Abstract
Salmonella infection in humans can become chronic, which leads to low-grade persistent inflammation. These chronic infections increase the risk of several gastrointestinal diseases, including cancer. Salmonella AvrA is a multifunctional protein that influences eukaryotic cell pathways by regulating ubiquitination and acetylation. In an animal model, we have demonstrated that infection with AvrA-expressing Salmonella induces beta-catenin signals and enhances colonic tumorigenesis. Beta-catenin signaling is a key player in intestinal proliferation and tumorigenesis. The relative contributions of AvrA-induced proliferation and inflammation on tumorigenesis, however, are unknown. STAT3 is activated in chronically inflamed intestines in human inflammatory bowel diseases and in colitis-associated colon cancer. In the current study, mice were colonized with Salmonella AvrA-sufficient or AvrA-deficient bacterial strains. Then, inflammation-associated colon cancer was induced through the use of azoxymethane/dextran sulfate sodium. We determined that AvrA-expressing bacteria activated the STAT3 pathway, which is predicted to enhance proliferation and promote tumorigenesis. Transcriptional activity of STAT3 and its target genes were upregulated by Salmonella expressing AvrA, thus promoting proliferation and intestinal tumorigenesis. Our findings provide new insights regarding a STAT3-dependent mechanism by which the specific bacterial product AvrA enhances the development of infection-associated colon cancer. These insights might suggest future biomarkers to risk assessment and early detection of infection-related cancer.
Introduction
Accumulating evidence suggests that chronic infection and the ensuing inflammation contribute to tumor initiation and tumor progression [1], [2]. Several studies have implicated colonic bacteria in the pathogenesis of colon cancer. A recent study in mice showed that adenomas cause barrier defects in the colonic epithelium allowing microbial products to drive interleukin (IL)-23/IL-17–mediated tumor growth [3]. Another study demonstrated that a human colonic commensal bacterium promoted tumorigenesis via activation of T-helper type 17 T-cell responses [4]. Colitis was shown to promote tumorigenesis by altering microbial composition and inducing the expansion of microorganisms with genotoxic capabilities [5]. Specifically, deletion of the polypetide synthase genotoxin from Escherichia coli NC101 decreased tumor load and tumor invasion in azoxymethane (AOM)-treated IL-10 knockout mice. These studies have highlighted the essential roles of bacteria and/or their products in colonic tumorigenesis.
Salmonella infection in humans can become chronic, which leads to low-grade persistent inflammation [2]. These chronic infections increase the risk of several gastrointestinal [6] diseases, including chronic cholecystitis and gallbladder cancer [7], [8]. Recently, Kato et al. reported that antibody against Salmonella flagellin was higher in colorectal cancer and precancer cases than controls in two distinct populations in the United States and the Netherlands and that dietary intake is the one of the mediating factors, suggesting a potential link of Salmonella to colorectal cancer [9]. In animal models, Salmonella and its derivatives have been observed invading transformed tissue more efficiently than normal tissue [10], [11]. Salmonella AvrA is a multifunctional protein that influences eukaryotic cell pathways by altering ubiquitination and acetylation of target proteins [12], [13], [14], [15], [16], [17], [18]. These posttranslational modifications modulate protein functions that regulate inflammation, epithelial apoptosis, and proliferation [12], [13], [14], [15], [16], [17], [18]. We recently reported that AvrA acts as a deubiquitinase to stabilize β-catenin. By suppressing β-catenin degradation, AvrA enhances intestinal epithelial proliferation [17] and promotes tumorigenesis [19].
Chronic infections activate multiple signaling pathways which contribute to inflammatory responses that promote tumor development [20]. In fact, epidemiologic studies and randomized clinical trials have provided strong evidence that use of aspirin and nonsteroidal anti-inflammatory drugs reduces the risk of colorectal cancer [21], [22]. In prior studies, we showed that AvrA activates β-catenin signaling, which is a key signal transduction event in intestinal proliferation and tumorigenesis [19]. However, the relative contributions of AvrA to proliferation and inflammation have not been dissected. STAT3 is known to be activated in chronic inflamed intestine in human inflammatory bowel diseases [23] and colitis-associated colon cancer [24]. At early stages of infection, STAT3 activation promoted inflammation, whereas at later stages, sustained STAT3 activation suppressed inflammatory responses and promoted proliferation [24]. Hyperproliferation alone, however, is insufficient to cause cancer, which requires both the initiation of mutagenic events that trigger cellular transformation as well as an activated microenvironment that supplies growth factors to support cellular survival, proliferation, and angiogenesis. Because AvrA promotes tumorigenesis and STAT3 is activated in tumor development, we speculated that AvrA might activate STAT3 in colonic tumorigenesis.
