Abstract
Background. Using Micral-test (MT) for screening microalbuminuria (MA) among type 2 diabetics (T2D) is helpful. We aimed at determining prevalence of MA and at describing the MT validity. Methods. We studied 182 T2D followed up in family medicine. Two 24-hour urinary quantitative assays of MA had been used as a gold standard. Results. Prevalence of MA was 23%, CI 95%: 16.9–29.1. MT validity was 77% for sensitivity, 88% for negative predictive value, and 0.2 for Kappa coefficient (p = 0.001). Among subjects having a blood pressure ≥130/80 mmHg, having a CHT/HDL ratio ≥ 3, being a T2D for more than 5 years, and being women, negative predictive values were, respectively, 91%, 89%, 95%, and 91%. The area under the ROC curve was 0.81 in men (p = 0.008) and 0.80 when diabetes duration exceeds 5 years (p = 0.001). The MA value at 100% Sp for MT was 35 mg/L. Conclusion. The use of MT in primary healthcare for yearly screening for MA in T2D must be accentuated especially when diabetes duration exceeds 5 years or when associated with other cardiovascular risks.
1. Introduction
Screening and management of early stage of diabetes are insured by the family practitioner, while Tunisian diabetics with complications are managed by specialists. Diabetic nephropathy (DN) is the commonest cause of end-stage renal disease (ESRD) [1]. Screening for microalbuminuria (MA) is required in primary healthcare centers to prevent the progression of this serious complication [2–5]. The prevalence of DN is increasing along with the diabetes prevalence [6]. It should be detected and treated by the family doctor, at the stage of MA which is potentially reversible and associated with an increased risk of cardiovascular morbidity and mortality [7, 8]. The determination of MA by the semiquantitative method, Micral-test (MT), is a rapid screening tool. It is the easiest method, using urinary strips and a spot urine specimen. It is simple and inexpensive [9, 10]. The aim of this study was to assess the validity of MT criteria to detect DN among subgroups of diabetics and therefore to establish prevalence of MIA in diabetics managed in primary healthcare centers.
2. Methods
2.1. Design
This is a cross-sectional study, conducted from January 2013 to December 2014, to screen diabetic patients for elevated urinary albumin excretion with Micral-test in general practice.
2.2. Patients
We have included type 2 diabetics (T2D) without known complications followed up in two primary healthcare centers based in Monastir (“Stah Jabeur” and “C2”). Patients with fever, transitory diabetes decompensation, urinary infection, hematuria, or DN managed by a specialist were excluded from the study.
2.3. Study Area
Monastir is a coastal city located in the central eastern part of Tunisia. It includes 7 primary healthcare centers. Over than 20,000 inhabitants are followed up in “Stah Jabeur” and “C2” centers. Those centers were chosen because they manage diabetics at early stage.
2.4. Methods
The participants benefited from screening using the MT, done at the primary healthcare center. For the gold standard, two quantitative assays of MA on the urine of 24 hours were performed at one-month interval, at the Biochemistry Laboratory of the University Hospital of Monastir. MA was considered pathological if urinary albumin concentration (UAC) was between 30 and 300 mg in the urine of 24 hours. A third quantitative assay was performed in case of discordance. The MT is a semiquantitative strip test done on a urine sample in order to determinate MA. A comparative color scale is used to assess the results of MT: at a concentration of 0 mg/L of microalbumin in the urine, the strip remains white; it turns to light pink at 20 mg/L, to dark pink at 50 mg/L, and finally to very dark pink at more than 100 mg/L. A therapeutic education session was conducted on the test's day in primary healthcare center to improve patients' participation to MT and quantitative assays. Answerable nurse received information about this study.
