHODS
Study population
CHS is a prospective study of cardiovascular risk factors among community-dwelling people 65 years of age or older (24). Individuals were recruited from Medicare eligibility lists and examined at 4 field centers across the United States (24, 25). The original cohort consisted of 5,201 individuals who enrolled in 1989–1990, with a supplementary cohort of 687 black participants enrolled in 1992–1993. Health evaluations were conducted for all participants using standardized protocols (24, 25).
In the present study, subjects with prevalent AF (n = 157) were excluded, as were those with prevalent cardiovascular disease (n = 1,410), because the latter can alter the usual relations of adiposity measures with adverse outcomes. Prevalent cardiovascular disease comprised coronary heart disease, stroke, transient ischemic attack, heart failure, and peripheral arterial disease. These conditions were ascertained through the use of questionnaires, review of medical records, or adjudication of prior events by specialized committees. After additional exclusion of participants with missing data on anthropometric variables (n = 45), there were 4,276 participants eligible for evaluation.
Baseline assessment of body size and composition
Anthropometric measurements were obtained by trained personnel using standardized methods (26). Height was measured in centimeters using a stadiometer. Weight was measured in pounds using a balance beam scale while participants were wearing examination gowns and no shoes. WC and HC were measured at the level of the umbilicus and the maximal protrusion of the gluteal muscles, respectively. BMI was calculated as weight in kilograms divided by height in meters squared. WHR was calculated as WC divided by HC. Participants also underwent bioelectrical impedance analysis. Resistance was measured at 50 kHz in the supine position using a TVI-10 Body Composition Analyzer (Danninger Medical, Columbus, Ohio) attached to 4 adhesive electrocardiographic electrodes placed in the standard distal positions on the dorsum of the right hand and foot. Fat-free mass was calculated as (6,710) × (ht2/R) + (3.1 × S) + 3.9, where ht2 was standing height in meters squared, R was resistance in ohms, and S was sex (0 = women, 1 = men) (27). Fat mass was calculated as body weight minus fat-free mass.
Definition of other covariates
Participants answered standard questionnaires regarding smoking, alcohol intake, and self-assessed health status. Recent smokers were defined as those who quit within the past 5 years. Physical activity level was determined using a validated questionnaire (28). Blood samples collected after a 12-hour overnight fast were processed for biochemical testing (29). Diabetes was defined as having a fasting glucose level of 126 mg/dL or higher or use of hypoglycemic therapy. Echocardiography performed at baseline on the original cohort, but not the supplementary cohort, included M-mode measurement of left atrial anteroposterior diameter (24).
AF events
Annual study clinic visits were conducted for all CHS participants for 10 years, with interim telephone contacts every 6 months. Thereafter, telephone contacts were made every 6 months. In addition, information on all hospitalizations was collected. Incident cases of AF were diagnosed through June 2008 from 12-lead electrocardiograms taken at the annual study clinic visits (30) or from discharge diagnosis codes, except when these accompanied coronary artery bypass or valve replacement surgery (31). Prior work in CHS has shown that the positive predictive value of hospital discharge codes for diagnosing AF was 98.6% compared with direct review of medical records (31).
Statistical analysis
Levels of baseline covariates were described across sex-specific quintiles of height. Spearman correlation coefficients among measures of body size and composition were computed. The functional forms of the associations of anthropometric and bioelectric impedance variables with AF were evaluated using penalized cubic splines (32).
Cox models were used to estimate the relative risk of AF associated with BMI, weight, height, WC, HC, WHR, fat mass, and fat-free mass. On the basis of assessment of penalized cubic splines, BMI was modeled using a linear plus quadratic term, whereas all other measures were modeled using linear terms. For each measure of body size/composition, 3 models were evaluated: 1) a model adjusted for demographic characteristics, including age, sex, and race; 2) a model additionally adjusted for the confounders smoking status, physical activity level, alcohol consumption, estrogen therapy use, and serum creatinine level; and 3) a model further adjusted for mediators, such as hypertension and dysglycemia measures, lipid fractions, and C-reactive protein concentration. The additional impact of adjustment of individual anthropometric and bioelectric impedance parameters for other such parameters was subsequently examined in these models. Assessment for interactions of body size/composition measures by age, sex, or race involved inclusion of cross-product terms. In sensitivity analyses, we restricted evaluation to 1) never smokers and smokers who quit less than 5 years earlier; 2) participants with good or better self-reported health; and 3) events that occurred after 3 years of follow-up. Additional secondary analyses were adjusted for echocardiographic left atrial diameter or incident myocardial infarction and heart failure as time-dependent covariates.
The proportion of missing data for demographic and clinical variables was low (<3%). Such missing values were imputed as described previously (33). Models were checked for violation of proportional hazards using Schoenfeld residual tests; none were found.