In the current study, we aimed to assess the role of bacterial protein AvrA in activating STAT3 in colon cancer development. We hypothesized that the bacterial effector AvrA would activate the STAT3 pathway to promote inflammation-associated colonic tumorigenesis. In prior studies, we established a mouse colon cancer model possessing Salmonella AvrA+ or AvrA− infection in the gastrointestinal tract [25]. We initiated colonic epithelial cell mutations with AOM and accelerated tumorigenesis with dextran sulfate sodium (DSS). To determine the effects of AvrA, we infected mice with bacterial strains expressing AvrA (parental PhoPc), AvrA-deficient strains (AvrA−), or PhoPc AvrA− strains complemented with a plasmid encoding AvrA (PhoPc AvrA−/AvrA+), as we previously described [15], [16], [17], [26], [27]. PhoP-PhoQ is a two-component regulatory system that controls the expression of over 40 genes essential for Salmonella typhimurium virulence and survival within macrophages. PhoPc is a PhoP-PhoQ constitutive mutation that increases the expression of PhoP-activated genes and represses the synthesis of 20 proteins encoded by PhoP-repressed genes [28]. We found that AvrA expression induced STAT3 activation and contributed to systemic and colonic inflammation in infected mice. Our findings provide important mechanistic insights into how a bacterial protein targets colonic epithelial cells and contributes to the development of colon cancer.
Results
Local Cytokines Accumulate in the AvrA-Expressed Tumor
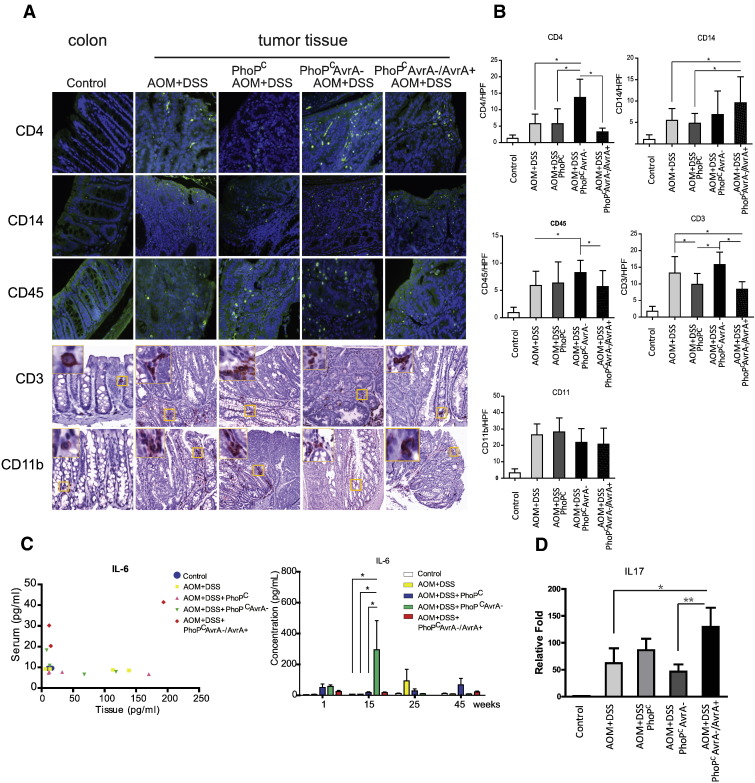
Our previous study showed the pathological changes associated with chronic Salmonella infection and differences in tumor development (adenoma versus carcinoma) and between AvrA − and AvrA + AOM/DSS experimental groups [19]. Many cytokines and chemokines are produced by activated stromata and immune cells during chronic inflammation in tumor development. These cytokines accumulate locally and can be detected in the tissue [29]. We assessed several lymphocyte markers in normal colon and colonic tumors (Figure 1A). Levels of CD4, CD14, CD45, and CD3 significantly increased in tumors. AvrA expression appeared to cause differential effects on these markers but had no influence on CD11 (Figure 1B). These data indicate that the local inflammation in the infected colon was associated with the AvrA expression level in Salmonella.
Figure 1.
Inflammatory cytokines in chronically Salmonella-infected colon in mice. (A) Immunocyte infiltration in control and infected colon cancer tissue by immunohistochemistry/immunofluorescence. (B) Quantitation of CD3-, CD4-, CD14-, CD45-, and CD11b-positive cells in tumors or control mucosa/high-power field. *P < .05. (C) Serum IL-6 versus tissue levels of IL-6 45 weeks postinfection. n = 4 mice/group. Serum IL-6 at indicated time points. (D) IL17 mRNA level was increased in the AvrA+-infected colon group. n = 4 mice/group.
The cancer microenvironment, reflecting a “wound that does not heal,” recruits these populations of lymphocytes and macrophage cells that are believed to contribute to cellular inflammation and tumor promotion. Along with tumor necrosis factor (TNF-α), IL-6 is one of the most commonly overexpressed cytokines in tumors. We compared the IL-6 levels from these groups in both serum and colonic mucosa (Figure 1C). Among the groups, we found that Salmonella expressing PhoPc AvrA−/AvrA+ induced the highest IL-6 serum levels 45 weeks postinfection (Figure 1C, right panel). We observed significant differences in IL-6 exclusively at 15 weeks postinfection, whereas there were no significant differences at 1, 25, and 45 weeks postinfection. Recently, IL-6 has been shown to play a key role in maintaining the balance between the regulatory subclass of T cells and Th17, effector T cells that produce IL-22, IL-17, IL-6, TNFα, and proinflammatory chemokines [30]. We then tested the IL-17 mRNA from the colonic mucosa. IL-17 expression was significantly increased in the AvrA + infected group (Figure 1D).