2.5. Parameters
The participants also benefited from multiple blood tests. The studied parameters were sociodemographic data (age, gender), information about diabetes (diabetes duration, fasting blood glucose levels, glycated hemoglobin (HbA1C)), and risk factors associated with T2D (blood pressure, total cholesterol, and HDL cholesterol (HDL-C)). Total cholesterol to HDL-C ratio was calculated; the patient was considered as being at low risk of overload if this ratio was less than 3, at intermediate risk between 3 and 4.4, and at high risk if more than 4.5 [11]. Controlled glycemia is defined as the ADA [12]. Renal function was assessed by the measurement of glomerular filtration rate (GFR) calculated using the MDRD formula (modification of diet and renal disease). Some variables influence AUC, as was found in the literature. So we have analyzed validity criteria of MT according to demographic variables, blood pressure, CH/HDL-C ratio, and T2D duration.
2.6. Statistical Analysis
Statistical data were performed on SPSS 17.0 computer software. Sensitivity and specificity were calculated to determine the diagnostic properties of MT in predicting a UAC greater than or equal to 30 mg/24 hours. To determine the confidence intervals for sensitivity and specificity, we used the guidelines of Gardner and Altman. The coefficient Kappa (κ) was used to assess the concordance between the MT and the quantitative measurement of MIA. A value of p < 0.05 was considered as the cut-off of significance. The cut-off point for 100% specificity and the equilibrium point between sensibility and specificity were determined. We assessed, according to the subgroups, diagnostic sensitivity, specificity, positive and negative predictive values, Kappa coefficient, positive likelihood ratio, and negative likelihood ratio. The ROC curves of MT in diurnal random urine specimen for screening of MA according to the subgroups were plotted.
3. Results
3.1. Description of the Study's Population
We have included 182 T2D among whom 122 were followed up in the primary healthcare center “Stah Jabeur” and 60 in “C2” center. The mean age of the patients was 61.4 years (SD: 11.9). Females represented 69.2% of the studied population (sex ratio = 0.44) and 62% had hypertension. The mean duration of diabetes was 8.1 years (SD: 6.4). Fasting blood glucose levels value was <7 mmol/L in 31% of the cases and the HbA1C <7% in 30% of T2D. Blood pressure was ≥130/80 mmHg in 66% of the studied population and 49% of the patients were obese. Mild renal impairment was present in 47.6% of diabetics. The prevalence of MA was 23%; CI 95% = 16.9–29.1.
3.2. Variability of Micral-Test Validity
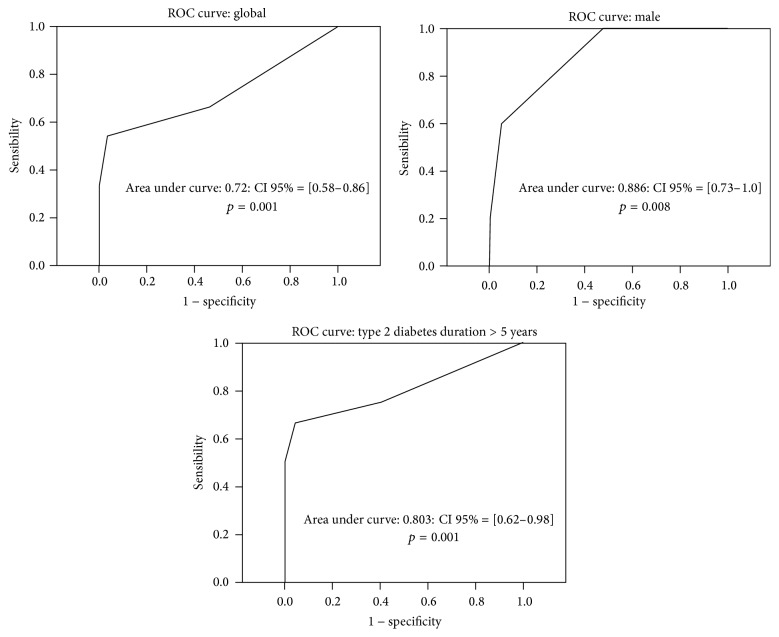
The MT had 77% sensitivity, CI 95%: 65.5–88.9, 46% specificity, and a negative predictive value (NPV) of 88%. The positive likelihood ratio was 1.4 and the negative likelihood ratio was 0.5. The validity of MT was better in women (NPV = 91%; κ = 0.3; p = 0.008), subjects having a blood pressure ≥130/80 mmHg (NPV = 91%; κ = 0.24; p = 0.005) or a total CHT/HDL ratio ≥3 (NPV = 89%; κ = 0.36; p = 0.01), and patients who have had T2D for more than 5 years (NPV = 95%; κ = 0.38; p = 0.001) (Table 1). The cut-off point for 100% specificity was 35 mg/L for all studied subgroups. The equilibrium point between sensitivity and specificity was 10 mg/L (Table 2). The area under the ROC curves was 0.72: CI 95% = 0.581–0.863 (p = 0.001) for the entire study population. It was 0.81 in men (p = 0.008) and 0.80 when diabetes duration exceeds 5 years (p = 0.001) (Figure 1).
