Introduction
Monilethrix is an autosomal dominant genodermatosis characterized by hair fragility, keratosis pilaris, and pathognomonic beading of the hair shaft. Hair fragility may lead to hair breakage and the appearance of generalized hair loss.1
The gene for autosome-dominant monilethrix has been mapped to the epithelial keratin gene cluster on 12q13,2 and point mutations have been found in the hair cortex–specific keratin genes KRT86, KRT83, and KRT81.3, 4 Mutations in desmoglein 4 are responsible for an autosomal recessive variant of monilethrix.5
Monilethrix has considerable inter- and intrafamilial variations in age of onset, severity, and natural history.4 Most often, hair is normal at birth and is progressively replaced by short, fragile, brittle hair during the first months of life. Hair breakage secondary to hair fragility may be accompanied by follicular keratosis most commonly on the occiput. Friction from wigs and their adhesives may exacerbate hair loss. Eyebrows, eyelashes, pubic, axillary, and general body hair may be affected along with the scalp.
In most patients, the hair loss persists with little change throughout life. Spontaneous improvement or complete recovery has been reported during pregnancy.6
There is no cure for monilethrix. Reduction in hairdressing trauma may diminish weathering and improve severely affected cases.1 Two women with monilethrix who responded to 6 months of treatment with low-dose oral minoxidil are described.
Case series
Case 1
A 40-year-old woman presented with lifelong fragile, sparse, and thin hair. She had monilethrix diagnosed in childhood. She reported striking improvement in hair volume and length during each pregnancy but relapsed within 12 months postpartum.
Comorbidities included Crohn's disease and impaired hearing. Her daughter and 2 sons also had monilethrix and suffered from sparse and fragile hair.
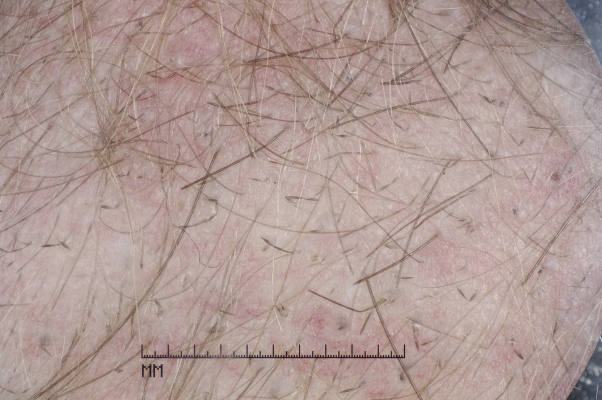
On examination, she had brittle, lustreless, fragile, short hair all over the scalp. Her hair turned to powder when rolled between fingers. She was noted to have small keratotic papules on the nape of the neck. There were no nail or tooth abnormalities detected. Dermoscopy (Fig 1) confirmed the diagnosis of monilethrix.
Fig 1.
Patient 1. Dermascopic image of the occipital scalp shows numerous broken hairs. Beaded hairs pathognomic of monilethrix can be seen.
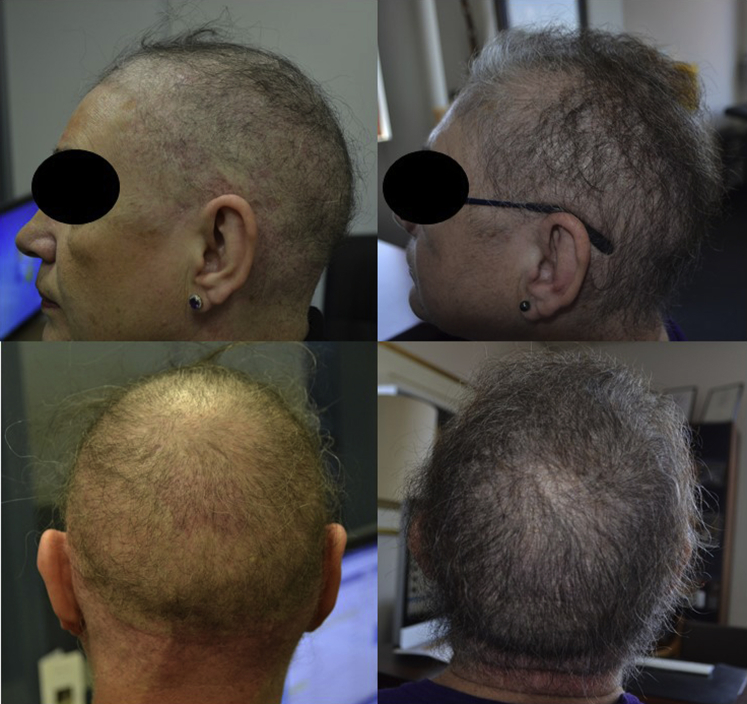
Treatment started with oral minoxidil, 0.25 mg daily. Review after 6 months found significant hair growth with reduced breakage and increased hair volume and length (Fig 2). The patient did not experience any side effects or complication of the treatment. The patient continued on the treatment and is reviewed at 3-month intervals. The initial improvement in hair density has been maintained and no side effects have been noted after 2 years additional follow-up.