The STAT3 Pathway is Activated in the Salmonella Chronically Infected Colon
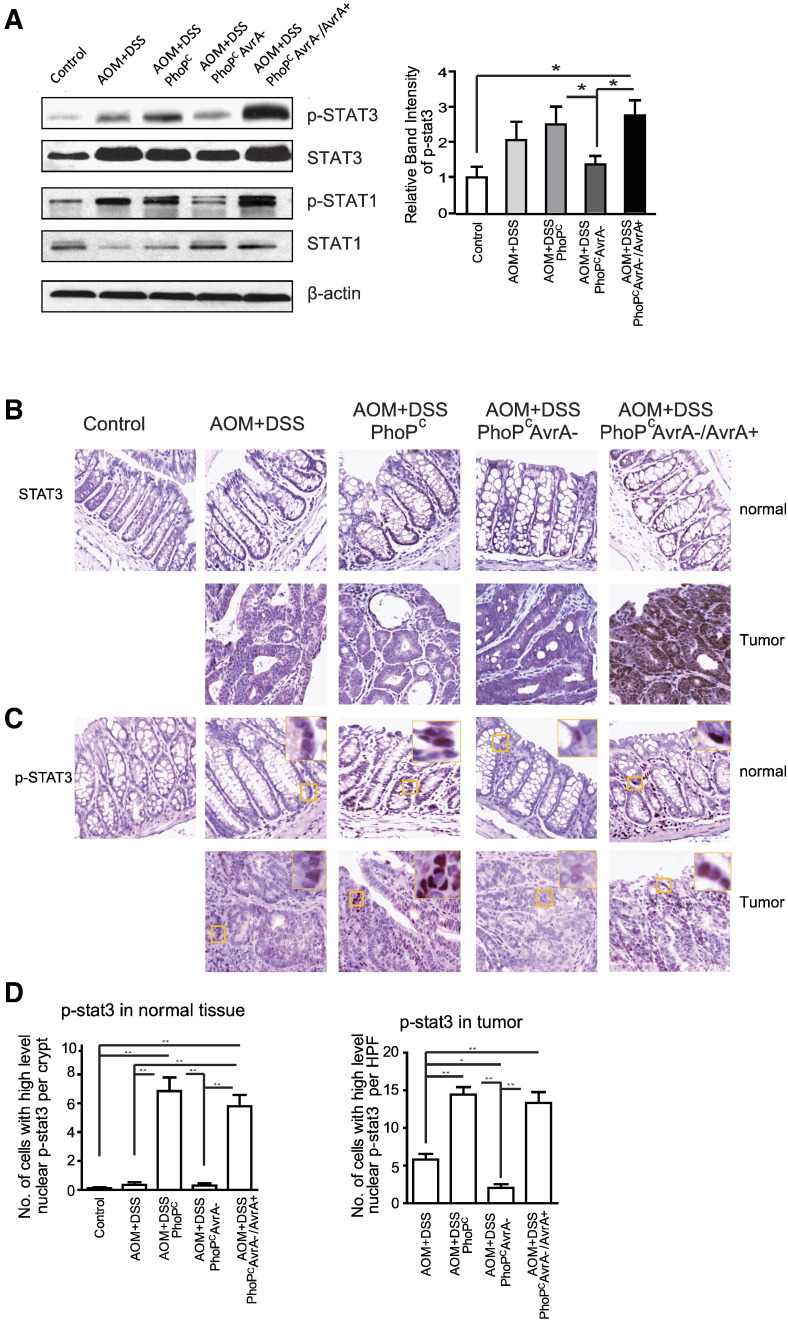
STAT proteins, especially STAT3, are crucial for both the extrinsic and the intrinsic pathways underlying cancer inflammation. IL-6 signals are mediated in part by activation of STAT3, a transcription factor important in cancer development. Based on elevated cytokine profiles in tissue and serum from infected mice, we presumed that the STAT pathway might be activated by infection, which promotes epithelial cancer. We confirmed this hypothesis by Western blotting that showed consistently enhanced phosphorylated STAT3 (p-STAT3) and total STAT3 in colonocytes from PhoPCAvrA −/AvrA + infected mice (Figure 2A). There was no consistent change in p-STAT1 in the infected mice (Figure 2A). We then examined the distribution of STAT3 and p-STAT3 in colon from control and infected mice (Figure 2B). We found significantly higher level of p-STAT3 in tumors from mice infected with AvrA-expressing bacteria compared with those in uninfected mice (Figure 3C). The quantitation of pSTAT3 was shown in Figure 3D. Nuclear pSTAT3 was increased in the “normal” colon tissue and tumors from mice infected with Salmonella expressing AvrA.
Figure 2.
AvrA modulates STAT3 activation in AOM/DSS colonic tumors. (A, left panel) Representative Western blots of STAT1/3 and p-STAT1/3 in control colons and tumors. (Right panel) Quantitation of nuclear pSAT3. (B and C) Immunostaining of p-STAT3 and STAT3 in control colons and tumors from indicated groups. (D) Quantitation of nuclear p-STAT3 in normal and tumor tissue in the indicated groups. Data are means ± SD. n = 5 mice/group. *P < .05, **P < .01.