Table 1.
Assessment of validity of Micral-test according to subgroups of diabetics.
| Cut-off value: 20 mg/L | Sensibility (%) | Specificity (%) | Kappa | p | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|
| Entire population | 77.2 CI 95% [65.5–88.9] |
46.3 CI 95% [32.4–60.2] |
0.2 | 0.001 | 88.4 | 27.8 | |
| Gender: | |||||||
| (i) Male | 66.6% | 46.6% | 0.103 | 0.008 | 77.7% | 1.2 | 0.74 |
| (ii) Female | 77% | 66% | 0.323 | 0.006 | 90.6% | 2.26 | 0.34 |
| BP ≥ 130/80 mmHg | 81% | 54.1% | 0.236 | 0.005 | 90.7% | 1.76 | 0.35 |
| CHT/C-HDL ratio ≥ 3 | 83.3% | 60.1% | 0.363 | 0.011 | 89.4% | 2.07 | 0.28 |
| T2D duration > 5 years | 91% | 60.6% | 0.378 | 0.001 | 95.2% | 2.27 | 0.15 |
NPV: negative predictive value.
PLR: positive likelihood ratio.
NLR: negative likelihood ratio.
Table 2.
Sensitivity and specificity among subgroups depending on the chosen point.
| Cut-off point for 100% specificity for MT | Equilibrium point between sensibility and specificity | |
|---|---|---|
| Entire population | 35 mg/L | 10 mg/L |
| Sensitivity | 54.2% | 66.7% |
| Specificity | 100% | 53.6% |
| Gender: male | 35 mg/L | 10 mg/L |
| Sensitivity | 60% | 100% |
| Specificity | 100% | 52.4% |
| BP ≥ 130/80 mmHg | 35 mg/L | 10 mg/L |
| Sensitivity | 52.4% | 66.7% |
| Specificity | 100% | 53.6% |
| CHT/C-HDL ratio ≥ 3 | 35 mg/L | 10 mg/L |
| Sensitivity | 58.3% | 58.3% |
| Specificity | 100% | 57.5% |
| T2D duration >5 years | 35 mg/L | 10 mg/L |
| Sensitivity | 66.7% | 75% |
| Specificity | 100% | 60% |
Figure 1.
The area under the ROC curve of Micral-test (mg/L) in microalbuminuria screening.
4. Discussion
The highlight of this study is that it was conducted in first-line therapy for diabetes in early stage. It allowed us to determine the accuracy of the MT in the detection of MA among diabetics subgroups and to estimate MA prevalence. This subject was little discussed in the Tunisian studies contrary to world literature [9, 10, 13–17].
