Abstract
Pancreatic cancer reveals the worst prognosis among human cancers with little improvement in its clinical outcome in the last three decades. We previously suggested that polypeptide N-acetylgalactosaminyltransferase 6 (GALNT6), which catalyzes O-type glycosylation of Mucin 1, might be a promising molecular target for drug development for breast cancer. In this study, we report upregulation of GALNT6 in pancreatic cancer cells where Mucin proteins are highly O-glycosylated. We found that knockdown of GALNT6 with small interfering RNA in pancreatic cancer cells decreased the amount of Mucin 4 protein as well as that of its transcript, reduced the levels of human epidermal growth factor receptor 2 and extracellular signal–regulated kinase, and significantly reduced pancreatic cancer cell viability. Interestingly, knockdown of GALNT6 caused drastic morphological changes of pancreatic cells, accompanied with the cadherin switching from P-cadherin to E-cadherin. Considering important roles of Mucin 4 in growth and invasion, our findings imply that targeting GALNT6 is a very promising therapeutic strategy for treatment of pancreatic cancer patients who still have very limited treatment modalities.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States and has kept the lowest 5-year survival rate among common cancers due to the difficulty of diagnosis at an early stage, a progressive phenotype, and limited options of treatment with relatively low efficacy [1]. Current treatment options of pancreatic cancer are based on either gemcitabine or a combination regimen called FOLFIRINOX, which is composed of folinic acid (FOL), 5-fluorouracil (F), irinotecan (IRIN), and oxaliplatin (OX) [2]. However, because these treatment modalities are not highly effective, more effective and less toxic treatment modalities should be developed for pancreatic cancer patients.
Accumulating evidences have suggested that mucins are involved in development, invasiveness, metastasis, and drug resistance of pancreatic cancer [3]. Mucins, which are present as a membrane-bound form or as a secreted form, enhance several oncogenic signal pathways and suppress cell adhesion [4]. Particularly, in pancreatic cancer, Mucin 4 protein expression is known to be elevated and extensively modified with O-linked oligosaccharides (O-glycosylation) [3]. Increase of O-glycosylated Mucin 4 was correlated with poor prognosis in patients with pancreatic cancer [5], [6].
The O-glycosylation is one of common posttranslational protein modifications in cancer cells, and aberrant O-glycosylation is generally considered to enhance functions of oncogenic proteins [7]. For example, O-glycosylation is indicated to play important roles in protein processing, stability, secretion, and thus activation of signaling pathways [8]. Such abnormal O-glycosylation in cancer cells is attributed to upregulated expression of polypeptide N-acetylgalactosaminyl transferase (GALNT) family proteins that are involved in the initiation of the O-glycosylation by transferring N-acetyl-alpha-D-galactosamine (GalNAc) to target substrates [9]. We previously reported that GALNT6 was highly transactivated and stabilized Mucin1 protein through the O-glycosylation in breast cancer cells [10]. However, the underlying mechanism to cause aberrant O-glycosylation of Mucins in pancreatic cancer has not been fully investigated.
In the present study, we demonstrate that GALNT6 is overexpressed in a subset of pancreatic cancer cell lines examined and plays imperative roles in growth and invasion of pancreatic cancer cells. We also reveal evidences supporting that GALNT6 is essential for O-glycosylation and stabilization of Mucin 4 protein, and that knockdown of GALNT6 causes drastic morphological changes of pancreatic cells accompanied with the cadherin switching from P-cadherin to E-cadherin. Our results imply that GALNT6 is a promising molecular target for drug development for pancreatic cancer.
Material and Methods
Cell Lines
Human pancreatic cancer cell lines ASPC-1, Capan-2, HPAF-II, Panc02.03, Panc08.13, PANC-1, and PL45 were purchased from the American Type Culture Collection (Rockville, MD). KP-1N, MiaPaCa-2, and SUIT-2 cells were purchased from the Japanese Collection of Research BioResources Cell Bank (Suita, Japan). KLM-1, PK-45P, and PK-59 cells were provided by the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University. KP4 cell was provided by RIKEN BioResource Center (Tsukuba, Japan). The stocks of cell lines that had been deposited in liquid nitrogen were used in this study. All cells were cultured at 37°C in atmosphere of humidified air with 5% of CO2 and in appropriate culture media (Life Technologies, Grand Island, NY), such as RPMI1640 for ASPC-1, KLM-1, KP-1N, Panc02.03, Panc08.13, PK-45P, and PK-59; DMEM for KP4, PANC-1, and PL45; EMEM for HPAF-II, MiaPaCa-2, and SUIT-2; and McCoy for Capan-2. Each medium was supplemented with 15% (for Panc02.03 and Panc08.13) or 10% (for other cell lines) of fetal bovine serum (Fisher Scientific, Pittsburgh, PA) and 1% of antibiotic-antimycotic solution (Fisher Scientific).