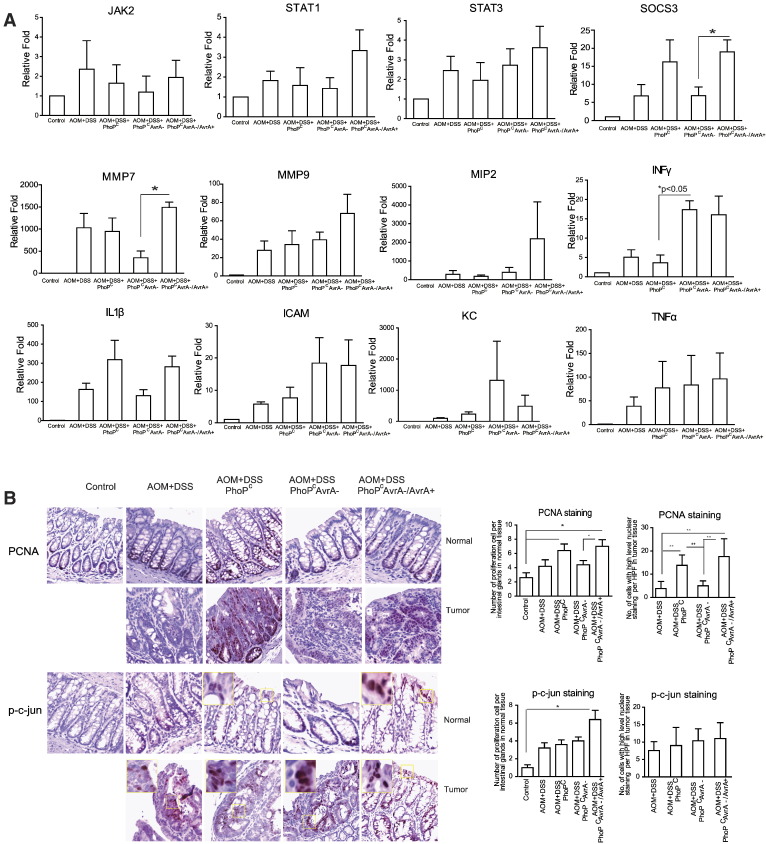
Figure 3.
AvrA modulates target genes of the STAT3 pathway in AOM/DSS colonic tumors. (A) mRNA expression of target genes of the STAT3 pathway in the AOM/DSS colon post–Salmonella infection. The total RNA from intestinal mucosa was extracted for a real-time PCR 45 weeks postinfection. Transcripts were assayed in triplicate. Data are means ± SD of n= 5 mice in each group. (B) The effect of AvrA on intestinal proliferation post–Salmonella infection. PCNA and p-c-Jun staining in colon 45 weeks postinfection.
AvrA Activates the Target Genes of STAT3 Signaling Pathway in the Salmonella Infected Intestine
To assess the biological relevance of STAT3 signaling, we examined the transcript levels of STAT. We did not find any changes of JAK2, STAT1, and STAT3 at the mRNA level associated with AvrA in tumors and adjacent tissue by real-time polymerase chain reaction (PCR) (Figure 3A). The STAT3 target genes, MMP7 and SOC3, were significantly increased, which appeared to correlate with AvrA expression (Figure 3A). The inflammatory cytokine INFγ was significantly enhanced in intestine infected with the AvrA-deficient Salmonella, whereas mRNA expression levels of MIP, IL-1β, ICAM, KC, and TNF-α did not appear to correlate with AvrA expression in Salmonella (Figure 3A).
Activation of STAT3 Signaling Contributes to Proliferation
In normal-appearing tissue close to tumors and in tumors, we found that the proliferation marker PCNA was significantly increased in colon infected with AvrA-expressing bacteria compared with uninfected mice (Figure 3B). Interestingly, we also found that STAT3 activation was associated with phosphoactive c-Jun redistribution in bacterially infected colons. Phosphoactive c-Jun was detected on colonic crypt surfaces in mice infected with AvrA-expressing bacteria (Figure 3B).