Last, we evaluated the adequacy of ratio measures (BMI, WHR) relative to their component body size measures in relation to incident AF. Specifically, we log-transformed body size variables to construct multiplicative Cox models, because body size variables tend to be multiplicatively related, providing a means to determine whether these ratio measures capture most of the predictive information expressed by their numerator and denominator variables modeled separately. Because BMI is given by the regression of ln(weight) on ln(height), which yields the result that 2 × ln(height) is the linear predictor of ln(weight) (and thus weight/height2 is on average a constant), if inclusion of both ln(height) and ln(weight) in a Cox model yields coefficients for AF that are not in the ratio of 1 to −2, then use of ln(BMI) results in a loss information. The same holds for WHR if the Cox model coefficients for ln(WC) and ln(HC) are not in the ratio of 1 to −1. We examined these coefficients to gain insight into the associations of the corresponding ratio measures BMI and WHR with AF.
RESULTS
Baseline characteristics
The mean age of participants in the cohort was 72.4 (standard deviation, 5.4) years; 61.7% were men and 15.3% were black. The distribution of baseline covariates by sex-specific quintiles of height is shown in Table 1. Increasing sex-specific quintiles of height were associated with younger age; a higher prevelance of blacks; lower systolic blood pressure and antihypertensive therapy; less frequent fair/poor health status; more physical activity; and lower low-density lipoprotein levels.
Table 1.
Demographic and Clinical Characteristics of Study Participants by Sex-Specific Quintiles of Height, Cardiovascular Health Study, 1989–2008
| Covariate | Quintile of Height |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a (n = 918) |
2b (n = 833) |
3c (n = 859) |
4d (n = 850) |
5e (n = 816) |
|||||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Age, years | 74.2 (6.0) | 73.0 (5.7) | 72.2 (5.2) | 71.4 (4.8) | 70.9 (4.6) | ||||||||||
| Male sex | 360 | 39.2 | 313 | 37.6 | 349 | 40.6 | 291 | 34.2 | 327 | 40.1 | |||||
| Black race | 129 | 14.1 | 119 | 14.3 | 134 | 15.6 | 136 | 16.0 | 135 | 16.5 | |||||
| Systolic blood pressure, mm Hg | 140 (22) | 137 (21) | 137 (21) | 133 (21) | 134 (21) | ||||||||||
| Diastolic blood pressure, mm Hg | 71 (11) | 71 (11) | 71 (11) | 71 (11) | 73 (12) | ||||||||||
| Antihypertensive medication use | 368 | 40.1 | 340 | 40.8 | 323 | 37.6 | 325 | 38.2 | 299 | 36.6 | |||||
| Impaired fasting glucose level | 109 | 13.8 | 105 | 14.3 | 106 | 14.2 | 96 | 13.3 | 98 | 14.1 | |||||
| Diabetes | 128 | 13.9 | 99 | 11.9 | 111 | 12.9 | 128 | 15.1 | 120 | 14.7 | |||||
| Smoking status | |||||||||||||||
| Never | 481 | 52.4 | 416 | 49.9 | 411 | 47.8 | 378 | 44.5 | 366 | 44.9 | |||||
| Former | 351 | 38.2 | 303 | 36.4 | 342 | 39.8 | 350 | 41.2 | 351 | 43.0 | |||||
| Current | 86 | 9.4 | 114 | 13.7 | 106 | 12.3 | 122 | 14.4 | 99 | 12.1 | |||||
| No. of alcoholic drinks per week | |||||||||||||||
| 0 | 500 | 54.5 | 416 | 49.9 | 396 | 46.1 | 391 | 46.0 | 384 | 47.1 | |||||
| <7 | 307 | 33.4 | 315 | 37.8 | 332 | 38.6 | 320 | 37.6 | 298 | 36.5 | |||||
| 7–13 | 50 | 5.4 | 48 | 5.8 | 51 | 5.9 | 61 | 7.2 | 60 | 7.4 | |||||
| ≥14 | 61 | 6.6 | 54 | 6.5 | 80 | 9.3 | 78 | 9.2 | 74 | 9.1 | |||||
| Estrogen replacement (women) | 185 | 33.2 | 187 | 36.0 | 212 | 41.6 | 251 | 44.9 | 199 | 40.7 | |||||
| Self-reported health | |||||||||||||||
| Excellent/very good | 353 | 38.5 | 381 | 45.7 | 361 | 42.0 | 384 | 45.2 | 393 | 48.2 | |||||
| Good | 371 | 40.4 | 285 | 34.2 | 333 | 38.8 | 317 | 37.3 | 290 | 35.5 | |||||
| Fair/poor | 194 | 21.1 | 167 | 20.0 | 165 | 19.2 | 149 | 17.5 | 133 | 16.3 | |||||
| Physical activity, kcalf | 1,065 (324, 2,288) | 1,076 (431, 2,266) | 1,098 (406, 2,382) | 1,166 (508, 2,480) | 1,226 (480, 2,660) | ||||||||||
| LDL, mg/dL | 132 (38) | 131 (36) | 130 (36) | 128 (36) | 128 (36) | ||||||||||
| HDL, mg/dL | 56 (16) | 56 (16) | 56 (16) | 57 (16) | 54 (16) | ||||||||||
| Triglycerides, mg/dLf | 117 (91, 159) | 117 (90, 166) | 118 (91, 162) | 116 (89, 154) | 122 (93, 159) | ||||||||||
| Creatinine, mg/dL | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.2) | ||||||||||
| C-reactive protein, mg/Lf | 1.9 (1.0, 3.4) | 1.8 (0.9, 3.3) | 1.7 (0.9, 3.2) | 1.7 (0.9, 2.9) | 1.8 (0.9, 3.2) | ||||||||||
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
a For women, height = 124.0–154.0 cm; for men, height = 152.0–168.0 cm.