Gene Silencing by RNA Interference
To knock down endogenous GALNT6 expression in pancreatic cancer cells, we used small interfering RNAs (siRNAs) synthesized from Sigma-Aldrich (St. Louis, MO). KLM-1 and Panc02.03 cells were transfected with 200 pmol of oligo siRNA using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. The target sequences of siRNA are 5′-GAGAAAUCCUUCGGUGACA-3′ for si-GALNT6 and 5′-GCACUGUUUCAAUGCCUUU-3′ for si-GALNT6-2, as previously described [10]. The siRNA Universal Negative Control (Sigma-Aldrich) was used for si-control.
Cell Viability Assay
For methyl thiazolyl tetrazolium (MTT) assay, pancreatic cancer cells were seeded into 24-well plates at 5 × 104 cells per well and transfected with oligo siRNA. Then, cancer cells were cultured at 37°C under 5% CO2 for an additional 72 hours. The Cell Counting Kit–8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used for MTT reaction and examined the cell viability. After reaction for 1 to 3 hours, 100 μl of supernatant was transferred into a 96-well plate and read in the iMark microplate absorbance reader (Bio-Rad, Hercules, CA) at 450 nm of wavelength. All of these experiments were done in triplicate.
Cell Invasion Assay
To examine cell invasiveness, 1 × 106 cells were seeded on Transwell plates (Costar, Cambridge, MA) of 5-μm pore filters. After adding 500 μl of 10% serum-containing medium to the lower chamber of the well, pancreatic cancer cells were allowed to migrate for 72 hours. The migrated cells on the lower side of the membrane were photographed and counted with the ImageJ software [11].
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from KLM-1 and Panc02.03 cells using RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. Total RNA (1 μg) was reversely transcribed using SuperScript III First-Strand Synthesis System (Invitrogen) to generate cDNA. Aliquots of cDNA samples were quantified by the real-time RT-PCR method. The RT-PCR was performed using primers listed below using the ViiA 7 system (Life Technologies). The expression level of GALNT6 or MUC4 was normalized with that of GAPDH. The PCR primer sequences were 5′-GGATGAAACCTACCCCATCA-3′ and 5′-ACCGATGTGCTCAAAGTAGGA-3′ for GALNT6, 5′-GAGGAATGACCAGCTGCCTT-3′ and 5′-AGGGCCAGGGTGTCATAGAT-3′ for MUC4, and 5′-CGACCACTTTGTCAAGCTCA-3′ and 5′-GGTTGAGCACAGGGTACTTTATT-3′ for GAPDH.
Western Blot Analysis
To detect the expression of endogenous proteins in pancreatic cancer cells, we performed Western blots with normalization of protein amount by β-actin. Cells were lysed with the IP lysis buffer (Thermo Scientific, Waltham, MA) containing protease inhibitor cocktail III (Millipore, Billerica, MA). The proteins were separated by electrophoresis using the any KD SDS-PAGE gel (Bio-Rad) and transferred onto nitrocellulose membrane (GE Healthcare, Pittsburgh, PA). After blocking with the Block ACE solution (AbD Serotec, Raleigh, NC), the membranes were incubated with the first antibodies: anti-GALNT6 (Atlas Antibodies, Stockholm, Sweden), anti–Mucin 4 (Santa Cruz Biothechnology, Santa Cruz, CA), anti–E-cadherin (Abcam Biotech, Cambridge UK), anti–P-cadherin (Abcam Biotech), anti–extracellular signal-regulated kinase (ERK) (Abcam Biotech), anti–human epidermal growth factor receptor 2 (HER2) (Abcam Biotech), anti–β-actin (Sigma-Aldrich), anti–Histone H3 (Abcam Biotech), or anti-Tubulin antibody (Millipore). Finally, the membrane was incubated with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biothechnology), and protein bands were visualized by the enhanced chemiluminescence detection reagents (GE Healthcare). For the nuclear/cytoplasm fractionation, KLM-1 and Panc02.03 cells were lysed using NE-PER Nuclear and Cytoplasmic Extraction buffers (Thermo Scientific) for 10 minutes on ice and then quick-spun for 15 seconds to collect cytosolic lysate. Pellets were washed two times with cytoplasmic lysis buffer and then lysed with the nuclear lysis buffer for 45 minutes on ice. The final lysates were spun for 20 minutes at 14,000 rpm at 4°C to collect nuclear lysates.
Immunocytochemical Staining
Pancreatic cancer cells were seeded on Lab-Tek II chamber slide system (Nalge Nunc International, Penfield, NY) before transfection with oligo siRNAs. The cells were fixed with 4% paraformaldehyde in PBS for 15 minutes and rendered permeable with 0.1% Triton X-100 in PBS at 4°C for 2.5 minutes. Subsequently, the cells were covered with 3 % BSA in PBS at 4°C for 3 hours to block nonspecific hybridization followed by incubation with antibodies of anti-GALNT6 (1:100), anti–E-cadherin (1:100), or anti–β-catenin (1:100). After washing with PBS for three times, cells were stained by Alexa Fluor 488– or Alexa Fluor 594–conjugated secondary antibodies (Molecular Probe, Eugene, OR) diluted at 1:1000. Finally, nuclei were counterstained with 4′,6-diamidino-2-phenylindole, and cells were examined by SP5 Tandem Scanner Confocal Microscope (Leica Microsystems, Buffalo Grove, IL) at the University of Chicago Microscopy Core Facility. Cytoskeleton structure was visualized by staining with Alexa Fluor 488–conjugated phalloidin (Molecular Probes).