AvrA Activates the STAT-3 Signaling Pathway in the Salmonella-Infected Colon Cancer Cells
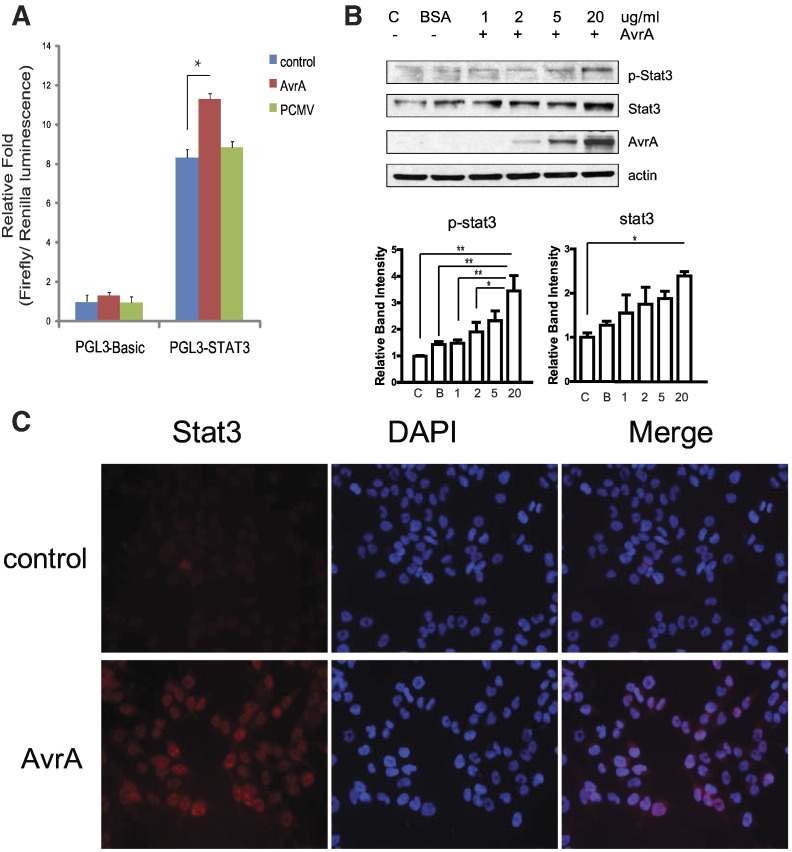
To directly test the ability of AvrA to activate STAT3, we examined colon cancer cells in cell cultures. We transfected human colonic epithelial HCT116 cells with an AvrA plasmid and a lucifierase reporter plasmid in vitro. We demonstrated that AvrA expression significantly increased the STAT-3 transcriptional activity as assessed by a STAT-3 promoter regulated-luciferase reporter (Figure 4A). Moreover, purified AvrA protein induced total STAT3 protein and p-STAT3 in a dose-dependent manner in human epithelial cells (Figure 4B). By Immunostaining, we detected the nuclear STAT-3 induced by AvrA treatment (Figure 4C, red staining for STAT3). Taken together, our in vitro studies indicate that AvrA activates the STAT3 signal pathway in Salmonella-infected colonocytes.
Figure 4.
Bacterial AvrA protein activates STAT3 signaling. (A) AvrA activates STAT3 promoters in HCT116 cells. HCT116 cells were transiently co-transfected with a STAT3 reporter pGL3-STAT3 and a p-CMVAvrA, or pGL3-empty vector and p-CMVAvrA using a lipofectin reagent. Each experiment was assayed in triplicate. * P < .05. (B) AvrA protein increases STAT3 and p-STAT3 in the HCT116 cells. Cells were treated with purified AvrA protein for 30 minutes. Cell lysates were collected for the Western blots. (C) HCT116 cells were treated with purified AvrA protein (20 μg/ml) for 1 hour. Immunofluorescence was performed using an anti-Stat3 antibody.
Discussion
In this study, we investigated the effects and mechanism of a chronic infection with S. typhimurium in an AOM/DSS colon cancer model. We discovered that Salmonella infection altered the distribution of multiple immune cell populations. The bacterial protein AvrA activated the STAT3 pathway and increased PCNA, a marker of proliferation in the colon. This novel study provides critical insights regarding the mechanisms of tumor promotion by AvrA involving colonic inflammation and proliferation. It is interesting to note that higher IL-6 levels in prediagnostic plasma samples have been associated with increased risk of subsequent colorectal cancer in both women and men mediated partly through obesity [6], [31]. Chronic STAT3 induces inflammation through increasing IL-6 and, in turn, can be induced by inflammation.
Cancer is defined by six hallmarks, namely, uncontrolled growth, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Increasing evidence suggests that inflammation should be included as a seventh feature on this list [2]. Many cytokines, angiogenic factors, and chemokines are produced by activated stromata and immune cells which accumulate during chronic inflammation [1]. These factors exert profound survival and growth effects on resident (neoplastic) epithelial cells, endothelial and stromal cells, as well as recruiting immune cells. Our data indicate that Salmonella AvrA upregulates IL-6, which in turn activates STAT3 and enhances proliferation. Solid tumors are commonly infiltrated with immune cells that provide a milieu for inflammation, which promotes tumor growth. We also found that profiles of colonic mucosal and serum cytokines, although altered by infection, are not closely correlated. It is known that STAT3 has a dual and self-perpetuating role in inflammation. At early stages of infection, STAT3 activation promoted inflammation; in contrast, at later stages, sustained STAT3 activation suppressed inflammation and promoted proliferation [24]. Our data for the changes of cytokines at different time courses postinfection (Figure 1 and data not shown) reflect the different roles of STAT3 in the progression of colon cancer. At early stages of infection (1-15 weeks) in the AOM/DSS mice, STAT3 activation may promote inflammation, whereas at later stages (45 weeks), sustained STAT3 activation suppressed inflammatory responses and promoted proliferation. As activators of transcription, the STATs upregulates the expression of genes, thus being involved in all steps of tumor development, including the induction and maintenance of an inflammatory microenvironment, as well as the malignant transformation and progression due to the maintenance of a proinflammatory state. Our data have shown that bacterial AvrA was able to regulate STAT3 activation and function in the intestine.
NF-κB activation accompanies STAT3 activation in epithelial tumorigenesis [32]. In the setting of acute Salmonella infections in mice, AvrA exerts an anti-inflammatory effect as it can suppress the NF-κB responses in mice. In colonic tumorigenesis, however, AvrA promotes tumor growth, and we have found that NF-κB is not persistently activated. Whether chronic infection with AvrA-expressing bacteria contributes to suppressing NF-κB despite promoting tumorigenesis will require further study.