b For women, height = 154.1–157.5 cm; for men, height = 168.3–171.5 cm.
c For women, height = 157.6–160.4 cm; for men, height = 171.6–175.0 cm.
d For women, height = 160.5–164.5 cm; for men, height = 175.1–178.5 cm.
e For women, height = 164.6–186.5 cm; for men, height = 178.6–193.1 cm.
f Value presented as median (interquartile range).
Table 2 presents the correlations among different measures of body size and composition stratified by sex. In both men and women, weight was highly correlated with BMI, WC, HC, and fat mass; moderately correlated with fat-free mass; and mild-moderately correlated with height and WHR. Findings were similar for BMI, except for its lack of correlation with height and its more modest correlations with fat-free mass. The correlations of height with WC, HC, and WHR in men and women were modest or nonexistent, but those with fat-free mass were moderately strong. In turn, WC and HC were highly correlated with each other and with fat mass, whereas correlations with fat-free mass were moderate.
Table 2.
Correlations Between Measures of Body Size and Composition, Cardiovascular Health Study, 1989–2008
| Sex and Measure | Height | BMI | Waist Circumference | Hip Circumference | WHR | Fat Mass | Fat-Free Mass |
|---|---|---|---|---|---|---|---|
| Women (n = 2,636) | |||||||
| Weight | 0.35 | 0.91 | 0.77 | 0.90 | 0.30 | 0.95 | 0.68 |
| Height | −0.03 | 0.08 | 0.19 | −0.05 | 0.24 | 0.47 | |
| BMI | 0.80 | 0.88 | 0.35 | 0.90 | 0.52 | ||
| Waist circumference | 0.74 | 0.76 | 0.77 | 0.45 | |||
| Hip circumference | 0.18 | 0.89 | 0.54 | ||||
| WHR | 0.30 | 0.15 | |||||
| Fat mass | 0.43 | ||||||
| Fat-free mass | |||||||
| Men (n = 1,640) | |||||||
| Weight | 0.44 | 0.86 | 0.84 | 0.86 | 0.42 | 0.86 | 0.67 |
| Height | −0.02 | 0.17 | 0.28 | −0.03 | 0.29 | 0.47 | |
| BMI | 0.84 | 0.81 | 0.49 | 0.79 | 0.48 | ||
| Waist circumference | 0.83 | 0.73 | 0.82 | 0.42 | |||
| Hip circumference | 0.26 | 0.78 | 0.51 | ||||
| WHR | 0.47 | 0.12 | |||||
| Fat mass | 0.24 | ||||||
| Fat-free mass |
Abbreviations: BMI, body mass index; WHR, waist-to-hip ratio.
Relationship with AF
During a median follow-up of 13 years, 1,050 cases of incident AF occurred. Using cubic spline analysis, we demonstrated that the association of BMI with incident AF was U-shaped in nature (P = 0.020 for nonlinearity) (Figure 1). This U-shaped relationship, which persisted in the model that was adjusted for demographic and clinical confounders, had its nadir at a BMI of 23.8, with risk of AF increasing at higher and lower values. By contrast, none of the other measures of adiposity—weight, WC, HC, WHR, fat mass, and fat-free mass—showed significant departure from a linear relationship with incident AF.
Figure 1.
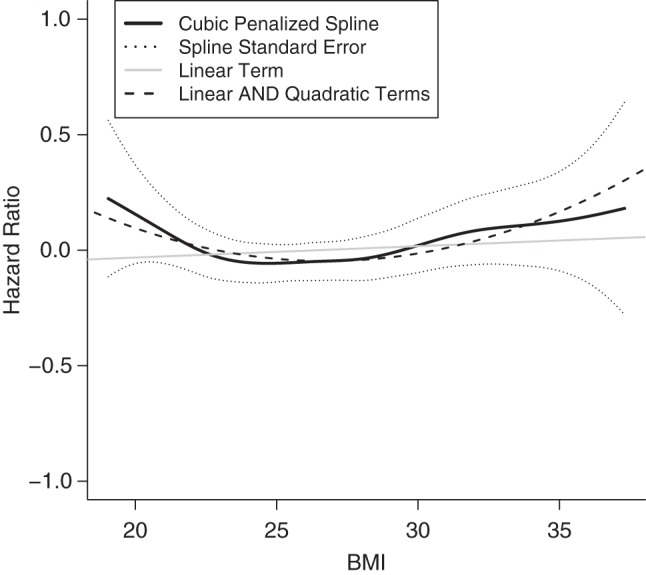
Plot of logarithm of the hazard ratio for atrial fibrillation versus body mass index (BMI), Cardiovascular Health Study, 1989–2008. The y-axis gives the logarithm of the hazard ratio; the x-axis gives BMI as weight in kilograms divided by height in meters squared. The plot shows the unadjusted linear term, linear and quadratic terms, and penalized cubic spline for BMI.