In vitro O-Glycosylation
Recombinant GALNT6 protein was purchased from Abnova (Walnut, CA), and MUC4 peptide (TSSASTGHATPLPVTD) was synthesized from GenScript (Piscataway, NJ). Then 5 μM of the MUC4 peptide was incubated with 0.5 μg of recombinant GALNT6 protein in 25 mM of Tris-HCl (pH 7.4), 10 mM of MnCl2, and w/wo 50 μM of UDP-GalNAc at 37°C for 12 hours. The resultant peptides were desalted by Zip-tip C18 (Merck Millipore, Billerica, MA), mixed with 4 mg/ml α-cyano-4-hydroxycinnamic acid, and analyzed with 4800 Plus MALDI-TOF (matrix-assisted laser desorption ionization time-of-flight) mass spectrometer (Sciex, Framingham, MA).
Statistical Analysis
Statistical significance was calculated by Student's t test using Prism 6 software (GraphPad, San Diego, CA). A difference of P < .05 was considered to be significant.
Results
GALNT6 and Mucin 4 Expression in Pancreatic Cancer
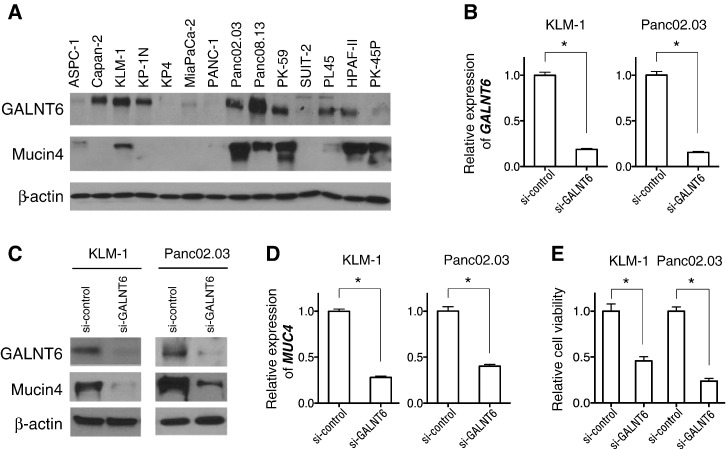
We first examined expression levels of GALNT6 in pancreatic cancers through publically available gene expression datasets. The Oncomine database revealed that GALNT6 is highly expressed in pancreatic cancer among 18 human cancer types listed (Supplementary Figure 1). Then, we confirmed by Western blot analyses high levels of GALNT6 expression in 8 of 14 pancreatic cancer cell lines (Figure 1A). We also examined expression levels of Mucin 4, a dominantly expressed Mucin protein in pancreatic cancer [3], and found that Mucin 4 protein levels are relatively higher in the pancreatic cancer cell lines with higher GALNT6 expression (Figure 1A).
Figure 1.
Expression levels and knockdown effects of GALNT6 in pancreatic cancer cells. (A) Expression levels of endogenous GALNT6 and Mucin 4 proteins in 14 pancreatic cancer cell lines were examined by Western blot analysis. (B) The expression levels of GALNT6 and MUC4 were successfully downregulated in KLM-1 and Panc02.03 cells 72 hours after transfection with si-GALNT6 compared with si-control. (C) GALNT6 and Mucin 4 protein levels were reduced in KLM-1 and Panc02.03 cells 72 hours after transfection with si-GALNT6 compared with si-control. (D) The transcriptional level of MUC4 was downregulated by GALNT6 knockdown. (E) Cell viability of KLM-1 and Panc02.03 cells was assessed by MTT assays 7 days after transfection with si-GALNT6 or si-control. Asterisks indicate the statistical significance with P value of < .001.
Knockdown Effects of Endogenous GALNT6
To investigate the biological function of GALNT6 in pancreatic cancer cells, we used siRNA to knock down GALNT6 expression using two pancreatic cancer cell lines, KLM-1 and Panc02.03, in which GALNT6 was highly expressed (Figure 1A). Knockdown of GALNT6 by siRNA successfully decreased GALNT6 expression in these two pancreatic cancer cells compared with those transfected with si-control (Figure 1B). Concordantly, Western blot analyses showed significant reduction of GALNT6 protein in KLM-1 and Panc02.03 cells (Figure 1C). Interestingly, we found that both protein level and transcriptional expression level of Mucin 4 were significantly decreased by GALNT6 knockdown (Figure 1, C and D). Furthermore, knockdown of GALNT6 significantly reduced cell viability (Figure 1E), which was verified by transfection of an additional siRNA targeting GALNT6 (Supplementary Figure 2). These results indicated that GALNT6 is likely to be essential for proliferation and/or survival of pancreatic cancer cells.