A study revealed that myeloid differentiation primary response gene 88 (MyD88) mediates microbe-induced TLR signaling and regulates Myc oncogene expression and related IEC tumor growth in a susceptible host [33]. We did not see MYD88 protein differences, indicating that the effects of AvrA were not mediated by changes in MYD88 expression (data not shown). MyD88-independent activation of ERK by epidermal growth factor increased p-ERK and c-Myc and restored the multiple intestinal neoplasia (Min) phenotype in Apc(min/+)/Myd88(−/−) mice. However, we did not find that AvrA modulated activation of ERK in the infected mice (data not shown). We did not examine Salmonella-infected Apc mutant min mice because these mice have constitutive β-catenin activation arising from Apc loss.
Wnt/β-catenin and STAT3 are known to cross-talk to maintain murine embryonic stem cells in an undifferentiated state [34]. STAT3 promoter possesses a β-catenin/Tcf cis-acting element in human cells and is upregulated by Wnt signaling in esophageal cancers [35]. Conversely, STAT3 binds β-catenin promoters and activates β-catenin transcription in human breast cancer cells [36]. We have found that AvrA induces both β-catenin and STAT3 signals, which could be through deubiquitination [17]. But further studies will be needed to determine the more proximal target in vivo.
In summary, this study reveals a novel mechanism linking bacterial AvrA protein to host oncogenic signals that promote colon cancer development (Figure 5). Salmonella effector AvrA persistently upregulates the STAT-3 signaling pathway. We further identified several STAT3 target genes that were upregulated by Salmonella expressing AvrA, predicted to promote proliferation and intestinal tumorigenesis (Figure 5). Our previous study showed the role of β-catenin in chronic Salmonella infection and differences between AvrA − and AvrA + AOM/DSS experimental groups in tumor development [19]. β-catenin–STAT3 may co-regulate one another in a positive feedback loop (Figure 5). Thus, our results bring us one step closer to unraveling infection-related dysregulation of host signaling in cancer. Signaling pathways, which connect infection, inflammation, and cancer, moreover, have emerged as attractive targets for prevention and therapy. These insights might suggest future biomarkers to risk assessment and early detection of infection-related cancer.
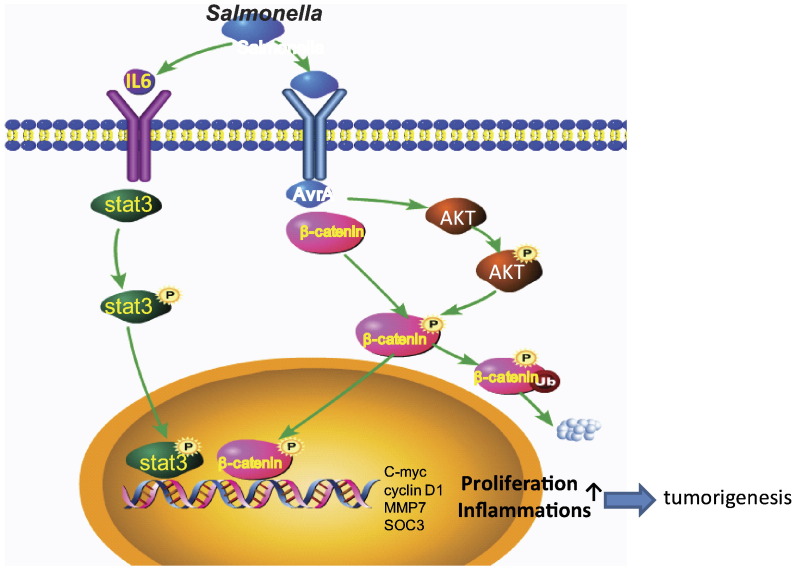
Figure 5.
The working model of STAT3 and AvrA interaction in chronic intestinal Salmonella infected in colon cancer. Salmonella effector AvrA is secreted through the Salmonella Type Three Secretion System and persistently upregulates the STAT-3 signaling pathway. Several STAT3 target genes were upregulated by Salmonella expressing AvrA. β-catenin–STAT3 may co-regulate one another in a positive feedback loop, thus promoting proliferation and intestinal tumorigenesis.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of Rochester University Committee on Animal Resources committee (UCAR 2007-065). All efforts were made to minimize suffering.
Bacterial Strains and Growth Condition
Bacterial strains used in this study included Salmonella mutant strains PhoPc [28], PhoPcAvrA−, and PhoPcAvrA−/AvrA+. Nonagitated microaerophilic bacterial cultures were prepared by inoculating 10 ml of Luria-Bertani broth with 0.01 ml of stationary-phase culture followed by overnight incubation (~ 18 hours) at 37°C, as previously described [16].