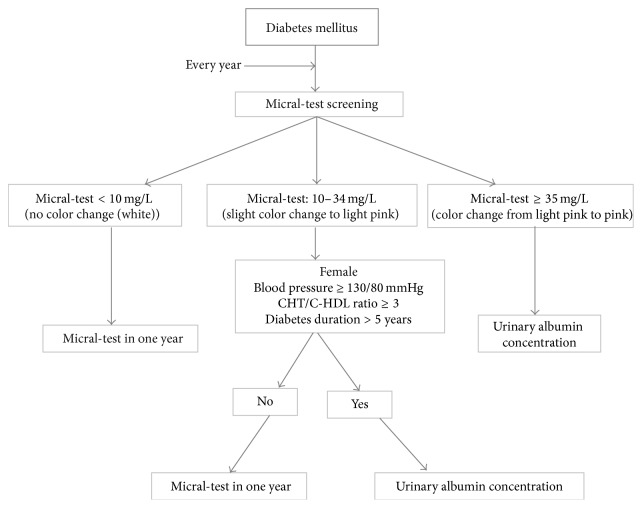
Our diabetic population was characterized by a female majority, as described in the literature [18, 19]. Different distributions were found though [20, 21]. The mean age (61.4 years) and the mean duration of diabetes (8.1 years (SD: 6.4)) were similar to those reported by other authors [13, 22]. We found that 30% of diabetics had a good glycemic control, and this result matches those observed in other Arab countries (Morocco, Kuwait, and Emirates) [13, 23, 24]. The glycemic control was better in China [25]. The review of literature shows a great variability in the prevalence of MA in T2D (Table 3); this prevalence ranged from 11.5 to 58.2% [13, 26]. This variation can be mainly attributed to the genetic and the ethnic dissimilarities between the studied groups [27] and to the difference in the classifications system. Among the studied population, 36 patients had MA (23%). This finding is comparable to those obtained in the United Kingdom Prospective Diabetes Study (UKPDS) [28] and in Adler et al.'s study [28] and Arfa et al.'s work [29] done in Tunisian specialized departments. MA's prevalence was lower in an Argentinean study [25] and greater in Canadian and Moroccan studies [13, 30, 31]. Several methods were used for the detection of the MA. The quantitative methods include the determination of the urinary albumin concentration (UAC) and the urinary albumin-to-creatinine ratio (UACR) in a first morning urine sample, 24 h urine collection, or timed urine collection. These two methods are performed in the laboratory by radioimmunoassay, radial immunodiffusion, enzyme immunoassay (ELISA), or immunoturbidimetric means. Among semiquantitative methods, the MT has been the most used screening tool for the detection of MA in many countries [32]. This test involves the immunochromatographic principle. Initially, the labeled antibody is combined with the sample albumin. Then, during the migration, excess conjugate is retained by the immobilized human albumin. Finally, the complex spread by migration on the strip to a revelation area. A more or less intense pink color appears in the play area based on the initial concentration of albumin [33, 34]. In different studies (Table 4), the MT precision varied from 67 to 96.7% and the specificity ranged from 46 to 96% [9, 32, 33, 35–39]. Table 5 shows a comparison between Micral-test and Multistix® commonly used for detection of DN in Tunisia. Our results are similar to Incerti's findings [36] for the same cut-off (20 mg/L). The MT validity was better in men (p = 0.008), subjects having a BP ≥130/80 mmHg (p = 0.005) or a cholesterol/C-HDL ratio ≥3 (p = 0.011), and patients who have had T2D for more than 5 years (p = 0.001). The point for 100% specificity (0% false positives) was 35 mg/L among all subgroups. The equilibrium point between sensibility and specificity was 10 mg/L; it was between 26 and 32 mg/L in Incerti et al.'s study [36]. Several studies have shown that the MT is commonly used worldwide, that it is a good screening tool for MA [32, 33, 36, 37, 39], and that positive results should be confirmed and quantified by a reference assay [33, 36]. Few authors have discussed the limitation of the diagnosis value of MT due to the rate of false positive [40], high costs, and the possibility of false negative results [32, 34]. Taking into consideration accuracy and cost, the measurement of the UAC in a random urine specimen, having 15 mg/L as the cut-off point for diagnosis, or the UACR on first morning micturition was considered by other authors as the best choice for MA screening patients with diabetes [36, 41]. Several advantages of MT have been described such as the economic benefit [42], the rapidity of the test (total time of 3 minutes), and its validity and reliability as a screening method for albuminuria on a single urine sample. It does not require 24-hour urine collection, feasible on a morning urine sample and especially adequate in the elderly [9, 32, 33]. We propose the algorithm (Figure 2) for screening ND.
