Abstract
Key points
Strenuous endurance exercise induces transient functional and biochemical cardiac perturbations that persist for 24–48 h.
The magnitude and time‐course of exercise‐induced reductions in ventricular function and increases in cardiac injury markers are influenced by the intensity and duration of exercise.
In a human experimental model, exercise‐induced reductions in ventricular strain and increases in cardiac troponin are greater, and persist for longer, when exercise is performed within the heavy‐ compared to moderate‐intensity exercise domain, despite matching for total mechanical work.
The results of the present study help us better understand the dose–response relationship between endurance exercise and acute cardiac stress/injury, a finding that has implications for the prescription of day‐to‐day endurance exercise regimes.
Abstract
Strenuous endurance exercise induces transient cardiac perturbations with ambiguous health outcomes. The present study investigated the magnitude and time‐course of exercise‐induced functional and biochemical cardiac perturbations by manipulating the exercise intensity–duration matrix. Echocardiograph‐derived left (LV) and right (RV) ventricular global longitudinal strain (GLS), and serum high‐sensitivity cardiac troponin (hs‐cTnI) concentration, were examined in 10 males (age: 27 ± 4 years; : 4.0 ± 0.8 l min−1) before, throughout (50%, 75% and 100%), and during recovery (1, 3, 6 and 24 h) from two exercise trials. The two exercise trials consisted of 90 and 120 min of heavy‐ and moderate‐intensity cycling, respectively, with total mechanical work matched. LVGLS decreased (P < 0.01) during the 90 min trial only, with reductions peaking at 1 h post (pre: −19.9 ± 0.6%; 1 h post: −18.5 ± 0.7%) and persisting for >24 h into recovery. RVGLS decreased (P < 0.05) during both exercise trials with reductions in the 90 min trial peaking at 1 h post (pre: −27.5 ± 0.7%; 1 h post: −25.1 ± 0.8%) and persisting for >24 h into recovery. Serum hs‐cTnI increased (P < 0.01) during both exercise trials, with concentrations peaking at 3 h post but only exceeding cardio‐healthy reference limits (14 ng l−1) in the 90 min trial (pre: 4.2 ± 2.4 ng l−1; 3 h post: 25.1 ± 7.9 ng l−1). Exercise‐induced reductions in ventricular strain and increases in cardiac injury markers persist for 24 h following exercise that is typical of day‐to‐day endurance exercise training; however, the magnitude and time‐course of this response can be altered by manipulating the intensity–duration matrix.
Key points
Strenuous endurance exercise induces transient functional and biochemical cardiac perturbations that persist for 24–48 h.
The magnitude and time‐course of exercise‐induced reductions in ventricular function and increases in cardiac injury markers are influenced by the intensity and duration of exercise.
In a human experimental model, exercise‐induced reductions in ventricular strain and increases in cardiac troponin are greater, and persist for longer, when exercise is performed within the heavy‐ compared to moderate‐intensity exercise domain, despite matching for total mechanical work.
The results of the present study help us better understand the dose–response relationship between endurance exercise and acute cardiac stress/injury, a finding that has implications for the prescription of day‐to‐day endurance exercise regimes.
Abbreviations
- A
late diastolic filling velocity
- cTn
cardiac troponin
- E
early diastolic filling velocity
- EDV
end diastolic volume
- ESV
end systolic volume
- E′
early diastolic myocardial tissue velocity
- FAC
fractional area change
- GET
gas exchange threshold
- GLS
global longitudinal strain
- HF
high frequency
- LF
low frequency
- LV
left ventricular
- RV
right ventricular
- S′
systolic myocardial tissue velocity
- SV
stroke volume
Introduction
The cardiovascular health benefits of regular moderate‐intensity exercise are numerous and profound (Shephard & Balady, 1999; Myers, 2003). However, it remains unclear whether prolonged high‐intensity exercise, which is known to provoke temporary (24–48 h) cardiac dysfunction (Oxborough et al. 2006; La Gerche et al. 2008; Chan‐Dewar et al. 2010; Stewart et al. 2015) and the release of cardiac‐specific injury biomarkers (Scherr et al. 2011; Shave & Oxborough, 2012; Stewart et al. 2014), confers additional cardiovascular benefits or even negates the gains associated with moderate‐intensity exercise (La Gerche & Heidbuchel, 2014; Levine, 2014).
Echocardiograph‐derived myocardial strain, a sensitive index of contractile function (Dandel et al. 2009), is often used to assess exercise‐induced functional cardiac perturbations (Oxborough et al. 2011, 2012). Transient reductions in left (LV) and right (RV) ventricular strain following an ultra‐endurance exercise (>18 min), and the release of cardiac injury biomarkers such as cardiac troponin (cTn), have been reported extensively (Whyte et al. 2005; Middleton et al. 2007; La Gerche et al. 2008; Scott et al. 2009; George et al. 2010, 2012). More recently, reduced LV and RV strain and a substantial release of cTn have been observed following shorter duration (60 min) high‐intensity exercise (Stewart et al. 2015). These functional cardiac perturbations are particularly evident when pre‐to‐post comparisons are performed under modest exercise stress rather than at rest (Claessen et al. 2014; Stewart et al. 2015). Furthermore, reductions in ventricular strain and an attenuated contractile reserve following endurance exercise manifest more profoundly in the RV than the LV (La Gerche et al. 2012; Claessen et al. 2014), secondary to a disproportionate rise in RV wall stress during exercise (La Gerche et al. 2011).
The magnitudes of exercise‐induced reductions in ventricular strain and increases in cTn are augmented with an increasing exercise intensity and/or duration (Banks et al. 2010) and are inversely proportional to prior exercise experience (Whyte et al. 2005). Sustained increases in myocardial wall stress and adrenergic stimuli during exercise, particularly when exceeding that which an individual is accustomed to, may perturb cardiac function by transiently reducing β‐adrenergic sensitivity (Banks et al. 2010), altering myocyte membrane permeability and/or provoking inflammation (Scherr et al. 2011; La Gerche et al. 2015). Given that the upper limit of the moderate‐intensity exercise domain demarks a physiological threshold for the regulation of homeostasis and cellular stress (Pringle et al. 2003), exercising above this threshold may have a profound influence on exercise‐induced reductions in ventricular strain and increases in cTn. This finding has implications for the prescription of day‐to‐day endurance exercise regimes.
Accordingly, the present study examined the time‐course development (throughout exercise and during recovery) of reductions in ventricular strain and increases in cTn evoked by exercise bouts performed within and above the moderate‐intensity domain, which are typical of day‐to‐day endurance training. It was hypothesized that perturbations to cardiac function and biomarker release would be particularly evident during and/or following heavy‐ compared to moderate‐intensity exercise, despite matching for total mechanical work.
Methods
Ethical clearance
The experimental procedures were approved by the Griffith University Human Research Ethics Committee in accordance with the Declaration of Helsinki. All subjects provided their written informed consent.
Subjects
Recreationally active men (aged 21–35 years) were recruited for the study via recruitment flyers distributed at local cycling and triathlon clubs and sporting events. Pre‐participation health screening, in accordance with the American College of Sports Medicine (American College of Sports Medicine, 2013), ensured that subjects were apparently healthy non‐smokers, and had no history of cardiopulmonary, metabolic or neuromuscular disorders. Subjects were not taking any medications and were actively engaging in endurance sports events (e.g. cycling and triathlon) at the time of participation in the study.