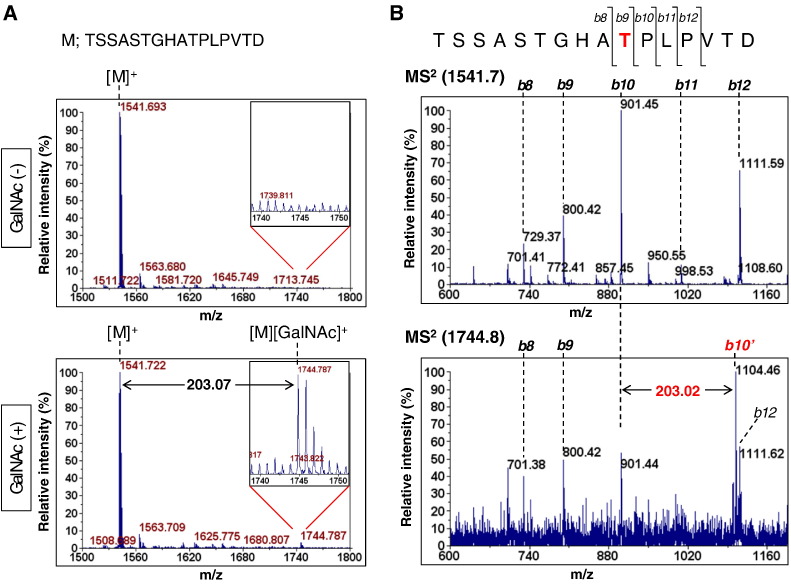
O-Glycosylation of Mucin 4 by GALNT6
Mucin 4 protein contains tandemly repeated peptides that are extensively O-glycosylated in pancreatic cancer cells [3]. Furthermore, previous reports showed that the GALNT family is directly involved in the O-glycosylation of Mucin 4 [12]. To examine whether GALNT6 can O-glycosylate Mucin 4, we performed in vitro O-glycosylation assay using recombinant GALNT6 protein and a Mucin 4 peptide (MUC4) corresponding to the core amino acids of tandemly repeated sequences (TSSASTGHATPLPVTD). As a result of MALDI-TOF mass spectrometry (MS) analysis, a molecule, which was 203.07 Da larger (m/z = 1744.79) than unmodified MUC4 peptide (m/z = 1541.72), was detected specifically in a UDP-GalNAc (+) sample (Figure 2A), indicating O-glycosylation of the MUC4 peptide by GALNT6. Furthermore, subsequent MS/MS analysis identified the shift of b10 fragment from m/z of 901.45 corresponding to an unmodified MUC4 peptide to b10’ position with m/z of 1104.46 corresponding to an O-glycosylated MUC4 peptide (Figure 2B). This result illustrated that the 10th threonine was predominantly O-glycosylated by GALNT6.
Figure 2.
O-glycosylation of Mucin 4 by GALNT6. (A) MALDI-TOF MS spectra of MUC4 peptides after O-glycosylation by GALNT6 with or without UDP-GalNAc. The upper right inserts show magnified spectra of m/z = 1740-1750. M; TSSASTGHATPLPVTD peptide. (B) MALDI-TOF-TOF MS/MS spectra of unmodified MUC4 peptide (top) and O-glycosylated one (bottom). The b10 or b10’ peak indicates [TSSASTGHAT]+ or [TSSASTGHAT][GalNAc]+ fragment ion, respectively.
