Abstract
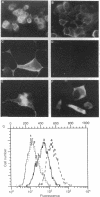
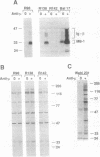
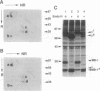
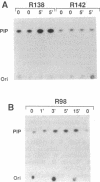
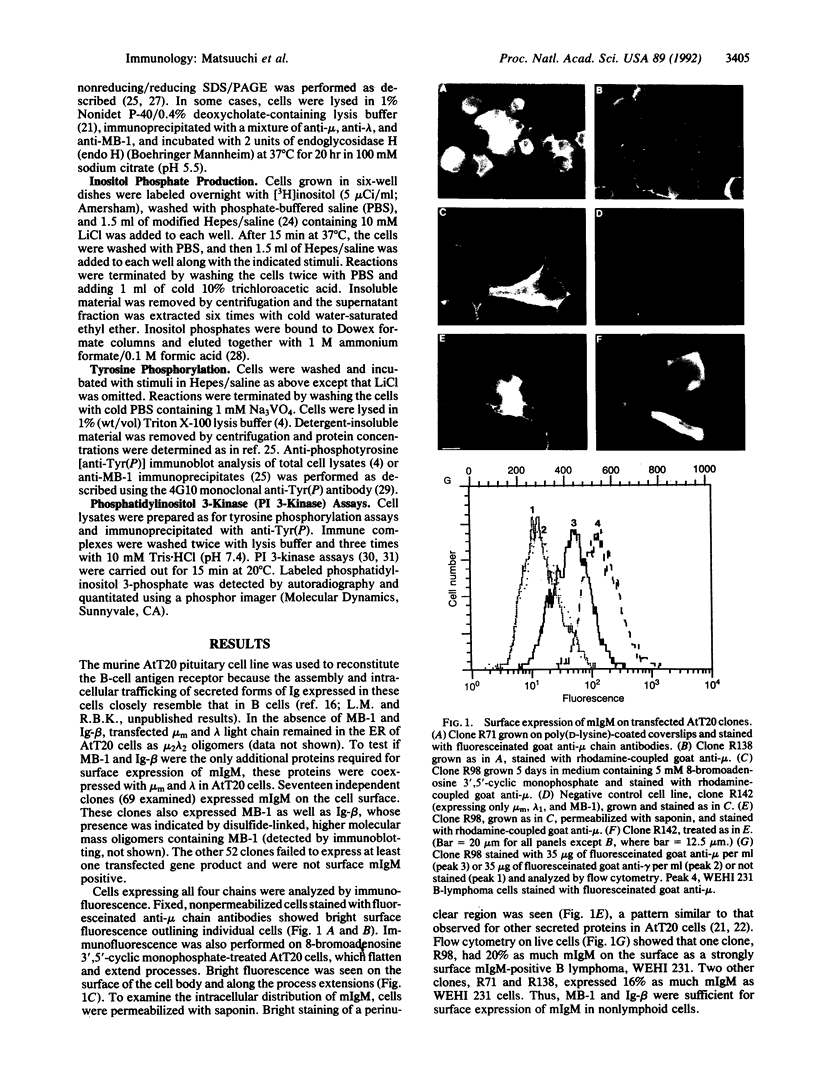
The B-cell antigen receptors consist of membrane immunoglobulins (mIgs) noncovalently associated with two accessory proteins, MB-1 and Ig-beta. We used transfection into a nonlymphoid cell line to test whether MB-1 and Ig-beta were sufficient to promote cell surface expression of mIgM capable of signal transduction. Expression of MB-1 and Ig-beta, but not MB-1 alone, allowed high-level surface expression of mIgM in the AtT20 endocrine cell line, which presumably lacks other B-cell-specific components. The reconstituted antigen receptor was capable of mediating some of the signaling reactions characteristic of mIgM in B lymphocytes. Crosslinking mIgM on transfected AtT20 cells stimulated tyrosine phosphorylation of MB-1 and Ig-beta and also increased the amount of phosphatidylinositol 3-kinase activity that could be precipitated with anti-phosphotyrosine antibodies. When total cell lysates were analyzed by anti-phosphotyrosine immunoblotting, however, no induced phosphorylation of more abundant proteins was detected. Moreover, crosslinking of the receptor in AtT20 cells did not stimulate inositol phospholipid breakdown. Thus, the transfected B-cell antigen receptor could initiate some signal transduction events but AtT20 cells may lack components required for other signaling events associated with mIgM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger K. R., Cantley L. C. Novel polyphosphoinositides in cell growth and activation. Cancer Cells. 1991 Jul;3(7):263–270. [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brunswick M., Samelson L. E., Mond J. J. Surface immunoglobulin crosslinking activates a tyrosine kinase pathway in B cells that is independent of protein kinase C. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1311–1314. doi: 10.1073/pnas.88.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess T. L., Craik C. S., Kelly R. B. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985 Aug;101(2):639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey K. A., DeFranco A. L. Cross-linking membrane IgM induces production of inositol trisphosphate and inositol tetrakisphosphate in WEHI-231 B lymphoma cells. J Immunol. 1987 Jun 1;138(11):3935–3942. [PubMed] [Google Scholar]