Study design
All subjects visited the laboratory on three separate occasions after abstaining from exercise for a minimum of 48 h before each visit. During the first visit, subjects underwent pre‐participation health screening and performed an incremental cycling test for determination of the gas exchange threshold (GET) and peak exercise values (oxygen uptake and heart rate). During the subsequent visits, subjects performed two laboratory‐based endurance cycling trials separated by 1 week. The endurance cycling trials consisted of a 90 and 120 min cycling protocol performed above (heavy‐intensity) and below (moderate‐intensity) the GET, respectively, followed by 6 h of monitored recovery and an additional monitoring session 24 h post‐exercise. Electrocardiograph and echocardiograph measurements were performed, and blood samples collected, before (baseline) and during the cycling trials (50%, 75% and 100% of cycling time), and during recovery from exercise (1, 3, 6 and 24 h). At baseline and during recovery, electrocardiograph and echocardiograph recordings were performed at rest and during a standardized low‐intensity exercise challenge on a semi‐recumbent cycling ergometer. During the cycling trials (50%, 75% and 100% of cycling time), subjects were transiently relocated to a semi‐recumbent cycle ergometer for electrocardiograph and echocardiograph recordings during the standardized low‐intensity exercise challenge.
The standardized low‐intensity exercise challenge served to control for any changes in blood pressure that typically manifests during acute recovery from exercise (i.e. post‐exercise hypotension), and thus ‘normalized’, to some extent, the loading conditions on the heart for comparisons throughout exercise and during recovery. Furthermore, the rest to exercise change in cardiac functional parameters (i.e. the difference between values attained during rest and the standardized exercise challenge, when metabolic and functional requirements are increased) allowed for comparisons of ventricular functional augmentation between baseline and during recovery. All experimental tests were performed in a controlled laboratory environment (22 °C and ∼55% humidity).
Incremental exercise test
Incremental exercise tests were performed on an upright, electronically‐braked cycle ergometer (Excalibur Sport, Lode BV, Groningen, The Netherlands) and comprised 6 min of warm‐up at 80 W, before the workload was increased by 15 W every 30 s until the subject reached volitional fatigue. Peak heart rate and oxygen uptake, measured via 12‐lead ECG and indirect calorimetry (Ultima CardiO2; MGC Diagnostics, St Paul, MN, USA), were calculated as the average of the highest two consecutive 30 s bin‐averaged values attained during the test. The GET was determined using the modified V‐slope method (Schneider et al. 1993).
Endurance cycling trials
All subjects performed two separate constant‐load cycling trials on an upright electronically‐braked cycle ergometer that consisted of either 90 min of exercise performed above (heavy‐intensity), or 120 min of exercise performed within, the moderate‐intensity exercise domain. The power output attained at the GET for each individual was used to demarcate the upper boundary of the moderate‐intensity exercise domain (Wasserman & Whipp, 1975; Whipp et al. 1981). The 90 min cycling protocol was performed at a power output equal to 110% of that attained at the GET for each individual, whereas the 120 min trial was performed at a power output equal to 80% GET; total mechanical work performed during the two cycling trials was matched for each individual. The cycling trials were separated by 1 week and their order was randomized. Heart rate and blood pressure were measured at 10 min intervals throughout the cycling trials and total myocardial work was estimated as the integral of the rate pressure product and exercise time. Oxygen uptake was measured every 30 min throughout the cycling trials. Subjects were instructed to drink water ad libitum and to consume ∼20 ml kg–1 of Gatorade (PepsiCo, Purchase, NY, USA) throughout each cycling trial.
Electrocardiography
Cardiac rhythm was monitored continuously during the cycling trials using a 12‐lead ECG (Mortara X‐Scribe; Mortara Instruments, WI, USA). Lead II electrocardiograph signals were extracted and recorded at 1000 Hz using a data acquisition system (MP100; BIOPAC Systems, Goleta, CA, USA). Beat‐by‐beat cardiac intervals (RR interval) were subsequently extracted from the raw ECG traces for designated time periods (10 min of quiet supine rest at baseline and at 1, 3, 6 and 24 h post‐exercise) and heart rate variability parameters were derived to estimate cardiac autonomic activity before and during recovery from exercise (Stewart et al. 2014).
Time‐ and frequency‐domain characteristics of the RR intervals were calculated using Matlab, version 7.7.0 (The Mathworks Inc., Natick, MA, USA). Time‐domain parameters included the mean interval and the root mean square of successive differences in intervals. Frequency‐domain parameters were derived from the power spectral density data of RR interval signals using autoregression techniques (Malik et al. 1996). Low frequency (LF: 0.04–0.15 Hz) and high frequency (HF: 0.15–0.4 Hz) parameters were normalized to total power and the LF:HF ratio was calculated (Malik et al. 1996).
Rest and exercise echocardiography
All two‐dimensional echocardiography (GE Vivid E9 and Imaging System; GE Healthcare, Milwaukee, WI, USA) was performed by the same cardiac sonographer in accordance with the American Society of Echocardiography guidelines (Lang et al. 2015). A minimum of three cardiac cycles were captured and analysed offline using EchoPac, version 11 (GE Healthcare) by a single cardiologist (AY) with specialist training in speckle‐tracking analysis, and using reproducibility data reported previously (Yamada et al. 2014).
All echocardiograph images were acquired with the subject placed semi‐supine (30°) and oriented in a partial left decubitus position (30°) on a recumbent cycle ergometer (E‐Bike EL; GE Healthcare). Resting images were acquired after the subject was placed in position and remained stationary for a minimum of 10 min. The standardized low‐intensity exercise challenge consisted of semi‐recumbent cycling at individualized power outputs to achieve a target heart rate of ∼100 beats min–1 when cycling at a cadence of 60 rev min–1. A target heart rate of 100 beats min–1 was selected to stimulate vagal withdrawal at the same time as still permitting optimum frame rate requirements for speckle‐tracking analysis. The power output for each individual was established during the initial standardized low‐intensity exercise challenge at baseline and held constant thereafter. Exercise‐echocardiograph images were acquired after at least 3 min of cycling to ensure that subjects had reached a steady‐state heart rate.
All two‐dimensional and tissue Doppler images were acquired from standard parasternal and apical windows at frame rates of 50–80 frames s–1, with system settings adjusted to ensure optimal signal‐to‐noise ratios and endocardial delineation. LV end‐diastolic (EDV) and end‐systolic (ESV) volumes were determined using the Simpson biplane method and the ejection fraction quantified as the percentage change between LV EDV and ESV. Stroke volume (SV) was derived from Doppler waveforms acquired at the LV outflow tract, and cardiac output was calculated. Systemic vascular resistance was estimated from mean arterial blood pressure divided by cardiac output. Peak early (E) and late (A) LV filling velocities were measured from apical long‐axis views at the level of the mitral valve leaflets, and the E‐to‐A ratio was derived. Wall motion velocities of the LV were measured during early filling (E′) from apical four‐chamber views at the level of the mitral valve annulus, with values averaged for the septal and lateral walls. RV end‐diastolic and end‐systolic areas were traced on the apical four‐chamber image (focused on the RV), and the fractional area change (FAC) was derived. Wall motion velocity of the RV during ejection (S′) was determined for the lateral free‐wall at the level of the tricuspid annulus. Ventricular strain was analysed using semi‐automated speckle‐tracking techniques as described previously (Stewart et al. 2015). LV global longitudinal strain (GLS) was determined by averaging longitudinal strain from apical four‐chamber, two‐chamber and long‐axis images (Geyer et al. 2010). RV GLS strain was determined as the average of three RV free wall segments (Chow et al. 2008; Teske et al. 2008).
Blood sampling
Blood samples were collected from a forearm vein via an indwelling cannula. Haematocrit was determined from fresh whole blood using an automated blood cell counter (Coulter Counter; Coulter Electronics, Luton, UK). Serum was isolated from whole blood by centrifugation at 1000 g for 10 min, and stored at −80°C for subsequent analysis. An Abbott Architect ci16200 analyser (Abbott Diagnostics, Abbott Park, IL, USA) was used for the determination of serum electrolyte concentrations (calcium, magnesium, potassium and sodium) and inflammatory markers (cortisol and C‐reactive protein). High‐sensitivity cardiac troponin I (hs‐cTnI) analysis was performed using the Abbott Architect STAT hs‐cTnI assay on the Abbott ci16200 analyser. To examine the effect of the standardized low‐intensity exercise challenge on haematological parameters, blood samples were taken after the initial exercise challenge and compared with baseline measures.