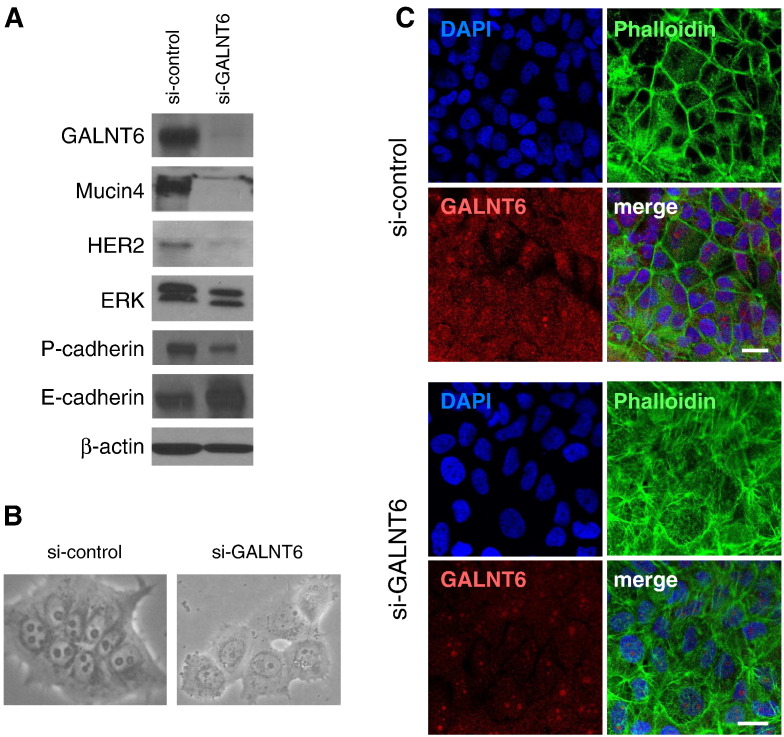
Suppression of Mucin 4 Signal Pathways by Knockdown of GALNT6
Becuase Mucin 4 protein was significantly reduced by knockdown of GALNT6, we further examined HER2 and ERK proteins, which were reported to interact with Mucin 4 and play important roles in pancreatic cancer cell proliferation and survival [13]. We observed that GALNT6 knockdown significantly affected protein levels of HER2 and ERK in KLM-1 pancreatic cancer cells (Figure 3A). We also observed drastic morphological changes in KLM-1 cell by GALNT6 knockdown compared with the cells transfected with si-control as shown in Figure 3B. Hence, we examined protein levels of the cadherin molecules and found that knockdown of GALNT6 caused the cadherin switching, decrease of P-cadherin, and increase of E-cadherin (Figure 3A). These morphologic alterations were further assessed by immunostaining with a fluorescence-labeled phalloidin which interacts with F-actin and clarified changes in the cytoskeleton structure. The staining of F-actin was restricted to cellular membrane in the si-control cells, but that of F-actin was markedly increased and dispersed in the cytoplasm of the GALNT6-knockdown cells (Figure 3C).
Figure 3.
Suppression of Mucin 4 signal pathways by GALNT6 knockdown. (A) Silencing of GALNT6 expression diminished Mucin 4 protein and its downstream molecules HER2, ERK, and P-cadherin proteins, which was accompanied by increase in E-cadherin protein level. (B) Seventy-two hours after treatment with si-control or si-GALNT6, microscopic observation was conducted to monitor cellular morphological changes. (C) Cell morphology was further investigated by immunostaining with a fluorescence-labeled phalloidin (green) at 4 days after transfection with si-control (top) or si-GALNT6 (bottom). Scale bars indicate 10 μm.
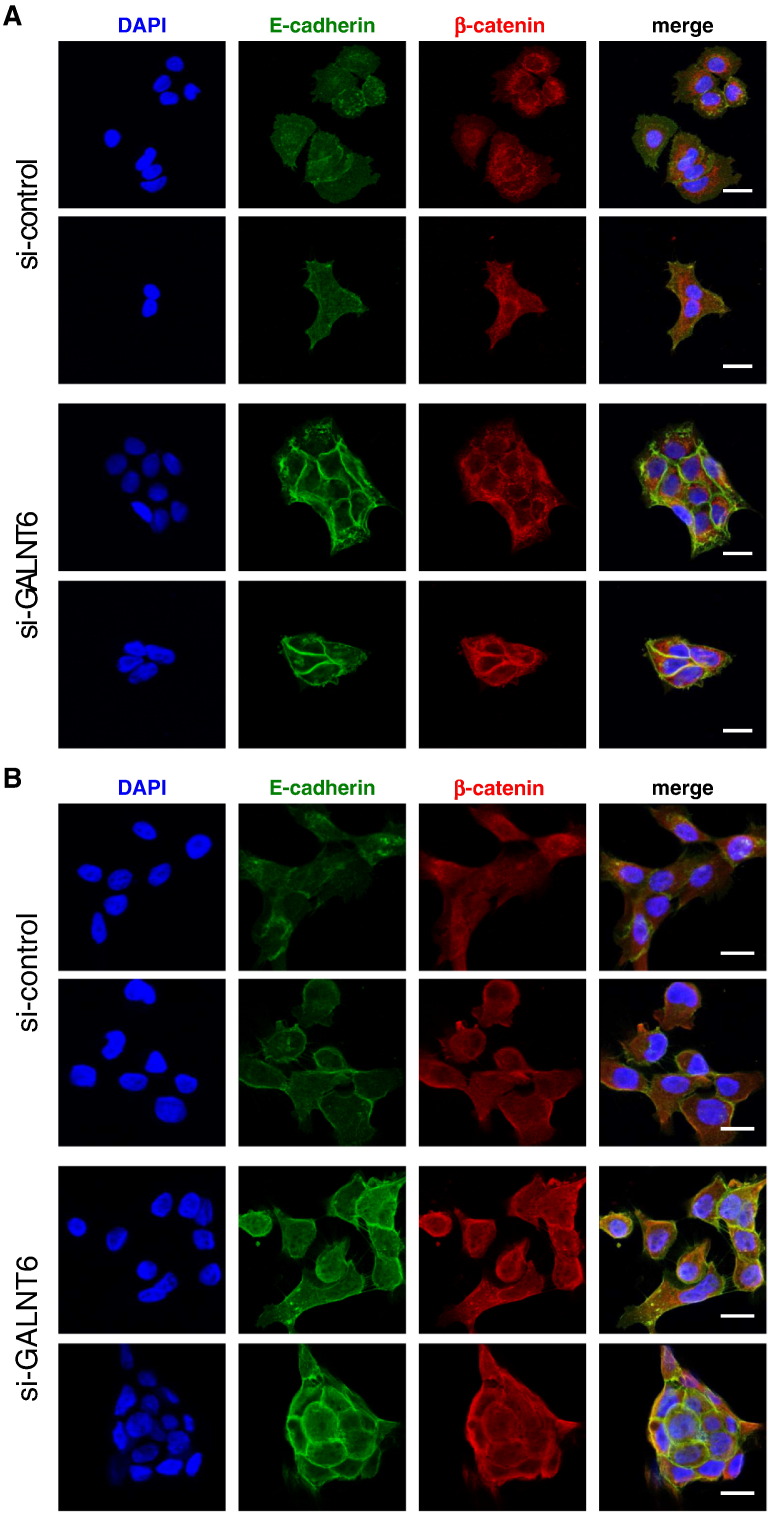
Cell Morphological Changes by GALNT6 Knockdown