Salmonella-Infected Colon Cancer Mouse Model
Animal experiments were performed by using specific pathogen-free female C57BL/6 mice (Taconic) that were 6 to 7 weeks old, as previously described [27]. Water and food were withdrawn 4 hours before oral gavage with 7.5 mg/mouse of streptomycin (100 μl of sterile solution). Afterward, animals were supplied with water and food ad libitum. Twenty hours after streptomycin treatment, water and food were withdrawn again for 4 hours before the mice were infected with 1 × 106 CFU of S. typhimurium (100-μl suspension in Hank’s balanced salt solution) or treated with sterile Hank’s balanced salt solution (control) by oral gavage, as previously described [25]. After Salmonella gavage, the mice were injected with 10 mg/kg body weight of AOM (Sigma-Aldrich) by intraperitoneal injection as described by Greten and colleagues [37]. After a 7-day recovery period, the animals were started on the first of four cycles of 1% DSS ad libitum [19]. Sixteen week later, mice received second AOM injection and another three cycles of 1% DSS in the drinking water. At 1, 15, 25, 35, and 45 weeks after Salmonella gavage, tissue samples were collected. For tumor enumeration and tumor dimensions, investigators were blinded to treatment conditions and used a stereodissecting microscope. Severity of inflammation and dysplasia were scored by a gastrointestinal pathologist (Z. Z.) blinded to treatment conditions using (hematoxylin and eosin) stained microscopic sections [38] from colons prepared as Swiss rolls. Histologic inflammatory scores were determined using a previously validated scoring system [39]. Histologic assessment of intestinal tumors in rodents was performed following recommended guidelines [40] as recently reported by our laboratory [19].
Histological Testing
Tissues were fixed in 10% neutral buffered formaldehyde for 4 to 12 hours, transferred into 70% ethanol, and processed by standard techniques. Sections (5 μm) were stained with hematoxylin and eosin. For immunostaining, antigens were retrieved by boiling for 10 minutes in 10 mM citrate (pH 6.0). Immunohistochemistry was done as previously described [17], [41]. The slides were stained with anti–pho-c-Jun, anti–pho-stat3, anti-stat3 (Cell Signal, Beverly, MA), anti-PCNA, anti-CD3, and anti-CD11b (Santa Cruz Biotechnology Inc., CA) antibodies.
Immunoblotting
Mouse intestinal mucosa, including most proximal and distal regions, was collected by scraping as previously described [27]. Mouse epithelial cells were broken in a lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA pH 8.0, 0.2 mM sodium orthovanadate, and protease inhibitor cocktail) (Roche, Nutley, NJ). Immunoblotting was performed with primary antibodies: anti–phospho-Stat1, anti–phospho-Stat3, anti–phospho-Jak2, anti-Stat1, anti-Stat3, and anti-Jak2 (Cell Signal, Beverly, MA), or anti–beta-actin (Sigma-Aldrich, Milwaukee, WI) antibodies and secondary antibodies visualized by ECL [42].
Immunofluorescence
Intestinal tissues were freshly isolated and embedded in paraffin wax after fixation with a 10% neutral buffered formalin. Immunohistochemistry was performed on paraffin-embedded sections (4 μm) of mouse colons. After preparation of the slides as described previously [17], slides were incubated in 3% hydrogen peroxide for 20 minutes at room temperature to block endogenous peroxidase activity, followed by incubation for 20 minutes in 5% BSA with 0.1% saponin in PBS to reduce nonspecific background. The slides were stained with primary antibodies to CD4, CD14, or CD45 (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Specimens were examined with a Leica SP5 Laser Scanning confocal microscope.
Real-Time Quantitative PCR Analysis
The total RNA from intestinal mucosa was extracted for a real-time PCR 45 weeks postinfection using TRIzol reagent (Invitrogen, Grand Island, NY). The RNA integrity was verified by electrophoresis. RNA reverse transcription was performed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. The RT cDNA reaction products were subjected to quantitative real-time PCR using CTFX 96 Real-Time System (Bio-Rad, Hercules, CA) and SYBR Green Supermix (Bio-Rad) according to the manufacturer's protocol. All expression levels were normalized to β-actin levels of the same sample. Percent expression was calculated as the ratio of the normalized value of each sample to that of the corresponding untreated control cells. All real-time PCRs were performed in triplicate. Optimal primer sequences were designed using Primer-BLAST or were obtained from Primer Bank primer pairs listed in Table 1.
Table 1.
Primers for real-time PCR.
| Name | Sequence (5' −> 3') | |
|---|---|---|
| Mmp7 | Forward | CTGCCACTGTCCCAGGAAG |
| Reverse | GGGAGAGTTTTCCAGTCATGG | |
| mmp9 | Forward | CTGGACAGCCAGACACTAAAG |
| Reverse | CTCGCGGCAAGTCTTCAGAG | |
| TNF alpha | Forward | CCCTCACACTCAGATCATCTTCT |
| Reverse | GCTACGACGTGGGCTACAG | |
| KC(CXCL1) | Forward | CTGGGATTCACCTCAAGAACATC |
| Reverse | CAGGGTCAAGGCAAGCCTC | |
| IL17 | Forward | AGCACACCCGTCTTCTCTC |
| Reverse | GCTGGAGTTCGCACTGTCC | |
| MIP2 | Forward | GAACAAAGGCAAGGCTAACTGA |
| Reverse | AACATAACAACATCTGGGCAAT | |
| ICAM | Forward | TGTGCTTTGAGAACTGTGGCA |
| Reverse | TGGCGGCTCAGTATCTCCTC | |
| IL 1β | Forward | GCAACTGTTCCTGAACTCAACT |
| Reverse | ATCTTTTGGGGTCCGTCAACT | |
| JAK2 | Forward | GGAATGGCCTGCCTTACAATG |
| Reverse | TGGCTCTATCTGCTTCACAGAAT | |
| STAT1 | Forward | TCACAGTGGTTCGAGCTTCAG |
| Reverse | CGAGACATCATAGGCAGCGTG | |
| STAT3 | Forward | CACCTTGGATTGAGAGTCAAGAC |
| Reverse | AGGAATCGGCTATATTGCTGGT | |
| Socs3 | Forward | TGCGCCTCAAGACCTTCAG |
| Reverse | GCTCCAGTAGAATCCGCTCTC | |
| Actin | Forward | TGTTACCAACTGGGACGACA |
| Reverse | CTGGGTCATCTTTTCACGGT |
Transfection and Luciferase Assay for STAT3 Transcriptional Activity
HCT1116 human colon cancer cells were transiently co-transfected with pGL3-STAT3&pCMV-AvrA or pGL3-Basic&pCMV-AvrA using lipofectin reagent according to the manufacturer’s instructions (Invitrogen, CA). PRL-TK vector was used as an internal control reporter. Luciferase activity was monitored using the dual luciferase assay system (Promega, Madison, WI). Experiments were done in triplicate with three repeats.