Statistical analysis
Normality of the data was determined using the Shapiro–Wilk test and all significance testing was performed using SPSS, version 21.0 (SPSS Inc., Chicago, IL, USA). Two‐way analyses of variance with repeated measures were performed to determine any differences in echocardiograph, heart rate variability and haematological parameters between the cycling trials (90 and 120 min) and over time (baseline, and during the cycling trials and recovery from exercise). Chamber‐specific strain values were included as within subject factors to examine differences between the left and right ventricles. Where significant main effects were observed, pairwise comparisons with Bonferroni adjustments were used to further examine differences between time periods (before exercise, throughout exercise at 50%, 75% and 100%, and during recovery from exercise at 1, 3, 6 and 24 h). P < 0.05 was considered statistically significant. Based on previous published work from our laboratory where we examined the myocardial responses (LV and RV function and biochemical parameters) to an acute bout of endurance exercise (Stewart et al. 2015), we calculated that a sample size of seven to nine participants would be required to yield a statistical power of ≥0.8 (with an α of <0.05). All data are reported as the mean ± SEM, unless stated otherwise.
Results
Subject characteristics
Ten recreationally active men (age: 27 ± 4 years; height: 1.81 ± 0.08 m; weight: 72.8 ± 10.8 kg) completed the study. Subject characteristics, incremental exercise test results and baseline echocardiographic data are presented in Table 1.
Table 1.
Subject characteristics
| Demographic and functional measures | |
|---|---|
| Age (years) | 27 ± 4 |
| Body mass index (kg m2) | 22.2 ± 2.5 |
| Mean arterial pressure (mmHg) | 88 ± 6 |
| Incremental exercise values | |
| Peak HR (beats min−1) | 180 ± 13 |
| Peak O2 uptake (ml kg−1 min−1) | 54.1 ± 5.9 |
| Peak power output (W) | 392 ± 70 |
| HR at GET (beats min−1) | 145 ± 13 |
| O2 uptake at GET (ml kg−1 min−1) | 37.5 ± 6.0 |
| Power output at GET (W) | 215 ± 55 |
| Cycling training history | |
| Years of training (years) | 10 ± 8 |
| Average hours per week (h week−1) | 8 ± 3 |
| Echocardiographic measures | |
| Interventricular septum (cm) | 0.9 ± 0.2 |
| LV outflow tract (cm) | 2.3 ± 0.2 |
| LV internal dimension (cm) | |
| Diastole | 5.4 ± 0.4 |
| Systole | 3.6 ± 0.3 |
| LV volume (ml) | |
| Diastole | 146 ± 32 |
| Systole | 54 ± 14 |
| LV ejection fraction (%) | 63 ± 3 |
| LV GLS (%) | −19.6 ± 1.7 |
| RV area (cm2) | |
| Diastole | 20.6 ± 4.0 |
| Systole | 10.5 ± 2.9 |
| RV fractional area change (%) | 0.49 ± 0.05 |
| RV GLS (%) | −26.6 ± 2.4 |
Data are the mean ± SD. HR, heart rate.
Endurance cycling trials
Power outputs during the 90 and 120 min cycling trials were 234 ± 17 W and 175 ± 13 W, respectively. Total mechanical work was not different between the endurance cycling trials (90 min: 1263 ± 92 kJ vs. 120 min: 1260 ± 95 kJ, P > 0.05). Oxygen uptake (90 min: 3.1 ± 0.18 l min−1 vs. 120 min: 2.6 ± 0.14 l min−1, P < 0.01) and heart rate (90 min: 160 ± 3 beats min–1 vs. 120 min: 145 ± 2 beats min–1, P < 0.01) were significantly higher in the 90 min trial. Total myocardial work was not different between the endurance cycling trials (90 min: 2.3 × 106 ± 8.7 × 104 vs. 120 min: 2.5 × 106 ± 8.5 × 104, P > 0.05). Body mass decreased significantly (P < 0.01) by ∼1.3% after the 90 min trial, and by ∼1.0% after the 120 min trial. The change in body mass was similar for both endurance cycling trials (P > 0.05).
Cardiac autonomic activity and haemodynamic responses
Both the time and frequency domain parameters of heart rate variability were significantly different from baseline at 1, 3 and 6 h following the 90 and 120 min cycling trials (Table 2), with the magnitude of change from baseline significantly (P < 0.01) greater for the 90 min trial. Heart rate variability parameters returned to baseline for both endurance cycling trials after 24 h of recovery. Resting arterial blood pressure and systemic vascular resistance (Table 3) were reduced at 1 and 3 h post‐cycling, with the magnitudes of change similar for both the endurance cycling trials. Resting arterial blood pressure and systemic vascular resistance were similar to baseline after 6 and 24 h of recovery for the 90 and 120 min trials. During the standardized exercise challenge, arterial blood pressure and systemic vascular resistance were comparable at all time points between the endurance cycling trials (Table 4).
Table 2.
Heart rate variability parameters at baseline and during recovery from exercise
| Baseline | 1 h | 3 h | 6 h | 24 h | ||
|---|---|---|---|---|---|---|
| Mean interval (ms) | 90 min | 1221 ± 69 | 1028 ± 96* | 1047 ± 95* | 1140 ± 91* | 1223 ± 101 |
| 120 min | 1231 ± 79 | 1133 ± 77*† | 1110 ± 82* | 1184 ± 97 | 1184 ± 70 | |
| RMSSD (ms) | 90 min | 72.7 ± 13.2 | 43.8 ± 9.8* | 43.7 ± 9.6* | 51.9 ± 11.7* | 72.7 ± 11.9 |
| 120 min | 75.5 ± 12.8 | 60.4 ± 12.3*† | 60.0 ± 10.6*† | 57.8 ± 11.2* | 71.8 ± 14.1 | |
| LF:HF | 90 min | 0.9 ± 0.3 | 3.4 ± 0.7* | 3.0 ± 0.8* | 2.0 ± 0.3* | 0.9 ± 0.2 |
| 120 min | 0.7 ± 0.2 | 1.5 ± 0.4*† | 1.5 ± 0.3*† | 1.6 ± 0.4* | 0.9 ± 0.1 |
Data are the mean ± SEM. RMSSD, root mean square of successive differences. *Significantly different from baseline (P < 0.05). †Significantly different from 90 min (P < 0.05).
Table 3.