We have demonstrated that knockdown of GALNT6 induced drastic changes in the cytoskeleton structure. To identify the mechanism underlying these alterations, we examined representative cell adhesion molecules, an E-cadherin/β-catenin complex by immunocytochemical staining. The knockdown of GALNT6 significantly increased staining of E-cadherin as well as β-catenin in the cell-to-cell adhesion area in KLM-1 (Figure 4A) and in Panc02.03 cells (Figure 4B). These findings were in concordant with our Western blot results from cellular fractions of cytoplasm and nucleus. Knockdown of GALNT6 increased amount of β-catenin protein, and most of the β-catenin protein was retained in the cytoplasm of KLM-1 cells (Supplementary Figure S3).
Figure 4.
Knockdown of GALNT6 induced morphological changes with increased cell adhesion complex. Immunocytochemistry of E-cadherin (green) and β-catenin (red) was conducted 72 hours after transfection with si-control (top) or si-GALNT6 (bottom) in KLM-1 cells (A) and Panc02.03 cells (B). 4′,6-Diamidino-2-phenylindole (blue) was costained to identify nucleus. Scale bars indicate 10 μm.
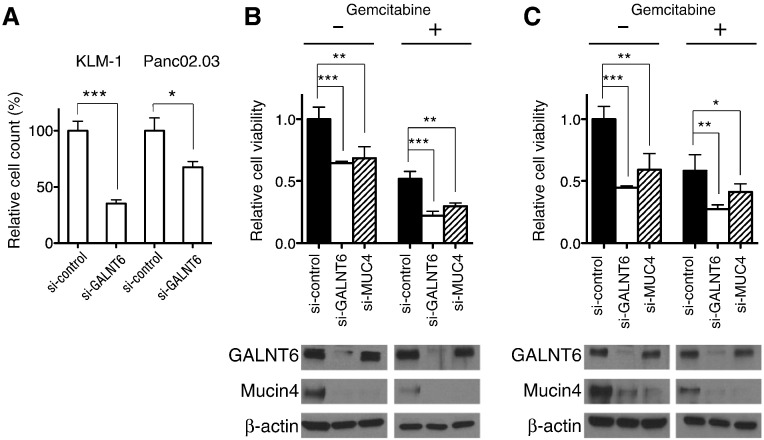
Decreased Invasion and Survival of Pancreatic Cancer Cells by GALNT6 Knockdown
Because upregulated Mucin 4 in pancreatic cancer cells was also indicated to enhance the invasiveness [13], we further examined knockdown effects of GALNT6 in the cell invasion using Transwell plates. Our results revealed that the invasive capacities of KLM-1 and Panc02.03 cells were significantly decreased by GALNT6 knockdown (Figure 5A). Because a critical role of Mucin 4 in resistance to gemcitabine through activation of HER2 and ERK pathways was indicated [14], we examined the effect of GALNT6 knockdown on the gemcitabine sensitivity of pancreatic cancer cells. We first measured the half-maximum inhibitory concentration (IC50) value for gemcitabine to be 41.5 nM for KLM-1 cells and 28.4 nM for Panc02.03 cells. Then, we treated the IC50 value of gemcitabine in the KLM-1 and Panc02.03 cell lines transfected with si-control, si-GALNT6, or si-MUC4. As expected, knockdown of either GALNT6 or MUC4 sensitized these two pancreatic cancer cell lines to gemcitabine (Figure 5, B and C). Because si-GALNT6 sensitized pancreatic cancer cells to gemcitabine more than si-MUC4, GALNT6 might have additional O-glycan substrates involved in the survival of pancreatic cancer cells.
Figure 5.