Table 3.
Microalbuminuria's prevalence in the literature.
| Study | Number of patients | MA measurement method | MA's prevalence (%) |
|---|---|---|---|
| Our study | 182 | 24-hour urinary UAC: 30–300 mg/d |
23% |
| Kuwait, 2008 [23] | 440 | Micral-test | 14.2% |
| Argentina, 2011 [26] | 88 | 24-hour urinary UAC: 30–300 mg/d |
46.2% |
| Albania, 2013 [43] | 222 | Matinal urine UACR: 30–300 mg/g |
38.6% |
| Italy, 1996 [41] | 1574 | Night urine UAC: 20–200 μg/min |
32.1% |
| Tunisia, 2014 [44] | 120 | — | 27.5% |
| Tunisia, 2008 [29] | 141 | 24-hour urinary UAC: 30–300 mg/d |
25.5% |
| Morocco, 2009 [13] | 728 | 24-hour urinary UAC: 30–300 mg/d |
11.5% |
UAC: urinary albumin concentration.
UACR: urinary albumin creatinine ratio.
Table 4.
Micral-test's validity in literature.
| Study | Number of patients | Sensibility (%) | Specificity (%) | NPV (%) | Area under curve |
|---|---|---|---|---|---|
| Our study | 182 | 77 | 46 | 88 | 0.72 |
| De Grauw et al. [35] | 401 | 67 | 93 | 0.84 | |
| Incerti et al. [36] | 278 | 90 | 46 | 0.84 | |
| Mogensen et al. [37] | 2228 | 96.7 | 71 | — | |
| Parikh et al. [38] | 444 | 88 | 80 | — | |
| Sarafidis et al. [39] | 165 | 70 | 83 | — | |
| Cortés-Sanabria et al. [9] | 71 | 83 | 96 | 88 | 0.91 |
| Larijani et al. [32] | 200 | 93 | 87 | 92 | |
| Le Floch et al. [33] | 302 | 79 | 81 | 95 | — |
| Mogensen et al. [37] | 530 | 78 | 77 |
NPV: negative predictive value.
Table 5.
Comparison of commonly used dipsticks for the detection of albuminuria.
| Micral-test | Multistix 10 SG, Bayer [45] | |
|---|---|---|
| Type | Semiquantitatives | Qualitative |
| Level of detection | 20 mg/L | 200 mg/L |
| Level of 100% specificity | 35 mg/L | — |
| Negative predictive value | 88% (20 mg/L) | 73.7% (200 mg/L) |
| Time for reading | 1 mn | 1 mn |
| Cost | 48.1£ | 8.59£ |
| Efficacy of ACE treatment at screening | Reversible stage of DN | Not reversible stage of DN |
ACE: angiotensin-converting enzyme (ACE) inhibitors; DN: diabetic nephropathy.
Figure 2.
Algorithm for screening nephropathy in diabetes mellitus with Micral-test.
5. Conclusion
Detecting MA in diabetic patients leads the family doctor to speed up the adaptation of the therapeutic goals in order to have the finest metabolic control, an improvement of health-related behaviors, and an optimal treatment of the associated risk factors. Screening for MA has to be improved in primary healthcare centers. The use of reliable, cheap, and easily semiquantitative methods such as the MT seems to be a promising strategy to achieve that purpose.