Haemodynamic and echocardiographic parameters measured during rest at baseline and during recovery from exercise
| Baseline | 1 h | 3 h | 6 h | 24 h | ||
|---|---|---|---|---|---|---|
| Haemodynamics | ||||||
| Mean arterial blood pressure (mmHg) | 90 min | 88 ± 2 | 80 ± 2* | 84 ± 2* | 85 ± 2 | 85 ± 2 |
| 120 min | 87 ± 2 | 79 ± 2* | 83 ± 2* | 86 ± 2 | 86 ± 2 | |
| Cardiac output (l min−1) | 90 min | 4.9 ± 0.4 | 6.0 ± 0.7* | 5.5 ± 0.5* | 5.3 ± 0.6 | 5.0 ± 0.4 |
| 120 min | 4.5 ± 0.3 | 5.3 ± 0.5* | 5.3 ± 0.4* | 4.9 ± 0.4 | 4.7 ± 0.4 | |
| Systemic vascular resistance (mmHg min l−1) | 90 min | 19.1 ± 1.8 | 15.3 ± 1.8* | 16.2 ± 1.4* | 17.8 ± 2.0 | 17.9 ± 1.6 |
| 120 min | 20.4 ± 1.9 | 16.2 ± 1.5* | 16.6 ± 1.5* | 19.0 ± 2.0 | 19.5 ± 1.8 | |
| Intracardiac pressure (E/E') | 90 min | 5.6 ± 0.5 | 5.4 ± 0.4 | 5.8 ± 0.4 | 5.3 ± 0.3 | 5.6 ± 0.4 |
| 120 min | 5.0 ± 0.3 | 4.9 ± 0.5 | 5.2 ± 0.4 | 5.2 ± 0.3 | 5.2 ± 0.3 | |
| Conventional echocardiography | ||||||
| LV end diastolic volume (ml) | 90 min | 148 ± 10 | 141 ± 8 | 143 ± 8 | 145 ± 9 | 146 ± 8 |
| 120 min | 145 ± 10 | 143 ± 8 | 145 ± 9 | 143 ± 8 | 146 ± 8 | |
| LV end systolic volume (ml) | 90 min | 50 ± 4 | 53 ± 5 | 52 ± 4 | 54 ± 4 | 54 ± 4 |
| 120 min | 51 ± 5 | 55 ± 5 | 51 ± 3 | 56 ± 4 | 53 ± 4 | |
| LV stroke volume (ml) | 90 min | 103 ± 6 | 88 ± 6* | 90 ± 6* | 94 ± 7* | 103 ± 7 |
| 120 min | 98 ± 4 | 88 ± 4* | 93 ± 4 | 89 ± 4* | 101 ± 5 | |
| RV fractional area change (%) | 90 min | 49.9 ± 1.5 | 47.9 ± 1.8* | 48.7 ± 2.1* | 47.8 ± 1.3* | 49.1 ± 1.2 |
| 120 min | 48.7 ± 1.6 | 48.6 ± 1.4 | 49.3 ± 1.4 | 48.4 ± 1.1 | 48.1 ± 1.4 |
Data are the mean ± SEM. *Significantly different from baseline (P < 0.05). †Significantly different from 90 min (P < 0.05).
Table 4.
Haemodynamic and echocardiographic parameters measured during a standardized exercise challenge at baseline, throughout the endurance cycling trial and during recovery from exercise
| Baseline | 50% | 75% | 100% | 1 h | 3 h | 6 h | 24 h | ||
|---|---|---|---|---|---|---|---|---|---|
| Haemodynamics | |||||||||
| Mean arterial blood pressure (mmHg) | 90 min | 94 ± 2 | 92 ± 2 | 92 ± 2 | 92 ± 2 | 93 ± 2 | 95 ± 3 | 95 ± 3 | 94 ± 3 |
| 120 min | 93 ± 3 | 93 ± 3 | 93 ± 3 | 93 ± 3 | 92 ± 2 | 94 ± 3 | 93 ± 3 | 95 ± 3 | |
| Cardiac output (l min−1) | 90 min | 12.0 ± 1.1 | 12.6 ± 1.0 | 12.5 ± 1.2 | 13.5 ± 1.2 | 12.9 ± 1.1 | 13.0 ± 0.9 | 12.7 ± 1.0 | 11.8 ± 0.7 |
| 120 min | 11.7 ± 0.8 | 11.8 ± 0.7 | 11.1 ± 0.6 | 11.9 ± 0.8 | 12.2 ± 0.8 | 12.6 ± 0.9 | 12.4 ± 0.8 | 11.5 ± 0.8 | |
| Systemic vascular resistance (mmHg min l−1) | 90 min | 8.6 ± 0.9 | 7.8 ± 0.8 | 7.9 ± 0.8 | 8.0 ± 0.9 | 7.8 ± 0.8 | 7.7 ± 0.6 | 8.0 ± 0.9 | 8.4 ± 0.7 |
| 120 min | 8.3 ± 0.7 | 8.2 ± 0.5 | 8.6 ± 0.6 | 8.1 ± 0.6 | 8.0 ± 0.8 | 7.8 ± 0.7 | 7.8 ± 0.6 | 8.6 ± 0.8 | |
| Intracardiac pressure (E/E') | 90 min | 6.3 ± 0.4 | 6.3 ± 0.4 | 6.1 ± 0.4 | 6.0 ± 0.3 | 6.0 ± 0.5 | 6.2 ± 0.4 | 6.4 ± 0.3 | 6.5 ± 0.4 |
| 120 min | 6.6 ± 0.4 | 6.5 ± 0.5 | 6.4 ± 0.5 | 6.2 ± 0.3 | 6.4 ± 0.4 | 6.2 ± 0.4 | 6.1 ± 0.3 | 6.3 ± 0.4 | |
| Conventional echocardiography | |||||||||
| LV end diastolic volume (ml) | 90 min | 151 ± 10 | 145 ± 8 | 145 ± 9 | 148 ± 9 | 144 ± 9 | 148 ± 9 | 145 ± 9 | 151 ± 8 |
| 120 min | 148 ± 9 | 151 ± 9 | 147 ± 9 | 150 ± 8 | 149 ± 9 | 146 ± 8 | 145 ± 8 | 146 ± 8 | |
| LV end systolic volume (ml) | 90 min | 42 ± 3 | 45 ± 4 | 45 ± 5 | 47 ± 4 | 45 ± 3 | 46 ± 4 | 43 ± 4 | 46 ± 3 |
| 120 min | 43 ± 3 | 49 ± 5 | 47 ± 4 | 45 ± 4 | 47 ± 4 | 45 ± 3 | 47 ± 4 | 45 ± 4 | |
| LV stroke volume (ml) | 90 min | 130 ± 9 | 115 ± 8* | 113 ± 8* | 117 ± 7* | 114 ± 8* | 120 ± 7* | 125 ± 8 | 123 ± 7 |
| 120 min | 128 ± 8 | 122 ± 6 | 117 ± 6* | 115 ± 7* | 120 ± 7 | 124 ± 6 | 123 ± 8 | 129 ± 8 | |
| RV fractional area change (%) | 90 min | 57.4 ± 1.2 | 52.5 ± 1.8* | 52.8 ± 1.6* | 53.3 ± 1.7* | 52.1 ± 1.9* | 54.0 ± 1.7* | 51.7 ± 1.4* | 53.4 ± 1.9 |
| 120 min | 56.9 ± 1.1 | 52.6 ± 1.6* | 52.8 ± 1.8* | 54.4 ± 1.8* | 52.4 ± 2.2* | 54.4 ± 1.6* | 51.9 ± 1.3* | 53.9 ± 1.7 |
Data are the mean ± SEM. *Significantly different from baseline (P < 0.05). †Significantly different from 90 min (P < 0.05).
Echocardiography during resting conditions
Conventional and speckle‐tracking echocardiographic data are presented in Figs 1 and 2 and Tables 3 and 4. LV volumes and E/E′ were not different from baseline following both endurance cycling trials. At rest, parameters of LV and RV systolic function (e.g. LVGLS, SV, RVGLS, RVFAC) were reduced and persisted for up to 24 h after the 90 min cycling trial, whereas only RV systolic function was reduced after the 120 min cycling trial (Fig. 1). During the 90 min trial, reductions in GLS at 1 h post‐exercise were greater in the RV than the LV (LV: 6.7% vs. RV: 12.6%, P < 0.01).
Figure 1. Resting measures of ventricular strain before and after endurance exercise .
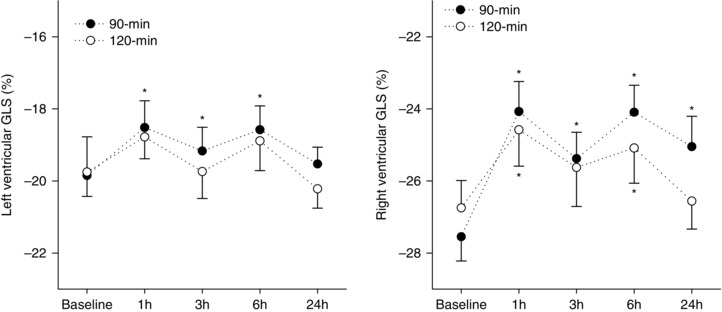
LV and RV GLS measured during rest at baseline and during recovery (1, 3, 6 and 24 h) from exercise performed above (90 min) or within (120 min) the moderate intensity exercise domain. Data are the mean ± SEM. *Significantly different from baseline.