Knockdown effects of GALNT6 in invasion and survival of pancreatic cancer cells. (A) Invasive ability of pancreatic cancer cells was assessed by the Transwell plate chamber assay. (B, C) Seventy-two hours after treatment with IC50 of gemcitabine, MTT assay was performed to examine cell viability of KLM-1 (B) and Panc02.03 (C) cells that were transfected with si-control, si-GALNT6, or si-MUC4. Asterisks indicated P value of < .001 (***), < .01 (**), or < .05 (*).
Discussion
Despite all the efforts and attempts in the last few decades, the outcomes of patients with pancreatic cancer have been improved little. The overall 5-year survival rate for pancreatic cancer patients is still less than 10%, and this disease is estimated to become the second leading cause of cancer-related death in the United States by 2030 [15]. Although molecular targeted therapies have been widely developed and applied for many types of cancer in the last two decades, no effective treatment has been developed for pancreatic cancer [16]. In this study, we put our effort into identification of a novel therapeutic target that can be applicable for drug development of pancreatic cancer treatment.
Mucins are known to be highly expressed in pancreatic cancers and play essential roles in activation of many oncogenic signals, resistance to anticancer drugs, and blocking drug delivery to cancer cell. Among them, Mucin 4 was reported to be significantly overexpressed and extensively O-glycosylated in pancreatic tumor cells [6]. Mucin 4 can associate with the HER2 receptor and affect tumorigenic processes including proliferation, apoptosis, and epithelial-mesenchymal transition [17]. High expression of Mucin 4 also enhances a Src kinase pathway leading to lysosomal degradation of E-cadherin. It was reported that knockdown of Mucin 4 enhanced formation of an E-cadherin/β-catenin complex and the membrane translocation of β-catenin, resulting in the downregulation of the Wnt/β-catenin signaling pathway in pancreatic cancer cells [18]. One of the possible mechanisms leading to abundant Mucin 4 protein in pancreatic cancer is the increase of protein stability caused by extensive O-glycosylations in the backbone tandem-repeat region. Recent studies suggested that O-glycosylation is one of the important modifications in cancer cells conducted by GALNT family enzymes that are involved in several cellular functions by catalyzing O-glycan substrates. Indeed, we previously demonstrated that GALNT6 was overexpressed in breast cancer cells and stabilized Mucin1 protein through O-glycosylation [10].
In this study, we first analyzed GALNT6 and Mucin 4 expression levels in pancreatic cancer cells and found that these proteins were coexpressed in nearly half of the cell lines. Our knockdown experiments suggested that GALNT6-induced O-glycosylation might be critically important to stabilize Mucin 4 protein. We also showed that MUC4 transcription level was decreased by knockdown of GALNT6. Although the mechanism of this transcriptional downregulation of Mucin 4 is unclear, we suspect some regulatory mechanism between Mucin 4 protein and MUC4 gene expression. In fact, it was suggested that the cytosolic domain of Mucin 4 could be translocated into the nucleus and interact with some transcription factors leading to increased expression of the MUC4 gene [19]. In addition, we assessed critical roles of GALNT6 in pancreatic cancer cells in the Mucin 4 signaling pathways by investigating protein levels of HER2 and ERK and found that protein levels of these two proteins were abrogated by knockdown of GALNT6. More interestingly, knockdown of GALNT6 resulted in drastic cell morphologic changes, round shape, and enlarged cell size, like the mesenchymal to epithelial transition, accompanied by the increase in the cell adhesion molecules E-cadherin and β-catenin.
Both E-cadherin and P-cadherin interact with β-catenin and form the cell adhesion complex linked to the actin cytoskeleton structure. Normal epithelial tissues show high expression of E-cadherin, but epithelial tumor cells often lose or reduce E-cadherin expression [20]. Transition of E-cadherin to P-cadherin, also called “cadherin switching,” is often related with tissue disorder, cellular dedifferentiation, and enhanced invasiveness of cancer cells [21]. Concordantly, we previously reported that P-cadherin was significantly upregulated in majority of human pancreatic cancers and its function was involved in the increased motility of pancreatic cancer cells [22]. Our results implied that knockdown of GALNT6 decreased P-cadherin expression and accelerated the cell adhesion complex including E-cadherin and β-catenin, resulting in reduction of the invasiveness of pancreatic cancer cells.
Gemcitabine is currently used as one of the major chemotherapeutic options to treat pancreatic cancer, but its clinical effect is very limited because of rapid acquirement of drug resistance. Mucin 4 is known to be involved in the chemoresistance and prolonged survival of cancer cells, and knockdown of Mucin 4 was shown to sensitize pancreatic cancer cells to gemcitabine through suppression of mitogen-activated protein kinase, c-Jun N-terminal kinase, and nuclear factor-κB pathways [23]. In concordance with these previous findings, our GALNT6 knockdown experiments with gemcitabine treatment revealed enhancement of pancreatic cancer cell death, compared with gemcitabine alone, probably through the reduction of Mucin 4 protein.
In summary, our results implicate that GALNT6 is essential in O-glycosylation and stabilization of Mucin 4 protein. Because O-glycosylation of Mucin 4 has critical roles in pancreatic carcinogenesis, its modifying enzyme GALNT6 is an attractive molecular target for the development of novel treatment for pancreatic cancer.