Acknowledgments
The authors thank the nurse staff of the healthcare centers Stah Jabeur and C2 in Monastir, where this study was conducted, for their invaluable contribution in implementing the study. Their special thanks extend to Dr. Jebara Hassen, Headmaster of the Regional Department of the Basic Healthcare in Monastir, for his help to supply the Micral-test. This work was financially supported by research committees in Monastir University.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Reutens A. T., Prentice L., Atkins R. The Epidemiology of Diabetes Mellitus. 2nd. John Wiley & Sons; 2008. The epidemiology of diabetic kidney disease; pp. 499–518. [Google Scholar]
- 2.Préneuf H. Néphropathies diabétiques. EMC—Nephrologie. 2011;1(1):1–16. doi: 10.1016/S1762-0945(11)33430-4. [DOI] [Google Scholar]
- 3.Ninomiya T., Perkovic V., De Galan B. E., et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. Journal of the American Society of Nephrology. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opie L. H., Parving H.-H. Diabetic nephropathy: can renoprotection be extrapolated to cardiovascular protection? Circulation. 2002;106(6):643–645. doi: 10.1161/01.cir.0000028099.24188.c4. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar N. S., Pfeffer M. A. Cardiovascular risk in chronic kidney disease. Kidney International. 2004;92:S11–S15. doi: 10.1111/j.1523-1755.2004.09203.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Dijk P. C. W., Jager K. J., Stengel B., Grönhagen-Riska C., Feest T. G., Briggs J. D. Renal replacement therapy for diabetic end-stage renal disease: data from 10 registries in Europe (1991–2000) Kidney International. 2005;67(4):1489–1499. doi: 10.1111/j.1523-1755.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 7.Pylypchuk G., Beaubien E. Diabetic nephropathy. Prevention and early referral. Canadian Family Physician. 2000;46:636–642. [PMC free article] [PubMed] [Google Scholar]
- 8.Ritz E. Diabetic nephropathy. Saudi Journal of Kidney Diseases and Transplantation. 2006;17(4):481–490. [PubMed] [Google Scholar]
- 9.Cortés-Sanabria L., Martínez-Ramírez H. R., Hernández J. L., Rojas-Campos E., Canales-Muñoz J. L., Cueto-Manzano A. M. Utility of the Dipstick Micraltest II in the screening of microalbuminuria of diabetes mellitus type 2 and essential hypertension. Revista de Investigación Clínica. 2006;58:p. 190. [PubMed] [Google Scholar]
- 10.Szymanowicz A., Blanc-Bernard E., Roche C., Neyron M. J., Perrin M., Nourdine K. Evaluation du micral test en vue du dépistage de la microalbuminurie en biologie délocalisée. Immuno-Analyse et Biologie Spécialisée. 2008;23:109–115. [Google Scholar]
- 11.Giannuzi P., Wood D. A. Guidelines on primary prevention of cardiovascular disease. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:S1–S113. [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2012;36(supplement 1):S11–S66. doi: 10.2337/dc13-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouattar T., Ahid S., Benasila S., et al. The factors for progression of the diabetic nephropathy: management and evolution. Nephrologie et Therapeutique. 2009;5(3):181–187. doi: 10.1016/j.nephro.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Marre M., Girault A., Vasmant D. Prévalence de la microalbuminurie chez les diabétiques de type 2 français suivis par leur médecin généraliste. Diabète et Métabolisme. 1995;21:34–40. [PubMed] [Google Scholar]
- 15.Sabouret P., Denolle T., Krempf M., Om S., Laville M. Enquête microalb: dépistage de la microalbuminurie chez l'hypertendu diabétique de type 2 par le médecin généraliste en France métropolitaine. Archives des Maladies du Coeur et des Vaisseaux. 2006;99:712–717. [PubMed] [Google Scholar]