Fig 2.
Patient 1. Monilethrix. Lateral and posterior views of the scalp before and after 6 months of treatment with low-dose oral minoxidil.
Case 2
A 35-year-old woman with fragile, thin, and sparse hair since childhood secondary to monilethrix presented for assessment and treatment. She had a positive family history of monilethrix affecting her son, daughter, and grandson.
Examination found brittle, lustreless, fragile short hair all over the scalp, keratotic papules on the occipital scalp, and keratosis pilaris. No nail or dental abnormalities were present. Dermoscopy found elliptical nodes separated by narrower internodes (Fig 3).
Fig 3.
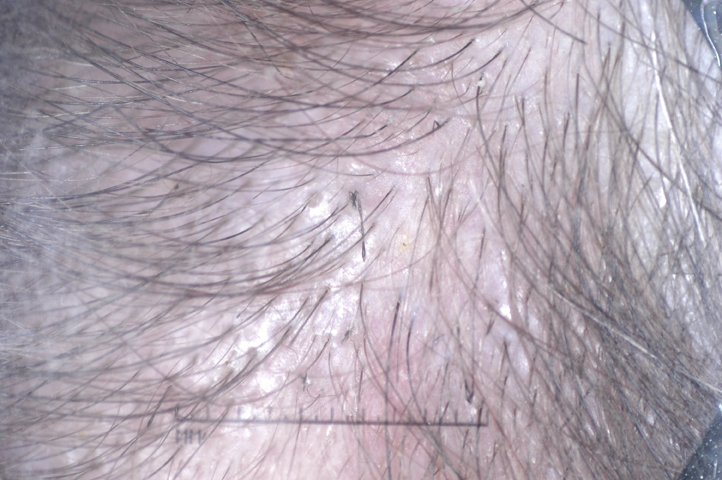
Patient 2. Dermascopic image of the mid-frontal scalp shows broken hairs. Beaded hairs pathognomic of monilethrix can be seen.
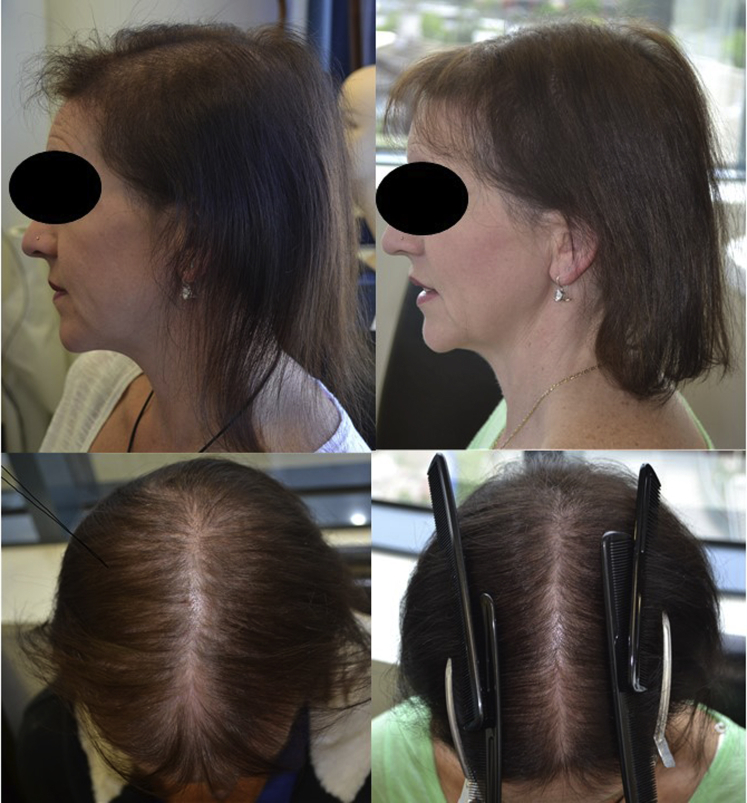
She started oral minoxidil, 0.25 mg daily. On examination 3 months after the initial consultation, hair shedding decreased significantly. However, the hair density remained unchanged. Minoxidil was increased to 0.5 mg daily. There was a significant improvement in hair density on review at 6 months (Fig 4).