Statistical Analysis
Data are expressed as mean ± SD. All statistical tests were two-sided. P values of less than .05 were considered to be statistically significant. For cecum data, four areas of mouse body were checked to obtain the average infection score. Then, multiple comparisons of mean infection score were performed using analysis of variance. Following analysis of overall group differences on the mean scores at P ≤ .05, alpha levels were adjusted using the Tukey correction for multiple comparisons. As the study involved a longitudinal design, the generalized estimating equations were utilized to provide statistical inference [43]. The analysis was conducted using the Genmod procedure in SAS. The overall treatment effects were modeled using the time by treatment interaction term through intake (week 0) to week 45 [25]. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Acknowledgement
We thank Kendy Li for helping with proofreading. This work was supported by the American Cancer Society (RSG-09-075-01-MBC).
Footnotes
Conflicts of Interest: The authors have no conflicts of Interest.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17–mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer. 2013;108:1891–1898. doi: 10.1038/bjc.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol. 2006;93:633–639. doi: 10.1002/jso.20530. [DOI] [PubMed] [Google Scholar]
- 9.Kato I, Boleij A, Kortman GA, Roelofs R, Djuric Z, Severson RK, Tjalsma H. Partial associations of dietary iron, smoking and intestinal bacteria with colorectal cancer risk. Nutr Cancer. 2013;65:169–177. doi: 10.1080/01635581.2013.748922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrach N, Zhao M, Porwollik S, Hoffman RM, McClelland M. Salmonella promoters preferentially activated inside tumors. Cancer Res. 2008;68:4827–4832. doi: 10.1158/0008-5472.CAN-08-0552. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 13.Du F, Galan JE. Selective inhibition of type III secretion activated signaling by the Salmonella effector AvrA. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardt WD, Galan JE. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci U S A. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao AP, Petrof EO, Kuppireddi S, Zhao Y, Xia Y, Claud EC, Sun J. Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of beta-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G220–G227. doi: 10.1152/ajpgi.00498.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ye Z, Petrof EO, Boone D, Claud EC, Sun J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol. 2007;171:882–892. doi: 10.2353/ajpath.2007.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Lu R, Wu S, Sun J. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 2010;584:911–916. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu R, Wu S, Zhang Y, Xia Y, Liu X, Zheng Y, Chen H, Schaefer KL, Zhou Z, Bissonnette M. 2014. Enteric bacterial protein AvrA promotes colonic tumorigenesis and activates colonic beta-catenin signaling pathway oncogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dube C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–375. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 23.Plevy S. A STAT need for human immunologic studies to understand inflammatory bowel disease. Am J Gastroenterol. 2005;100:73–74. doi: 10.1111/j.1572-0241.2005.41382.x. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu R, Wu S, Liu X, Xia Y, Zhang YG, Sun J. Chronic effects of a Salmonella type III secretion effector protein AvrA in vivo. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G129–G137. doi: 10.1152/ajpgi.00515.2004. [DOI] [PubMed] [Google Scholar]
- 27.Duan Y, Liao AP, Kuppireddi S, Ye Z, Ciancio MJ, Sun J. beta-Catenin activity negatively regulates bacteria-induced inflammation. Lab Invest. 2007;87:613–624. doi: 10.1038/labinvest.3700545. [DOI] [PubMed] [Google Scholar]
- 28.Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer — more than a "gut" feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 31.Ho GY, Wang T, Gunter MJ, Strickler HD, Cushman M, Kaplan RC, Wassertheil-Smoller S, Xue X, Rajpathak SN, Chlebowski RT. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72:3029–3037. doi: 10.1158/0008-5472.CAN-11-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, Herdman S, Varki N, Corr M, Lee J. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Yan S, Zhou C, Zhang W, Zhang G, Zhao X, Yang S, Wang Y, Lu N, Zhu H, Xu N. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett. 2008;271:85–97. doi: 10.1016/j.canlet.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Armanious H, Gelebart P, Mackey J, Ma Y, Lai R. STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. Int J Clin Exp Pathol. 2010;3:654–664. [PMC free article] [PubMed] [Google Scholar]
- 37.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10–deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 41.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 43.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]