Table 3 presents the risk estimates for the various measures of body size and composition considered in relation to incident AF. For BMI, risk increases modeled using linear plus quadratic terms are presented for levels 1 standard deviation above and below the mean value, whereas risk estimates for the remaining measures modeled linearly correspond to 1-standard-deviation increments throughout their distribution. All measures except for WHR exhibited significant associations with AF after adjustment for potential confounders. In the case of weight, height, WC, HC, fat mass, and fat-free mass, these associations were positive and, though not directly comparable to the standardized risk estimates around the mean BMI value, tended to be stronger in magnitude. Height exhibited the largest standardized risk estimate for AF of all measures, and its lower 95% confidence bound exceeded the upper 95% confidence bounds for WC, HC, and fat mass. Like height relative to weight, fat-free mass also had a numerically higher risk estimate than did fat mass, but there was broad overlap of the corresponding 95% confidence intervals. After additional adjustment for putative mediators, the relationship between BMI and AF ceased to be significant, whereas those for weight, height, WC, HC, fat mass, and fat-free mass persisted. For all of the latter measures, associations were attenuated after such adjustment, with the exception of height.
Table 3.
Relationships of Measures of Body Size and Composition With Incident Atrial Fibrillation, Cardiovascular Health Study 1989–2008
| Measure | Model 1a |
Model 2b |
Model 3c |
|||
|---|---|---|---|---|---|---|
| HRd | 95% CI | HRd | 95% CI | HRd | 95% CI | |
| Body mass index | 1.10 | 1.03, 1.16 | 1.08 | 1.02, 1.15 | 1.03 | 0.96, 1.10 |
| Weight | 1.21 | 1.13, 1.30 | 1.21 | 1.13, 1.29 | 1.17 | 1.08, 1.26 |
| Height | 1.37 | 1.25, 1.50 | 1.38 | 1.25, 1.51 | 1.40 | 1.28, 1.54 |
| Waist circumference | 1.15 | 1.08, 1.23 | 1.14 | 1.07, 1.22 | 1.10 | 1.02, 1.18 |
| Hip circumference | 1.17 | 1.10, 1.25 | 1.17 | 1.10, 1.24 | 1.13 | 1.06, 1.21 |
| Waist-to-hip ratio | 1.04 | 0.97, 1.11 | 1.02 | 0.96, 1.10 | 1.00 | 0.93, 1.07 |
| Fat mass | 1.17 | 1.10, 1.25 | 1.16 | 1.09, 1.24 | 1.12 | 1.04, 1.20 |
| Fat-free mass | 1.26 | 1.13, 1.39 | 1.26 | 1.13, 1.40 | 1.23 | 1.11, 1.37 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age, sex, and race.
b Adjusted for age, sex, race, smoking status, physical activity level, alcohol consumption, estrogen therapy use, and serum creatinine level.
c Adjusted for age, sex, race, smoking status, physical activity level, alcohol consumption, estrogen therapy use, serum creatinine level, systolic blood pressure, antihypertensive medication use, impaired fasting glucose level, diabetes, low-density lipoprotein level, high-density lipoprotein level, triglyceride level, and C-reactive protein concentration.
d All measures were modeled linearly, except for body mass index (weight (kg)/height (m)2), which was modeled with linear and quadratic terms. For body mass index, risk estimates are per 1–standard-deviation increment (4.7) from the mean (26.6). For other measures, risk estimates are per 1–standard-deviation increments across the range: for weight, 14.6 kg; height, 9.4 cm; waist circumference, 13.2 cm; hip circumference, 10.0 cm; waist-to-hip ratio, 0.09 units; fat mass, 10.8 kg; and fat-free mass, 9.2 kg.
We next examined whether the individual associations of BMI, weight, WC, and HC with AF remained significant after we accounted for each of the others or for height, as appropriate, in addition to other potential confounders. As shown in Table 4, BMI ceased to be significantly associated with AF after adjustment for WC and became instead inversely associated after adjustment for HC. For weight, adjustment for height substantially attenuated the association with AF, but there was little if any change after adjustment for WC or HC. Conversely, adjustment for weight abolished the associations of WC and HC with the outcome. The relationship between height and AF was minimally affected after adjustment for weight, WC, or HC, whereas risk estimates for WC and HC were modestly attenuated after adjustment for height. Of note, the association with weight became nonsignificant with concurrent adjustment for height, WC, and HC, but that for height was not meaningfully changed by concurrent adjustment for weight, WC, and HC (data not shown). In turn, mutual adjustment of WC and HC abolished the association of WC with AF but only negligibly affected the association of HC with AF. In addition, the relations of fat mass and fat-free mass with AF were attenuated after adjustment for each other. In contrast to fat mass, however, the association for fat-free mass was strongly attenuated after adjustment for height (but not vice versa; data not shown).