Authorship contributions
Y. N. planned and supervised the entire project; J. P. made the study design and interpreted results; Y. E. T. designed all experiments and performed data analysis; T. K. supported real-time PCR experiments; M. J. supported cell culture and sample preparation; Y. H. and K. U. performed MS analysis. Y. E. T., J. P., and Y. N. wrote this article.
Disclosure of Potential Conflicts of Interest
Y. N. is a stockholder and a scientific advisor of OncoTherapy Science, Inc. J. P. is a scientific advisor of OncoTherapy Science, Inc.
Acknowledgements
We thank Dr. Vytas Bindokas and Microscopy Core Facility of the University of Chicago for technical support. This study was supported partly by the funding from OncoTherapy Science, Inc.
Footnotes
This study was supported partly by the funding from OncoTherapy Science, Inc.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2016.03.005.
Appendix A. Supplementary data
Supplementary Figure 1. Expression of GALNT6 in pancreatic cancer. (A) Oncomine database shows high expression of GALNT6 in pancreatic cancer (arrow) compared with other types of cancer. (B) Oncomine database shows high expression of GALNT6 in pancreatic cancers (arrow) compared with normal pancreas.
Supplementary Figure 2. Knockdown effects of GALNT6 in pancreatic cancer cells. Expression level of GALNT6 protein and cell viability were assessed in KLM-1 and Panc02.03 cells after transfection with si-control or si-GALNT6-2. Asterisks indicate P value of < .001.
Supplementary Figure 3. Western blot analysis using cytoplasm and nucleus fractions. Expression of endogenous proteins was examined after fractionation of cytoplasmic and nuclear components from KLM-1 pancreatic cancer cells. Histone H3 and Tubulin were used as loading controls.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10(10):607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9(12):874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westgaard A, Schjølberg AR, Cvancarova M, Eide TJ, Clausen OP, Gladhaug IP. Differentiation markers in pancreatic head adenocarcinomas: MUC1 and MUC4 expression indicates poor prognosis in pancreatobiliary differentiated tumours. Histopathology. 2009;54(3):337–347. doi: 10.1111/j.1365-2559.2009.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, Mandel U, Clausen H, Yu F, Hollingsworth MA. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin Cancer Res. 2013;19(8):1981–1993. doi: 10.1158/1078-0432.CCR-12-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14(4–5):525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 8.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7(6):599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patani N, Jiang W, Mokbel K. Prognostic utility of glycosyltransferase expression in breast cancer. Cancer Genomics Proteomics. 2008;5(6):333–340. [PubMed] [Google Scholar]
- 10.Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, Hirata K, Nakamura Y, Katagiri T. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70(7):2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 11.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanisch FG, Reis CA, Clausen H, Paulsen H. Evidence for glycosylation-dependent activities of polypeptide N-acetylgalactosaminyltransferases rGalNAc-T2 and -T4 on mucin glycopeptides. Glycobiology. 2011;11(9):731–740. doi: 10.1093/glycob/11.9.731. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68(7):2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101(7):1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 16.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 17.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29(20):2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhi X, Tao J, Xie K, Zhu Y, Li Z, Tang J, Wang W, Xu H, Zhang J, Xu Z. MUC4-induced nuclear translocation of β-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346(1):104–113. doi: 10.1016/j.canlet.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Cullen PJ. Post-translational regulation of signaling mucins. Curr Opin Struct Biol. 2011;21(5):590–596. doi: 10.1016/j.sbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1(6):a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paredes J, Figueiredo J, Albergaria A, Oliveira P, Carvalho J, Ribeiro AS, Caldeira J, Costa AM, Simões-Correia J, Oliveira MJ. Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim Biophys Acta. 2012;1826(2):297–311. doi: 10.1016/j.bbcan.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65(8):3092–3099. doi: 10.1158/0008.5472.CAN-04-3646. [DOI] [PubMed] [Google Scholar]
- 23.Skrypek N, Duchêne B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the concentrative nucleoside transporter family. Oncogene. 2013;32(13):1714–1723. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Expression of GALNT6 in pancreatic cancer. (A) Oncomine database shows high expression of GALNT6 in pancreatic cancer (arrow) compared with other types of cancer. (B) Oncomine database shows high expression of GALNT6 in pancreatic cancers (arrow) compared with normal pancreas.
Supplementary Figure 2. Knockdown effects of GALNT6 in pancreatic cancer cells. Expression level of GALNT6 protein and cell viability were assessed in KLM-1 and Panc02.03 cells after transfection with si-control or si-GALNT6-2. Asterisks indicate P value of < .001.
Supplementary Figure 3. Western blot analysis using cytoplasm and nucleus fractions. Expression of endogenous proteins was examined after fractionation of cytoplasmic and nuclear components from KLM-1 pancreatic cancer cells. Histone H3 and Tubulin were used as loading controls.