Figure 2. Exercising measures of ventricular strain before, throughout and after endurance exercise .
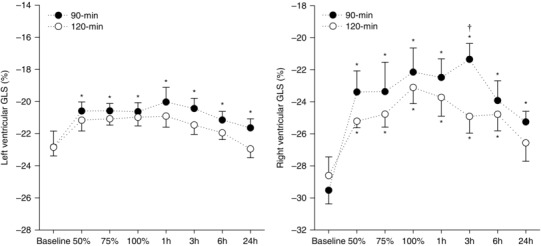
LV and RV GLS measured during a low‐intensity exercise challenge at baseline, and throughout (50%, 75% and 100%) and during recovery (1, 3, 6 and 24 h) from exercise performed above (90 min) or within (120 min) the moderate intensity exercise domain. Data are the mean ± SEM. *Significantly different from baseline. †Significantly different from the 120 min trial.
Echocardiography during the standardized exercise challenge
LV volumes and E/E′ were not different from baseline at any time‐point when assessed during the standardized exercise challenge (Table 4). Although LV volumes did not change significantly, LV EDV tended to decrease and LV ESV tended to increase throughout both endurance cycling trials. Consequently, SV decreased significantly relative to baseline by mid‐way through both endurance cycling trials persisting for 3 h following the 90 min cycling trial but returned to baseline 1 h following the 120 min trial. RV FAC decreased relative to baseline by mid‐way through both endurance cycling trials, with decrements persisting for 6 h into recovery (Table 4). LVGLS and RVGLS both decreased relative to baseline by mid‐way through the 90 min trial with decrements persisting for 24 h into recovery (Fig. 2). Only RVGLS was reduced throughout and following the 120 min trial but returned to baseline after 24 h of recovery. The magnitude of change in the augmentation of GLS (change from rest to during the standardized exercise challenge) was significantly different (P < 0.01) for the left and right ventricles (Fig. 3), with the greatest decrements induced by the 90 min trial. Augmentation of LV GLS was attenuated 1 h following the 90 min trial only, and returned to baseline at 3 h of recovery (Fig. 3). By contrast, augmentation of RV GLS was completely abolished following both exercise trials, although to a lesser extent during the 120 min trial; these changes persisted for 6 h following the 120 min cycling trial, and 24 h following the 90 min trial (Fig. 3).
Figure 3. Augmentation of ventricular strain before and after endurance exercise .
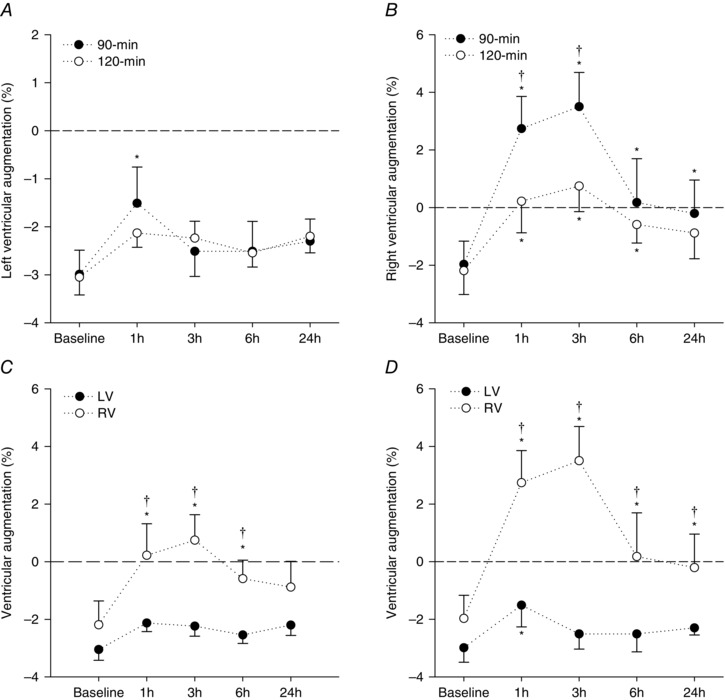
Augmentation of LV and RV GLS, namely the change in strain from rest to a low‐intensity exercise challenge, measured before (baseline) and during recovery (1, 3, 6 and 24 h) from exercise performed above (90 min) or within (120 min) the moderate intensity exercise domain. Data presented in (A) (LV GLS) and (B) (RV GLS) highlight the effect of the exercise conditions (90 min vs. 120 min trial) on cardiac responses to exercise. Data presented in (C) (120 min trial) and (D) (90 min trial) highlight the differing ventricular (LV vs. RV) responses to exercise. Note that augmentation of the RV strain is completely abolished during recovery from both cycling trials, whereas augmentation of the LV strain is only reduced following the 90 min trial. Data are the mean ± SEM. *Significantly different from baseline. †Significantly different between trials (120 min vs. 90 min) or between ventricles (LV vs. RV).
Haematological responses
Haematocrit and serum albumin were unchanged across all time points (Table 5) and were not different between the endurance cycling trials. Similarly, serum electrolyte concentrations were unchanged across all time points and not different between the endurance cycling trials (data range: Na: 136.4–139.1 mmol; K: 4.0–4.6 mmol; Ca: 2.3–2.5 mmol; Mg: 0.7–0.9 mmol). Serum hs‐cTnI increased during both trials (Fig. 4) but only exceeded cardio‐healthy population reference limits (14 ng l−1) in the 90 min trial. Hs‐cTnI concentrations reached peak values 3–6 h post‐cycling, and were significantly different between the two trials (P < 0.01). On average, peak cTnI concentration increased by 21 ng l−1 from baseline following the 90 min trial, and by 1.4 ng l−1 following the 120 min trial (Fig. 4). Similarly, exercise‐induced increases in cortisol and C‐reactive protein were greatest for the 90 min trial (Table 5). Haematological parameters did not change (P > 0.05) from baseline to immediately after the initial (baseline) standardized exercise challenge for both the 90 min trial (e.g. cTn: 4.3 ± 2.3 ng l−1 vs. 4.2 ± 2.3 ng l−1; Htc: 0.43 ± 0.01% vs. 0.43 ± 0.01%) and the 120 min trial (e.g. cTn: 2.9 ± 1.9 ng l−1 vs. 3.0 ± 2.0 ng l−1; Htc: 0.43 ± 0.01% vs. 0.43 ± 0.01%).
Table 5.
Haematological parameters at baseline, throughout exercise and during recovery from exercise
| Baseline | 50% | 75% | 100% | 1 h | 3 h | 6 h | 24 h | ||
|---|---|---|---|---|---|---|---|---|---|
| Haematocrit (%) | 90 min | 43.2 ± 1.1 | 43.5 ± 1.0 | 43.4 ± 0.9 | 43.3 ± 0.9 | 43.1 ± 1.1 | 42.6 ± 1.0 | 42.9 ± 1.0 | 42.7 ± 1.0 |
| 120 min | 43.0 ± 1.1 | 43.1 ± 1.1 | 43.1 ± 1.0 | 42.9 ± 1.0 | 43.0 ± 1.0 | 42.4 ± 1.2 | 42.7 ± 1.2 | 42.7 ± 1.1 | |
| Albumin (g dl−1) | 90 min | 44.8 ± 0.7 | 45.2 ± 0.6 | 45.3 ± 0.7 | 45.4 ± 0.7 | 45.1 ± 0.8 | 44.8 ± 0.7 | 45.4 ± 0.6 | 43.9 ± 0.7 |
| 120 min | 43.1 ± 0.7 | 43.4 ± 0.6 | 43.1 ± 0.7 | 43.9 ± 0.6 | 43.2 ± 0.7 | 42.7 ± 0.7 | 42.7 ± 0.6 | 43.0 ± 0.5 | |
| Cortisol (nmol l−1) | 90 min | 246 ± 33 | 422 ± 49* | 411 ± 48* | 425 ± 46* | 323 ± 39 | 185 ± 18* | 128 ± 29* | 278 ± 42 |
| 120 min | 256 ± 23 | 284 ± 45† | 254 ± 38† | 304 ± 49† | 223 ± 38† | 148 ± 15*† | 93 ± 23* | 232 ± 28 | |
| C‐reactive | 90 min | 0.50 ± 0.12 | 0.48 ± 0.11 | 0.50 ± 0.11 | 0.47 ± 0.11 | 0.48 ± 0.11 | 0.49 ± 0.11 | 0.67 ± 0.12* | 1.29 ± 0.31* |
| protein | 120 min | 0.47 ± 0.11 | 0.44 ± 0.12 | 0.44 ± 0.10 | 0.49 ± 0.11 | 0.51 ± 0.11 | 0.44 ± 0.09 | 0.53 ± 0.10 | 0.92 ± 0.15*† |
| (mg l−1) |
Data are the mean ± SEM. *Significantly different from baseline (P < 0.05). †Significantly different from 90 min (P < 0.05).