Table 4.
Relationship of Measures of Body Size and Composition With Incident Atrial Fibrillation After Adjustment, Cardiovascular Health Study, 1989–2008
| Measure | Model 2a |
|
|---|---|---|
| HRb | 95% CI | |
| Body mass index adjusted for waist circumference | 0.92 | 0.82, 1.03 |
| Body mass index adjusted for hip circumference | 0.80 | 0.70, 0.91 |
| Weight adjusted for height | 1.12 | 1.03, 1.21 |
| Weight adjusted for waist circumference | 1.24 | 1.10, 1.40 |
| Weight adjusted for hip circumference | 1.18 | 1.02, 1.36 |
| Height adjusted for weight | 1.30 | 1.18, 1.44 |
| Height adjusted for waist circumference | 1.35 | 1.23, 1.48 |
| Height adjusted for hip circumference | 1.33 | 1.21, 1.47 |
| Waist circumference adjusted for hip circumference | 1.02 | 0.91, 1.13 |
| Waist circumference adjusted for height | 1.11 | 1.04, 1.19 |
| Waist circumference adjusted for weight | 0.96 | 0.86, 1.08 |
| Hip circumference adjusted for waist circumference | 1.15 | 1.04, 1.28 |
| Hip circumference adjusted for height | 1.13 | 1.06, 1.20 |
| Hip circumference adjusted for weight | 1.03 | 0.90, 1.17 |
| Fat mass adjusted for fat-free mass | 1.12 | 1.05, 1.20 |
| Fat mass adjusted for height | 1.11 | 1.03, 1.18 |
| Fat-free mass adjusted for fat mass | 1.19 | 1.06, 1.33 |
| Fat-free mass adjusted for height | 1.08 | 0.96, 1.22 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age, sex, race, smoking status, physical activity level, alcohol consumption, estrogen therapy use, and serum creatinine level.
b All measures were modeled linearly, except for body mass index (weight (kg)/height (m)2), which was modeled with linear and quadratic terms. For body mass index, risk estimates are per 1–standard-deviation increment (4.7) from the mean (26.6). For other measures, risk estimates are per 1–standard-deviation increments across the range: for weight, 14.6 kg; height, 9.4 cm; waist circumference, 13.2 cm; hip circumference, 10.0 cm; waist-to-hip ratio, 0.09 units; fat mass, 10.8 kg; and fat-free mass, 9.2 kg.
Sensitivity analyses revealed that the associations of BMI, height, weight, WC, HC, fat mass, and fat-free mass with incident AF were similar after excluding current and recent smokers, participants with self-reported fair/poor health, or subjects who experienced AF in the first 3 years of follow-up. In additional analyses, adjustment for incident myocardial infarction and heart failure as time-varying covariates minimally attenuated the observed associations. Among participants in the original cohort, further adjustment for echocardiographic left atrial diameter resulted in mild-moderate attenuations of the associations for weight, WC and HC, and fat mass and fat-free mass but did not meaningfully affect that for height (Appendix Table 1). In addition, there was no evidence of effect modification by age, sex, or race for any of the body size and composition measures examined.
Last, the relationships of log-transformed height and weight with AF and of log-transformed WC and HC with AF were assessed. In the confounder-adjusted model, the regression coefficients of ln(weight) and ln(height) were 0.49 (95% confidence interval: 0.10, 0.89) and 4.81 (95% confidence interval: 3.02, 6.59), showing that the relationship of these individual components of BMI with AF is inconsistent with the expected 1 to −2 given by the ratio measure, such that use of BMI in lieu of height and weight individually leads to a loss of information in predicting AF. Similarly, the regression coefficients for ln(WC) and ln(HC) were 0.01 (95% confidence interval: −0.73, 0.76) and 1.62 (95% confidence interval: 0.55, 2.70), which were also incompatible with the expected 1 to −1 for WHR.
DISCUSSION
The present study of a large population-based cohort of older adults reveals several important findings. First, most measures of body size and composition were positively associated with incident AF in older persons, with the exception of BMI, which had a U-shaped association. Second, among all measures, height proved to be most strongly associated with incident AF, an association that was minimally altered by adjustment for other markers of body composition or cardiovascular risk factors. Third, assessment of logarithmically transformed weight and height yielded regression coefficients that differed from the expected 1 to −2 ratio given by the equation for BMI, indicating that inclusion of BMI in lieu of height and weight in AF risk models leads to a loss of predictive information.