- 16.Guerin A., Savary O., Del Puppo S. Enquête mira: épidémiologie de la microalbuminurie et des comorbidités associées chez le diabétique de type 2. Archives des Maladies du Coeur et des Vaisseaux. 2005;98:783–787. [PubMed] [Google Scholar]
- 17.Bendriss L., Lebbaq A., Jallal H., Mrani S., Khatouri A. Intérêt de la microalbuminurie au sein du syndrome métabolique dans la prédiction des évènements cardiovasculaires. Étude prospective à propos de 78 cas. Annales de Cardiologie et d'Angéiologie. 2012;61(1):15–19. doi: 10.1016/j.ancard.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Al-Adsani A. Risk factors associated with albuminuria in Kuwaiti adults with type 2 diabetes. Saudi Journal of Kidney Diseases and Transplantation. 2012;23(4):860–865. doi: 10.4103/1319-2442.98189. [DOI] [PubMed] [Google Scholar]
- 19.Krairittichai U., Potisat S., Jongsareejit A., Sattaputh C. Prevalence and risk factors of diabetic nephropathy among Thai patients with type 2 diabetes mellitus. Journal of the Medical Association of Thailand. 2011;94:S1–S5. [PubMed] [Google Scholar]
- 20.Prasad K. D., Rajaseker P. Study of microalbuminuria as a cardiovascular risk factor in type 2 diabetes mellitus. Asian Journal of Pharmaceutical and Clinical Research. 2012;5:42–43. [Google Scholar]
- 21.Rahamtalla F. A. Prevalence of microalbuminuria among sudanese type 2 diabetic patients at elmusbah center at ombadda—omdurman. IOSR Journal of Pharmacy. 2012;2(5):51–55. doi: 10.9790/3013-25205155. [DOI] [Google Scholar]
- 22.Parving H.-H., Lewis J. B., Ravid M., Remuzzi G., Hunsicker L. G. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney International. 2006;69(11):2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 23.Shebl M., Atteia M. High prevalence of MicroAlbuminuria (MA) in type 2 diabetic patients. Health Care. 2008;21:221–232. [Google Scholar]
- 24.Al-Maskari F., El-Sadig M., Obineche E. Prevalence and determinants of microalbuminuria among diabetic patients in the United Arab Emirates. BMC Nephrology. 2008;9, article 1 doi: 10.1186/1471-2369-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia W., Gao X., Pang C., et al. Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai diabetic complications study (SHDCS) Nephrology Dialysis Transplantation. 2009;24(12):3724–3731. doi: 10.1093/ndt/gfp349. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez Azcona P., Quaglia N. B. A higher prevalence of microalbuminuria in women in a diabetes management program at a public hospital in the south of Santa Fe province. Revista Argentina de Endocrinologia y Metabolismo. 2011;48(3):158–163. [Google Scholar]
- 27.Maeda S., Osawa N., Hayashi T., Tsukada S., Kobayashi M., Kikkawa R. Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney International. 2007;72(106):S43–S48. doi: 10.1038/sj.ki.5002385. [DOI] [PubMed] [Google Scholar]
- 28.Adler A. I., Stevens R. J., Manley S. E., Bilous R. W., Cull C. A., Holman R. R. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney International. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Arfa I., Abid A., Nouira S., et al. Lack of association between the angiotensin-converting enzyme gene (I/D) polymorphism and diabetic nephropathy in Tunisian type 2 diabetic patients. Journal of the Renin-Angiotensin-Aldosterone System. 2008;9(1):32–36. doi: 10.3317/jraas.2008.002. [DOI] [PubMed] [Google Scholar]
- 30.Zacharias J. M., Young T. K., Riediger N. D., Roulette J., Bruce S. G. Prevalence, risk factors and awareness of albuminuria on a Canadian First Nation: a community-based screening study. BMC Public Health. 2012;12, article 290 doi: 10.1186/1471-2458-12-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gæde P., Lund-Andersen H., Parving H.-H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. The New England Journal of Medicine. 2008;358(6):580–591. doi: 10.1056/nejmoa0706245. [DOI] [PubMed] [Google Scholar]