Figure 4. Cardiac troponin concentration before, throughout and after endurance exercise .
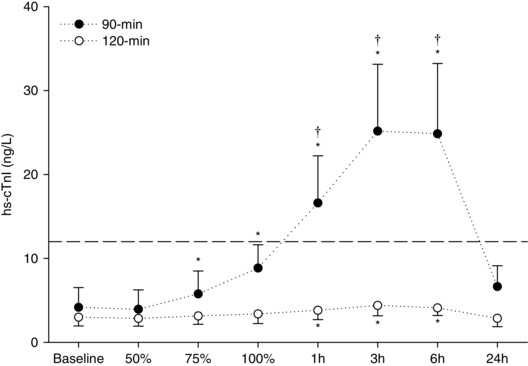
hs‐cTnI measured at baseline and throughout (50%, 75% and 100%) and during recovery (1, 3, 6 and 24 h) from exercise performed above (90 min) or within (120 min) the moderate intensity exercise domain. Data are the mean ± SEM. *Significantly different from baseline. †Significantly different from the 120 min trial.
Discussion
The results of the present study demonstrate that exercise, typical of day‐to‐day endurance training, significantly perturbs ventricular function and provokes a substantial release of cardiac‐specific biomarkers for up to 24 h post‐exercise. In a cohort of recreationally active men, manipulating the exercise intensity–duration matrix altered the magnitude and time‐course of exercise‐induced functional and biochemical perturbations. Specifically, exercise‐induced attenuation of ventricular strain and increases in cTn were greater, and persisted for longer, when exercise was performed at an intensity above (heavy‐intensity) compared to within the moderate‐intensity exercise domain, despite a similar amount of total work being performed. Furthermore, functional perturbations manifested most profoundly in the right ventricle.
Effects of exercise on LV and RV function
Despite comparable blood volume and preload (based on stable Htc, EDV and E/E′) throughout the experimental interventions, several parameters of LV and RV systolic function were reduced after 90 min of heavy‐intensity exercise when measured at rest, whereas only RV function was reduced after 120 min of moderate‐intensity exercise. However, these modest reductions in ventricular strain, measured under resting conditions, may not reveal the full extent of exercise‐induced perturbations in ventricular mechanical function. The post‐exercise hypotension and an augmented sympatho‐excitation during recovery may mask, to some extent, the influence of exercise on ventricular strain parameters, given the dependence of the latter on cardiac loading conditions and autonomic tone (Wang et al. 2011; Liu et al. 2013; Stewart et al. 2015). Indeed, we observed greater decrements in post‐exercise LV and RV function, which persisted for longer, when comparisons were performed during a standardized low‐intensity exercise challenge, where cardiac loading and functional metabolic requirements were normalized across time and interventions (i.e. the endurance cycling trials).
Interestingly, although LV and RV GLS both decreased during the 90 min cycling trial and persisted for 24 h into recovery, only RVGLS was reduced throughout and following the 120 min moderate‐intensity trial, although to a lesser extent than the 90 min trial. This suggests that exercise‐induced decrements in ventricular function are intensity‐dependent, given that total mechanical and myocardial work were equivalent between the two endurance cycling trials. Moreover, the moderate‐heavy intensity exercise boundary (i.e. the gas exchange threshold), which is known to have a profound effect on skeletal muscle homeostasis (Vanhatalo et al. 2010), may also demarcate a threshold for LV functional perturbations and an exacerbated magnitude of RV dysfunction. Although the mechanisms contributing to the intensity‐dependent functional cardiac perturbations are not clear, the similar and concomitant alterations in heart rate variability suggest an autonomic influence. Indeed, during exercise, there is a non‐linear relationship between sympathetic outflow and exercise intensity and/or heart rate (Pickering et al. 1972). Therefore, a ‘tolerable’ sympathetic load may exist, which, if exceeded for prolonged periods of time, will evoke a decline in β‐receptor sensitivity (Banks et al. 2010) and autonomic responsiveness (Seiler et al. 2007), inducing a decline in myocardial contractility.
Furthermore, the augmentation of LV strain was moderately reduced following the 90 min cycling trial, whereas RV augmentation was completely abolished following both 90 min of heavy‐intensity and 120 min of moderate‐intensity cycling. Although it is unclear to what extent a loss of free wall deformation may have impaired RV ejection in the present study, FAC was reduced in line with the decrements in RV strain, suggesting a diminished functional output. Moreover, a recent exercise‐magnetic resonance imaging study reported a decline in RV stroke volume following ultra‐endurance exercise, subsequent to increases in end‐diastolic and end‐systolic volumes (Claessen et al. 2014), without any evidence of LV dysfunction. The marked RV perturbations observed in the 90 min cycling trial, concomitant with only modest reductions in LV function, may reflect ventricular interactions that are only evident when exercise is performed at high intensities. Studies have previously reported an increased incidence of septal bounce secondary to RV dilatation following intense endurance sporting events, which may subsequently impede LV function (Whyte et al. 2005; Oxborough et al. 2011, 2012).
The disparate LV and RV functional perturbations observed in the present study, which were particularly evident following higher intensity exercise, suggest that the ‘myocardial load’ during acute exercise differs between ventricles. Indeed, the rise in afterload during exercise is relatively greater for the right side of the heart than the left (La Gerche et al. 2011). Greater haemodynamic loads, combined with the structural and geometrical differences between the left and right ventricles (La Gerche et al. 2011, 2012), may increase the susceptibility of the right ventricle to acute dysfunction following high‐intensity endurance exercise. Again, exceeding a tolerable volume and/or pressure load, most notably for the right ventricle, induces a greater decline in ventricular function, potentially by increasing cell membrane stress/inflammation (La Gerche et al. 2015) and altering β‐receptor sensitivity (Banks et al. 2010).
Effects of exercise on cardiac biomarker release
With the advent of highly sensitive assays for the detection of serum cTn concentrations, the release of cTn following exercise has been widely observed (Shave et al. 2002, 2007; Scherr et al. 2011; Stewart et al. 2015); however, the clinical implications of exercise‐induced cTn release are unclear. In the present study, an increase in cTn concentrations that peaked between 3 and 6 h post‐exercise was observed in all subjects. Interestingly, cardio‐healthy population reference limits (14 ng l−1) (Koerbin et al. 2013; Jarolim, 2015) were not exceeded by any subjects following 120 min of moderate‐intensity exercise. By contrast, 90% of the subjects recorded a peak serum cTnI concentration >14 ng l−1 following 90 min of heavy‐intensity exercise. As with the autonomic and functional perturbations observed in the present study, the upper boundary of the moderate‐intensity domain may demarcate an exercise intensity threshold for the release of cTn.