As far as we are aware, this is the largest study focused on older adults to report on a wide range of anthropometric variables, along with measures of fat mass and fat-free mass, in relation to incidence of AF. In previous studies largely comprising younger, middle-aged populations, investigators have documented that greater adiposity, as assessed principally by BMI (although also WC (12)), is associated with a higher risk of future AF. In these studies, BMI has often been assessed based on World Health Organization categories (10, 11), which may have obscured nonlinear associations. More recently, nonlinear associations with incident AF were detailed for BMI, WHR, and fat mass in a large cohort of middle-aged Danish adults (21), wherein values between 1 standard deviation and 2 standard deviations below the mean exhibited a horizontal slope. The more marked U-shaped association observed here occurred despite exclusion of prevalent cardiovascular disease, and it was not affected by sensitivity analyses in which we excluded participants with fair/poor self-reported health or AF during the initial 3 years of follow-up. Nonetheless, these differences likely relate to the fact that a lower BMI represents a marker of underlying illness in older adults rather than of health and fitness, as is typical of middle-aged individuals (34). It is possible that failure to detect nonlinear associations for WHR and fat mass here is due to the smaller sample size relative to the Danish study.
That use of BMI instead of height and weight separately for AF prediction leads to a loss of information is important because BMI has traditionally been relied upon as the anthropometric variable of choice for risk-adjustment or risk-prediction equations (9). Lately, a new multicohort AF prediction model has been developed that uses height and weight, instead of BMI, as predictors (35). It was noted that replacement of weight by BMI or WC therein led to similar model discrimination, but the question of whether BMI or WC remains the more pathophysiologically relevant measure remains unresolved.
Although BMI represents a useful measure of adiposity because it captures the component of weight that is independent of body height, this surrogate has notable limitations. Specifically, the relationship between BMI and body fat changes with age, such that older individuals have an increased ratio of fat mass to fat-free mass despite a constant BMI over time (36). Additionally, racial and ethnic differences in the relationship between BMI and body fat have been documented (37). Beyond these considerations, and perhaps of greatest importance to AF, inclusion of the square of height in the denominator of BMI dissipates height's very considerable contribution to the risk of this dysrhythmia.
In fact, our findings in older adults provide evidence that body size, rather than adiposity, may be the more dominant risk factor for AF incidence. This premise is supported by the stronger risk estimate observed for height than for weight upon mutual adjustment, as well as by a risk estimate for fat-free mass that was comparable to, if not stronger than, that for fat mass. It is also suggested by the persistence of a significant association with AF of HC but not WC upon mutual adjustment, although HC showed a stronger correlation than did WC with not just fat-free mass but also with fat mass.
Although these findings do not entirely agree with those from the only other previous study in which body size/composition measures in relation to AF were assessed (21), there are notable similarities. In the Danish cohort, the strength of the association between weight and AF was greater than that for height, but weight was also more strongly associated with AF than was BMI. Moreover, fat-free mass was more strongly associated with AF than was fat mass and, with mutual adjustment, the association was far more substantially attenuated for fat mass than for fat-free mass. These previous findings from a younger population (50–64 years of age) are consistent with the concept underscored here that BMI is a weaker determinant of AF than are height and weight assessed separately. The greater association for weight than for height, however, could reflect greater lean mass per unit of body weight in this age group than in our older participants. Interestingly, the strong association of fat-free mass with AF in the Danish cohort persisted after adjustment for height, which was not the case in our older population, perhaps again reflecting the lower lean body mass in elders and its closer dependence on height. The findings from the earlier report suggest that, compared with fat mass, lean body mass could be a stronger determinant of left atrial size, as it is for left ventricular mass (38), possibly because of its greater metabolic activity and oxygen delivery needs; however, the relationship in elders appears more largely driven by the relationship to body height than lean mass content per se.
Both larger body size and greater lean mass may therefore confer larger left atrial size, which is recognized as an important risk factor for AF (39). Such greater chamber size may foster greater ectopic activity as a trigger of the dysrhythmia while at the same time serving as a larger substrate to allow development of multiple re-entrant wavelets for its propagation (40, 41). Interestingly, however, the risk estimate for only fat-free mass, but not height, exhibited moderate attenuation upon adjustment for left atrial diameter here. Determining whether adjustment for left atrial volume, a more accurate measure of left atrial size that was not routinely obtained herein, dampens the association for height—as hypothesized—will require further investigation. Nonetheless, the association of AF with greater body proportions has implications for clinical practice, where indexation of left atrial volume to body surface area is recommended in order to differentiate pathophysiological processes from large body size alone (42). However, this practice may not be useful if body size in itself is a risk factor for the disease process, as our data suggest.
The more pronounced association for height observed here does not negate the role of adiposity as a risk factor for AF in this population. Apart from BMI, weight, and fat mass, both WC and HC were significantly associated with AF. Indeed, excess adiposity and AF have many shared risk factors, including diabetes, hypertension, and obstructive sleep apnea (12, 35, 43). Unlike height and lean-body mass, these and other (44–46) obesity-related factors can foster inflammation and fibrosis as underlying substrates for the dysrhythmia (40). Obesity-related factors can also promote left atrial enlargement (39), but this influence does not tend to be as sustained through the lifespan as it is for height and fat-free mass. The pathophysiologic changes associated with obesity, however, are difficult to capture using generalized body size and composition measures and will require further evaluation by examining specific fat depots and their respective contributions.