- 32.Larijani B., Hemati P., Javadi A., Mahmoudi M., Shafaee A. Screening for Microalbuminuria in the early detection of diabetic nephropathy: a cheap and simple method. Acta Medica Iranica. 2002;40:1–2. [Google Scholar]
- 33.Le Floch J. P., Marre M., Rodier M., Passa P. H. Interest of clinitek® microalbumin in screening for microalbuminuria: results of a multicentre study in 302 diabetic patients. Diabetes and Metabolism. 2001;27(1):36–39. [PubMed] [Google Scholar]
- 34.McFarlane P., Culleton B. La néphropathie chronique en présence de diabète. Canadian Journal of Diabetes. 2008;32:S139–S147. [Google Scholar]
- 35.De Grauw W. J. C., Van de Lisdonk E. H., Van den Hoogen H. J. M., et al. Screening for microalbuminuria in type 2 diabetic patients: the evaluation of a dipstick test in general practice. Diabetic Medicine. 1995;12(8):657–663. doi: 10.1111/j.1464-5491.1995.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 36.Incerti J., Zelmanovitz T., Camargo J. L., Gross J. L., de Azevedo M. J. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrology Dialysis Transplantation. 2005;20(11):2402–2407. doi: 10.1093/ndt/gfi074. [DOI] [PubMed] [Google Scholar]
- 37.Mogensen C. E., Viberti G. C., Peheim E., et al. Multicenter evaluation of the Micral-Test II test strip, an immunologic rapid test for the detection of microalbuminuria. Diabetes Care. 1997;20(11):1642–1646. doi: 10.2337/diacare.20.11.1642. [DOI] [PubMed] [Google Scholar]
- 38.Parikh C. R., Fischer M. J., Estacio R., Schrier R. W. Rapid microalbuminuria screening in type 2 diabetes mellitus: simplified approach with Micral test strips and specific gravity. Nephrology Dialysis Transplantation. 2004;19:1881–1885. doi: 10.1093/ndt/gfh300. [DOI] [PubMed] [Google Scholar]
- 39.Sarafidis P. A., Riehle J., Bogojevic Z., Basta E., Chugh A., Bakris G. L. A comparative evaluation of various methods for microalbuminuria screening. American Journal of Nephrology. 2008;28(2):324–329. doi: 10.1159/000111825. [DOI] [PubMed] [Google Scholar]
- 40.Webb D. J., Newman D. J., Chaturvedi N., Fuller J. H. The use of the Micral-Test strip to identify the presence of microalbuminuria in people with insulin dependent diabetes mellitus (IDDM) participating in the EUCLID study. Diabetes Research and Clinical Practice. 1996;31:93–102. doi: 10.1016/0168-8227(96)01208-9. [DOI] [PubMed] [Google Scholar]
- 41.Bruno G., Cavallo-Perin P., Bargero G., et al. Prevalence and risk factors for micro-and macroalbuminuria in an Italian population-based cohort of NIDDM subjects. Diabetes Care. 1996;19(1):43–47. doi: 10.2337/diacare.19.1.43. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs D., DeMott W., Grady H., Horvat R., Huestis D., Kasten B. Laboratory Test Handbook. Cleveland, Ohio, USA: Lexi-Comp; 1996. [Google Scholar]
- 43.Pasko N., Toti F., Zekollari E., Strakosha A., Kacori V., Thereska N. Prevalence of microalbuminuria in type 2 diabetes patients in Tirana, a preliminary multicenter study. Journal of Diabetes Mellitus. 2013;3(3):145–149. doi: 10.4236/jdm.2013.33022. [DOI] [Google Scholar]
- 44.Ben Ahmed H., Bouzid K., Hassine M., et al. Prévalence des facteurs de risque cardiovasculaire non conventionnels chez les sujets diabétiques tunisiens. La Presse Médicale. 2014;43(1):e9–e16. doi: 10.1016/j.lpm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Garcia C., Bordier L., Burnat P., et al. Urinary dipsticks must not be used to detect diabetes-induced incipient nephropathy. La Presse Médicale. 2006;35(7-8):1117–1121. doi: 10.1016/s0755-4982(06)74767-4. [DOI] [PubMed] [Google Scholar]