Although the mechanisms of exercise‐induced cTn release are unclear, unbound cTn may be released from the cytosol during periods of membrane stress or inflammation (Scherr et al. 2011), both of which may be exacerbated with increased exercise intensity. Indeed, both cortisol and hs‐CRP increased to a greater extent following 90 min of heavy‐intensity exercise than 120 min of moderate‐intensity exercise, despite comparable total mechanical and myocardial work being completed during the endurance cycling trials. The increased myocyte contraction rate and afterload pressures during higher‐intensity exercise would provoke greater myocardial wall stress and inflammation, leading to an increased release of cTn from the myoctes (Serrano‐Ostariz et al. 2009). Although the inflammatory markers reported in the present study are not cardiac‐specific, a positive relationship between pro‐inflammatory cytokines implicated in cardiac pathologies and increases in cardiac injury markers following acute exercise has been reported recently (La Gerche et al. 2015), and highlights the link between acute myocyte inflammation and the release of cTn following exercise.
Importantly, the magnitude of cTn release observed following exercise can reach clinically relevant levels and should be interpreted carefully. In the absence of additional clinical signs and symptoms of an adverse cardiovascular event, elevated cTn post‐exercise may not reflect underlying cardiac damage or pathology, but rather an acute physiological perturbation (Shave et al. 2010). Indeed, the time‐course of exercise‐induced cTn release, where values typically return to baseline by 24 h, follows a profile similar to that of acute inflammation and stress, potentially comprising a physiological response leading to adaption, rather than a pathological substrate leading to cell damage and adverse remodelling.
Methodological considerations
The present study implemented a standardized low‐intensity exercise challenge to control cardiac loading and metabolic requirements across time and cycling trials; thus, arterial blood pressure was similar across all time‐points. However, estimates of pulmonary artery pressure were not successfully obtained because of the low incidence of observed tricuspid regurgitation (n = 2 with data for all time‐points). In these participants, estimates of RV systolic pressure agree with previous work (La Gerche et al. 2010), and were similar between cycling trials and across all time‐points, both at rest (range 11.6–15.5 mmHg) and during the standardized exercise challenge (range 20.5–23.1 mmHg). Furthermore, to permit the manipulation of exercise intensity and duration at the same time as controlling total mechanical work, the study design incorporated constant‐load exercise, which does not reflect everyday physical activity patterns. Replicating the present study under a natural training environment would help extrapolate the findings to day‐to‐day training regimes.
Physiological and clinical perspectives
Although it remains unclear whether exercise‐induced cardiac perturbations constitute healthy physiological adaptations (Levine, 2014) or, in some individuals, provide a stimulus for adverse cardiac remodelling, there are suggestions that cumulative bouts of strenuous exercise without sufficient recovery may be detrimental to cardiac health (La Gerche et al. 2012). However, exercise‐induced reductions in ventricular strain are transient and seldom reach clinical cut‐off values (e.g. −20% for RV strain) that are indicative of cardiac abnormalities (Lang et al. 2015). Nonetheless, the upper limit of the moderate‐intensity exercise domain, shown in the present study to demarcate a threshold for exercise‐induced cardiac stress or injury markers, may represent an exercise intensity boundary that has a profound influence on cardiovascular health and/or adaptation. When endurance exercise training requires a large and fixed volume of mechanical work, reducing the intensity and prolonging the duration reduces the overall cardiac stress. However, endurance performance success, and therefore the goal of training, is not solely defined by the capacity of the cardiovascular system. Indeed, for peripheral muscular and biochemical adaptations, a high‐intensity exercise stimulus is required (Dudley et al. 1982). Nevertheless, the exercise stimulus required for ‘optimal’ cardiac remodelling remains to be clarified and is probably chamber‐specific given that greater RV (compared to LV) remodelling has been observed following prolonged endurance exercise training (Arbab‐Zadeh et al. 2014). The upper boundary of the moderate‐intensity exercise domain may provide an ‘intensity threshold’ for optimal exercise‐induced cardiac stress or adaptations.
Conclusions
There is currently debate over the effects of excessive endurance exercise practice on cardiac health. The present study highlights the transient nature of acute functional and biochemical cardiac perturbations following exercise that is typical of day‐to‐day endurance exercise training. Importantly, the magnitude and time‐course of exercise‐induced cardiac perturbations are intensity‐dependent and chamber‐specific, and can be manipulated by altering the exercise intensity–duration matrix about the upper boundary of the moderate‐intensity exercise domain.
Additional information
Competing interests
The authors declare that they have no competing interests.
Funding
AY was supported by a Griffith Health Institute ASI grant.
Author contributions
GMS, LJH, JJK, JC and SS conceived and designed the research. GMS, AY and SS performed the experiments. GMS, AY, GK and CW analysed the data. GMS, AY, JJK, GK, JC and SS interpreted the results of the experiments. GMS prepared the figures. GMS and SS drafted the manuscript. GMS, AY, JJK, JC and SS edited and revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- American College of Sports Medicine (2013). ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins, Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- Arbab‐Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams‐Huet B, Haykowsky MJ & Levine BD (2014). Cardiac remodelling in response to 1 year of intensive endurance training. Circulation 130, 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L, Sasson Z, Busato M & Goodman JM (2010). Impaired left and right ventricular function following prolonged exercise in young athletes: influence of exercise intensity and responses to dobutamine stress. J Appl Physiol 108, 112–119. [DOI] [PubMed] [Google Scholar]
- Chan‐Dewar F, Oxborough D, Shave R, Gregson W, Whyte G, Noakes T & George K (2010). Evidence of increased electro‐mechanical delay in the left and right ventricle after prolonged exercise. Eur J Appl Physiol 108, 581–587. [DOI] [PubMed] [Google Scholar]
- Chow PC, Liang XC, Cheung EW, Lam WW & Cheung YF (2008). New two‐dimensional global longitudinal strain and strain rate imaging for assessment of systemic right ventricular function. Heart 94, 855–859. [DOI] [PubMed] [Google Scholar]
- Claessen G, Claus P, Ghysels S, Vermeersch P, Dymarkowski S, LA Gerche A & Heidbuchel H (2014). Right ventricular fatigue developing during endurance exercise: an exercise cardiac magnetic resonance study. Med Sci Sports Exerc 46, 1717–1726. [DOI] [PubMed] [Google Scholar]
- Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N & Hetzer R (2009). Strain and strain rate imaging by echocardiography – basic concepts and clinical applicability. Curr Cardiol Rev 5, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM & Terjung RL (1982). Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 53, 844–850. [DOI] [PubMed] [Google Scholar]
- George K, Whyte GP, Green DJ, Oxborough D, Shave RE, Gaze D & Somauroo J (2012). The endurance athletes heart: acute stress and chronic adaptation. Br J Sports Med 46 (Suppl 1), i29–i36. [DOI] [PubMed] [Google Scholar]
- George KP, Naylor LH, Whyte GP, Shave RE, Oxborough D & Green DJ (2010). Diastolic function in healthy humans: non‐invasive assessment and the impact of acute and chronic exercise. Eur J Appl Physiol 108, 1–14. [DOI] [PubMed] [Google Scholar]
- Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J & Sengupta PP (2010). Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23, 351–369. [DOI] [PubMed] [Google Scholar]
- Jarolim P (2015). High sensitivity cardiac troponin assays in the clinical laboratories. Clin Chem Lab Med 53, 635–652. [DOI] [PubMed] [Google Scholar]