- Gold M. R., DeFranco A. L. Phorbol esters and dioctanoylglycerol block anti-IgM-stimulated phosphoinositide hydrolysis in the murine B cell lymphoma WEHI-231. J Immunol. 1987 Feb 1;138(3):868–876. [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Matsuuchi L., Kelly R. B., DeFranco A. L. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Lottspeich F., Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990 Dec;20(12):2795–2799. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Justement L. B., Campbell K. S., Chien N. C., Cambier J. C. Regulation of B cell antigen receptor signal transduction and phosphorylation by CD45. Science. 1991 Jun 28;252(5014):1839–1842. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- Kashiwamura S., Koyama T., Matsuo T., Steinmetz M., Kimoto M., Sakaguchi N. Structure of the murine mb-1 gene encoding a putative sIgM-associated molecule. J Immunol. 1990 Jul 1;145(1):337–343. [PubMed] [Google Scholar]
- Lane P. J., McConnell F. M., Schieven G. L., Clark E. A., Ledbetter J. A. The role of class II molecules in human B cell activation. Association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Immunol. 1990 May 15;144(10):3684–3692. [PubMed] [Google Scholar]
- Matsuo T., Kimoto M., Sakaguchi N. Direct identification of the putative surface IgM receptor-associated molecule encoded by murine B cell-specific mb-1 gene. J Immunol. 1991 Mar 1;146(5):1584–1590. [PubMed] [Google Scholar]
- Matsuuchi L., Buckley K. M., Lowe A. W., Kelly R. B. Targeting of secretory vesicles to cytoplasmic domains in AtT-20 and PC-12 cells. J Cell Biol. 1988 Feb;106(2):239–251. doi: 10.1083/jcb.106.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L., Kelly R. B. Constitutive and basal secretion from the endocrine cell line, AtT-20. J Cell Biol. 1991 Mar;112(5):843–852. doi: 10.1083/jcb.112.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Lau A. L., Cunningham D. D. Selective radiolabeling of cell surface proteins to a high specific activity. Biochemistry. 1987 Feb 10;26(3):743–750. doi: 10.1021/bi00377a014. [DOI] [PubMed] [Google Scholar]
- Travis A., Hagman J., Grosschedl R. Heterogeneously initiated transcription from the pre-B- and B-cell-specific mb-1 promoter: analysis of the requirement for upstream factor-binding sites and initiation site sequences. Mol Cell Biol. 1991 Nov;11(11):5756–5766. doi: 10.1128/mcb.11.11.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- de Silva A. M., Balch W. E., Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990 Sep;111(3):857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]