Fig 4.
Patient 2 is less severely affected than patient 1. Lateral and mid-frontal views of the scalp before and after 6 months of treatment with low-dose oral minoxidil.
The patient remains on the treatment and is reviewed at 3-month intervals. The initial improvement in hair density has been maintained, and no side effects have emerged after 18 months of additional follow-up.
Discussion
Monilethrix is a hair shaft fragility genodermatosis. Etretinate is found to reduce hair beading but does not improve hair fragility or hair density.7 Topical minoxidil 2% has been used to treat monilethrix. Saxena et al8 treated 3 sisters with 2% monilethrix solution twice daily and noted a reduction in fragility after 2.5 months and increased hair length at 6 months. Rossi et al9 treated 4 patients for 12 months with similar effects.
Minoxidil is known to induce hypertrichosis, prolong anagen duration, and increase hair diameter.8 Because the application of topical minoxidil can leave the hair dry and tangled and could potentially accentuate breakage of fragile hairs, both patients were treated off-label with low-dose oral minoxidil.
Little is known about the pharmacologic interaction between minoxidil and target cells in hair follicles. Several ways in which minoxidil could stimulate hair growth include increasing the linear growth rate of hair, increasing the diameter of the hair fiber, altering the hair cycle by either shortening of telogen or prolonging anagen, or a combination of these.10
Histologic studies show that treatment with minoxidil causes an increase in the proportion of follicles in anagen, a reduction in telogen follicles, and an increase in hair follicle size. It is also effective on proliferation and differentiation of normal human keratinocyte.10
Oral minoxidil is a promising treatment for hair loss associated with monilethrix. When used in low doses it appears to be well tolerated. Based on observations in male and female pattern hair loss, it is likely that that long-term maintenance treatment will be required to prevent relapse of hair loss.
Patients receiving long-term treatment should have regular monitoring of their blood pressure and pulse rate, as oral minoxidil has been associated with postural hypotension and tachycardia. Generally, these side effects and fluid retention are only seen at higher dosages. Facial hypertrichosis can occur with both oral and topical minoxidil. Facial hypertrichosis is dose related, and when mild is easily managed with waxing, depilatory creams, or laser hair removal.
Further studies are required to document safety and efficacy.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Sinclair R., De Berker D. Hereditary and congenital alopecia and hypotrichosis. In: Dawber R.P.R., editor. Diseases of the Scalp and Hair. Blackwell Science; Oxford: 1997. pp. 151–238. [Google Scholar]
- 2.Van Steensel M.A.M., Steijlen P.M., Bladergroen R.S., Vermeer M., van Geel M. A missense mutation in the type II hair keratin hHb3 is associated with monilethrix. J Med Genet. 2005;42:e19. doi: 10.1136/jmg.2004.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horev L., Djabali K., Green J. De novo mutations in monilethrix. Exp Dermatol. 2003;12:882–885. doi: 10.1111/j.0906-6705.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 4.De Cruz R., Horev L., Green J. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. B J Dermatol. 2012;166:20–26. doi: 10.1111/j.1365-2133.2012.10861.x. [DOI] [PubMed] [Google Scholar]
- 5.Zlotogorski A., Marek D., Horev L. An autosomal recessive form of monilethrix is caused by mutations in DSG4: clinical overlap with localized autosomal recessive hypotrichosis. J Invest Dermatol. 2006;126:1292–1296. doi: 10.1038/sj.jid.5700251. [DOI] [PubMed] [Google Scholar]
- 6.Summerly R., Donaldson E.M. Monilethrix. A family study. Br J Dermatol. 1962;74:387–391. doi: 10.1111/j.1365-2133.1962.tb13432.x. [DOI] [PubMed] [Google Scholar]
- 7.de Berker D., Dawber R.P.R. Monilethrix treated with oral retinoids. Clin Exp Dermatol. 2006;16:226–228. doi: 10.1111/j.1365-2230.1991.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Saxena U., Ramesh V., Misra R.S. Topical minoxidil in monilethrix. Dermatology. 1991;182:252–253. doi: 10.1159/000247808. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A., Iorio A., Scali E. Monilethrix treated with minoxidil. J Immunopathol Pharmacol. 2011;24:239–242. doi: 10.1177/039463201102400129. [DOI] [PubMed] [Google Scholar]
- 10.Messenger A.G., Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]