- Koerbin G, Abhayaratna WP, Potter JM, Apple FS, Jaffe AS, Ravalico TH & Hickman PE (2013). Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clin Biochem 46, 1636–1643. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H & Prior DL (2012). Exercise‐induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33, 998–1006. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI & Prior DL (2008). Biochemical and functional abnormalities of left and right ventricular function after ultra‐endurance exercise. Heart 94, 860–866. [DOI] [PubMed] [Google Scholar]
- La Gerche A & Heidbuchel H (2014). Can intensive exercise harm the heart? You can get too much of a good thing. Circulation 130, 992–1002. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI & Prior DL (2011). Disproportionate exercise load and remodelling of the athlete's right ventricle. Med Sci Sports Exerc 43, 974–981. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Inder WJ, Roberts TJ, Brosnan MJ, Heidbuchel H & Prior DL (2015). Relationship between inflammatory cytokines and indices of cardiac dysfunction following intense endurance exercise. PloS ONE 10: e0130031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbuchel H & Prior DL (2010). Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109, 1307–1317. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W & Voigt JU (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16, 233–270. [DOI] [PubMed] [Google Scholar]
- Levine BD (2014). Can intensive exercise harm the heart? The benefits of competitive endurance training for cardiovascular structure and function. Circulation 130, 987–991. [DOI] [PubMed] [Google Scholar]
- Liu S, Thomas SG, Sasson Z, Banks L, Busato M & Goodman JM (2013). Blood pressure reduction following prolonged exercise in young and middle‐aged endurance athletes. Eur J Prev Cardiol 20, 956–962. [DOI] [PubMed] [Google Scholar]
- Malik M, Bigger JT, Camm J, Kleiger RE, Malliani A, Moss AJ & Schwartz PJ (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17, 354–381. [PubMed] [Google Scholar]
- Middleton N, Shave R, George K, Whyte G, Simpson R, Florida‐James G & Gaze D (2007). Impact of repeated prolonged exercise bouts on cardiac function and biomarkers. Med Sci Sports Exerc 39, 83–90. [DOI] [PubMed] [Google Scholar]
- Myers J (2003). Cardiology patient pages. Exercise and cardiovascular health. Circulation 107, 2–5. [DOI] [PubMed] [Google Scholar]
- Oxborough D, Sharma S, Shave R, Whyte G, Birch K, Artis N, Batterham AM & George K (2012). The right ventricle of the endurance athlete: the relationship between morphology and deformation. J Am Soc Echocardiogr 25, 263–271. [DOI] [PubMed] [Google Scholar]
- Oxborough D, Shave R, Middleton N, Whyte G, Forster J & George K (2006). The impact of marathon running upon ventricular function as assessed by 2D, Doppler, and tissue‐Doppler echocardiography. Echocardiography 23, 635–641. [DOI] [PubMed] [Google Scholar]
- Oxborough D, Shave R, Warburton D, Williams K, Oxborough A, Charlesworth S, Foulds H, Hoffman MD, Birch K & George K (2011). Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise: exploratory insights from conventional two‐dimensional and speckle tracking echocardiography. Circ Cardiovas Imaging 4, 253–263. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Gribbin B, Petersen ES, Cunningham DJ & Sleight P (1972). Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res 30, 177–185. [DOI] [PubMed] [Google Scholar]
- Pringle JSM, Doust JH, Carter H, Tolfrey K, Campbell IT, Jones AM & Sakkas GK (2003). Oxygen uptake kinetics during moderate, heavy and severe intensity ‘submaximal’ exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol 89, 289–300. [DOI] [PubMed] [Google Scholar]
- Scherr J, Braun S, Schuster T, Hartmann C, Moehlenkamp S, Wolfarth B, Pressler A & Halle M (2011). 72‐h kinetics of high‐sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc 43, 1819–1827. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Phillips SE & Stoffolano S (1993). The simplified V‐slope method of detecting the gas exchange threshold. Med Sci Sports Exerc 25, 1180–1184. [PubMed] [Google Scholar]
- Scott JM, Esch BT, Shave R, Warburton DE, Gaze D & George K (2009). Cardiovascular consequences of completing a 160‐km ultramarathon. Med Sci Sports Exerc 41, 26–34. [DOI] [PubMed] [Google Scholar]
- Seiler S, Haugen O & Kuffel E (2007). Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc 39, 1366–1373. [DOI] [PubMed] [Google Scholar]
- Serrano‐Ostariz E, Terreros‐Blanco JL, Legaz‐Arrese A, George K, Shave R, Bocos‐Terraz P, Izquierdo‐Alvarez S, Bancalero JL, Echavarri JM, Quilez J, Aragones MT & Carranza‐Garcia LE (2009). The impact of exercise duration and intensity on the release of cardiac biomarkers. Scand J Med Sci Sports 21, 244–249. [DOI] [PubMed] [Google Scholar]
- Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D & Thompson PD (2010). Exercise‐induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol 56, 169–176. [DOI] [PubMed] [Google Scholar]
- Shave R, George KP, Atkinson G, Hart E, Middleton N, Whyte G, Gaze D & Collinson PO (2007). Exercise‐induced cardiac troponin T release: A meta‐analysis. Med Sci Sport Exer 39, 2099–2106. [DOI] [PubMed] [Google Scholar]
- Shave R & Oxborough D (2012). Exercise‐induced cardiac injury: evidence from novel imaging techniques and highly sensitive cardiac troponin assays. Prog Cardiovasc Dis 54, 407–415. [DOI] [PubMed] [Google Scholar]
- Shave RE, Dawson E, Whyte G, George K, Ball D, Gaze DC & Collinson PO (2002). Evidence of exercise‐induced cardiac dysfunction and elevated cTnT in separate cohorts competing in an ultra‐endurance mountain marathon race. Int J Sports Med 23, 489–494. [DOI] [PubMed] [Google Scholar]
- Shephard RJ & Balady GJ (1999). Exercise as cardiovascular therapy. Circulation 99, 963–972. [DOI] [PubMed] [Google Scholar]
- Stewart GM, Kavanagh JJ, Koerbin G, Simmonds MJ & Sabapathy S (2014). Cardiac electrical conduction, autonomic activity and biomarker release during recovery from prolonged strenuous exercise in trained male cyclists. Eur J Appl Physiol 114, 1–10. [DOI] [PubMed] [Google Scholar]
- Stewart GM, Yamada A, Haseler LJ, Kavanagh JJ, Koerbin G, Chan J & Sabapathy S (2015). Altered ventricular mechanics after 60 min of high‐intensity endurance exercise: Insights from exercise speckle‐tracking echocardiography. Am J Physiol Heart Circ Physiol 308, H875–H883. [DOI] [PubMed] [Google Scholar]
- Teske AJ, De Boeck BW, Olimulder M, Prakken NH, Doevendans PA & Cramer MJ (2008). Echocardiographic assessment of regional right ventricular function: a head‐to‐head comparison between 2‐dimensional and tissue Doppler‐derived strain analysis. J Am Soc Echocardiogr 21, 275–283. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ & Jones AM (2010). Influence of hyperoxia on muscle metabolic responses and the power‐duration relationship during severe‐intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95, 528–540. [DOI] [PubMed] [Google Scholar]
- Wang NC, Chicos A, Banthia S, Bergner DW, Lahiri MK, Ng J, Subacius H, Kadish AH & Goldberger JJ (2011). Persistent sympathoexcitation long after submaximal exercise in subjects with and without coronary artery disease. Am J Physiol Heart Circ Physiol 301, H912–H920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K & Whipp BJ (1975). Exercise physiology in health and disease. Am Rev Respir Dis 112, 219–249. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Davis JA, Torres F & Wasserman K (1981). A test to determine parameters of aerobic function during exercise. J Appl Physiol Respir Environ Exerc Physiol 50, 217–221. [DOI] [PubMed] [Google Scholar]
- Whyte G, George K, Shave R, Dawson E, Stephenson C, Edwards B, Gaze D, Oxborough D, Forster J & Simspon R (2005). Impact of marathon running on cardiac structure and function in recreational runners. Clin Sci 108, 73–80. [DOI] [PubMed] [Google Scholar]
- Yamada A, Luis SA, Sathianathan D, Khandheria BK, Cafaro J, Hamilton‐Craig CR, Platts DG, Haseler L, Burstow D & Chan J (2014). Reproducibility of regional and global longitudinal strains derived from two‐dimensional speckle‐tracking and Doppler tissue imaging between expert and novice readers during quantitative dobutamine stress echocardiography. J Am Soc Echocardiogr 27, 880–887. [DOI] [PubMed] [Google Scholar]