Our study has several limitations. The present results come from predominantly white older adults free of major comorbid conditions after the additional exclusion of overt cardiovascular disease, and the findings are not generalizable to less healthy or more ethnically distinct older populations. Furthermore, despite exploring the relationships of fat mass and fat-free mass with incident AF, we did not explore specific anatomical fat depots, which may be important for pathophysiologic mechanisms linking obesity and AF. In addition, these findings are based on annual electrocardiogram and discharge diagnoses of AF, and associations may have been attenuated by underdetection of paroxysmal AF. Notwithstanding these limitations, the validity of the documented associations is bolstered by our study's prospective, population-based nature, its standardized assessment of exposures and outcome, and a large number of incident AF events during long-term follow-up.
The predominance of height as an anthropometric risk factor for AF in elders documented here, and the demonstrated loss of information content for BMI relative to height and weight considered separately, have implications not only for epidemiologic studies but also for clinical practice. These findings indicate that the use of BMI should be abandoned in favor of height and weight in order to optimally incorporate body size, and not just adiposity, into risk assessment of AF in older populations and suggest that the recommendation of indexation of left atrial volume by body size requires re-evaluation as relates to the prognostic value of left atrial size for this dysrhythmia.
ACKNOWLEDGMENTS
Author affiliations: Division of Cardiology, Department of Medicine, Weill Cornell Medical College, New York, New York (Maria G. Karas); Department of Biostatistics, University of Washington, Seattle, Washington (Laura M. Yee, Mary L. Biggs, Richard A. Kronmal); Division of Aging, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Luc Djoussé); Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts (Kenneth J. Mukamal); Nephrology Section, Veterans Affairs San Diego Healthcare System, San Diego, California (Joachim H. Ix); Division of Nephrology, Department of Medicine, University of California San Diego, San Diego, California (Joachim H. Ix); Division of Preventive Medicine, Department of Family and Public Health, University of California San Diego, San Diego, California (Joachim H. Ix); Division of Geriatrics and Clinical Gerontology, National Institute on Aging, Bethesda, Maryland (Susan J. Zieman); New York Academy of Medicine, New York, New York (David S. Siscovick); Division of Cardiology, Department of Medicine, School of Medicine, University of Maryland, Baltimore, Maryland (John S. Gottdiener); Center for Human Genetics Research, Massachusetts General Hospital, Boston, Massachusetts (Michael A. Rosenberg); Cardiovascular Health Research Unit, Departments of Medicine and Epidemiology, University of Washington, Seattle, Washington (Susan R. Heckbert); Division of Cardiology, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York (Jorge R. Kizer); and Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York (Jorge R. Kizer).
A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://chs-nhlbi.org.
This study was supported by grant R01 HL094555 from the National Heart, Lung, and Blood Institute to L.D., K.J.M, J.H.I, S.J.Z., and J.R.K. M.A.R. was supported by grant K23 HL127296 from the National Heart, Lung, and Blood Institute. The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by grant AG023629 from the National Institute on Aging.
Conflict of interest: none declared.
Appendix Table 1.
Sensitivity Analysis of the Impact of Additional Adjustment for Echocardiographic Left Atrial Anteroposterior Diameter on the Relationship Between Measures of Body Size and Composition and Incident Atrial Fibrillation (Original Cohort Only, n = 3,789), Cardiovascular Health Study, 1989–2008
| Measure | Model 2a |
Model 2a and Left Atrial Diameter |
||
|---|---|---|---|---|
| HRb | 95% CI | HRb | 95% CI | |
| Body mass index | 1.00 | 0.93, 1.08 | 0.93 | 0.87, 1.02 |
| Height | 1.41 | 1.28, 1.55 | 1.40 | 1.27, 1.54 |
| Weight | 1.19 | 1.11, 1.29 | 1.12 | 1.04, 1.22 |
| Waist circumference | 1.07 | 0.99, 1.16 | 1.02 | 0.94, 1.10 |
| Hip circumference | 1.15 | 1.08, 1.23 | 1.10 | 1.02, 1.18 |
| Fat mass | 1.16 | 1.08, 1.24 | 1.09 | 1.01, 1.18 |
| Fat free mass | 1.25 | 1.10, 1.40 | 1.16 | 1.03, 1.31 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Adjusted for age, sex, race, smoking status, physical activity level, alcohol consumption, estrogen therapy use, and serum creatinine concentration.
b All measures were modeled linearly, except for body mass index (weight (kg)/height (m)2), which was modeled with linear and quadratic terms. For body mass index, risk estimates are per 1–standard-deviation increment (4.7) from the mean (26.6). For other measures, risk estimates are per 1–standard-deviation increments across the range: for weight, 14.6 kg; height, 9.4 cm; waist circumference, 13.2 cm; hip circumference, 10.0 cm; waist-to-hip ratio, 0.09 units; fat mass, 10.8 kg; and fat-free mass, 9.2 kg.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;1293:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;526:101–104, 106. [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;228:983–988. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ et al. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;278:936–941. [DOI] [PubMed] [Google Scholar]
- 5.Thacker EL, McKnight B, Psaty BM et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;812:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Wolf PA, D'Agostino RB et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;9810:946–952. [DOI] [PubMed] [Google Scholar]
- 7.Kelly T, Yang W, Chen CS et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;329:1431–1437. [DOI] [PubMed] [Google Scholar]
- 8.Dublin S, French B, Glazer NL et al. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;16621:2322–2328. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Aspelund T, Li G et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;17021:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanahita N, Messerli FH, Bangalore S et al. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;1552:310–315. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Parise H, Levy D et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;29220:2471–2477. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain AM, Agarwal SK, Ambrose M et al. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;1595:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottdiener JS, Reda DJ, Williams DW et al. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;293:651–658. [DOI] [PubMed] [Google Scholar]
- 14.Hubert HB, Feinleib M, McNamara PM et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;675:968–977. [DOI] [PubMed] [Google Scholar]
- 15.Kenchaiah S, Evans JC, Levy D et al. Obesity and the risk of heart failure. N Engl J Med. 2002;3475:305–313. [DOI] [PubMed] [Google Scholar]
- 16.Mokdad AH, Ford ES, Bowman BA et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;2891:76–79. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D'Agostino RB, Sullivan L et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;16216:1867–1872. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Krishnaswami S, Resnick H et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;262:372–379. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;6110:1059–1064. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg MA, Patton KK, Sotoodehnia N et al. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;3321:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost L, Benjamin EJ, Fenger-Grøn M et al. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring). 2014;226:1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain AM, Agarwal SK, Folsom AR et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;1071:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdecchia P, Dagenais G, Healey J et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. J Hypertens. 2012;305:1004–1014. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Borhani NO, Enright P et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;13:263–276. [DOI] [PubMed] [Google Scholar]
- 25.Psaty BM, Kuller LH, Bild D et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;54:270–277. [DOI] [PubMed] [Google Scholar]
- 26.Djoussé L, Bartz TM, Ix JH et al. Adiposity and incident heart failure in older adults: the cardiovascular health study. Obesity (Silver Spring). 2012;209:1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deurenberg P, van der Kooy K, Hautvast JG. The assessment of the body composition in the elderly by densitometry, anthropometry and bioelectrical impedance. Basic Life Sci. 1990;55:391–393. [DOI] [PubMed] [Google Scholar]
- 28.Taylor HL, Jacobs DR Jr, Schucker B et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;3112:741–755. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Cornell ES, Howard PR et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;412:264–270. [PubMed] [Google Scholar]
- 30.Rautaharju PM, MacInnis PJ, Warren JW et al. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;294:362–374. [PubMed] [Google Scholar]
- 31.Psaty BM, Manolio TA, Kuller LH et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;967:2455–2461. [DOI] [PubMed] [Google Scholar]
- 32.Hastie T, Tibshirani R. Generalized Additive Models. Boca Raton, FL: Chapman & Hall/CRC; 1990. [Google Scholar]
- 33.Arnold AM, Kronmal RA. Multiple imputation of baseline data in the Cardiovascular Health Study. Am J Epidemiol. 2003;1571:74–84. [DOI] [PubMed] [Google Scholar]
- 34.Lainscak M, von Haehling S, Doehner W et al. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;31:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso A, Krijthe BP, Aspelund T et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;22:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohn SH. In Vivo Body Composition Studies. London, United Kingdom: The Institute of Physical Sciences in Medicine; 1987. [Google Scholar]
- 37.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;2212:1164–1171. [DOI] [PubMed] [Google Scholar]
- 38.Bella JN, Devereux RB, Roman MJ et al. Relations of left ventricular mass to fat-free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation. 1998;9823:2538–2544. [DOI] [PubMed] [Google Scholar]
- 39.Abhayaratna WP, Seward JB, Appleton CP et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;4712:2357–2363. [DOI] [PubMed] [Google Scholar]
- 40.Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med. 2009;207:672–681. [DOI] [PubMed] [Google Scholar]
- 41.Conen D, Adam M, Roche F et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012;12619:2302–2308. [DOI] [PubMed] [Google Scholar]
- 42.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;281:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 43.Gami AS, Hodge DO, Herges RM et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;495:565–571. [DOI] [PubMed] [Google Scholar]
- 44.Messerli FH, Ventura HO, Reisin E et al. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982;661:55–60. [DOI] [PubMed] [Google Scholar]
- 45.Iacobellis G, Ribaudo MC, Leto G et al. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002;108:767–773. [DOI] [PubMed] [Google Scholar]
- 46.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl). 2001;791:21–29. [DOI] [PubMed] [Google Scholar]


