Abstract
Gene therapy has emerged as a powerful tool in targeting the molecular mechanisms implicated in heart failure. Refinements in vector technology, including the development of recombinant adeno-associated vectors, have allowed for safe, long-term, and efficient gene transfer to the myocardium. These advancements, coupled with evolving delivery techniques, have placed gene therapy as a viable therapeutic option for patients with heart failure. However, after much promise in early-phase clinical trials, the more recent larger clinical trials have shown disappointing results, thus forcing the field to re-evaluate current vectors, delivery systems, targets, and endpoints. We provide here an updated review of current cardiac gene therapy programmes that have been or are being translated into clinical trials.
Keywords: Gene therapy, Heart failure, Adeno-associated vectors, Sarcoplasmic reticulum calcium ATPase, Excitation–contraction coupling
Introduction
Heart Failure (HF) remains a leading cause of mortality (≈50% after 5 years) and hospitalizations throughout the world.1 It is estimated that over 23 million people worldwide are afflicted with HF and its prevalence will continue to grow in the next 10 years with the ageing of the population. Recent advances in the medical management and implantable devices have contributed to slow down the evolution of the disease, to decrease hospitalizations, and to prolong survival in patients with left ventricular (LV) systolic dysfunction.1,2 However, as the disease evolves, patients experience recurrent hospitalizations and disabling health conditions, thus further urging for innovative approaches to reverse the course of ventricular dysfunction.
Our understanding of the molecular changes in HF has increased significantly in the last 20 years, and novel targets have been identified. These signalling pathways have been difficult to manipulate pharmacologically and for this reason gene therapy has been used experimentally and clinically for targeting purposes. We report here on the latest developments in translating targets into viable clinical trials that require the identification of therapeutic effects, suitable vectors, appropriate methods of gene delivery, and a clinical trial design with well-defined populations and endpoints.
Targets
Excitation–contraction coupling is dysregulated at multiple levels in HF. Targeting the various channels, transporters, and critical proteins thus represent a way to restore contractile function. Other attempts have concerned angiogenesis, cytoprotection, and stem cell homing. We here review gene targets that are related to clinical trials described later, or the targets that were recently shown to be effective in large animal studies. Other targets that have been examined in large animals are reviewed elsewhere.3
Enhance cardiac muscle contractility
Targeting the β-adrenergic system
The β-adrenergic system is a key regulator of cardiac contractility. During HF, β-adrenergic receptors (β-ARs) are, however, down-regulated and de-sensitized, particularly because of the up-regulation of the critical protein GRK2.4 GRK2 belongs to a family of G protein-coupled receptor kinases (GRKs) and is the most abundantly expressed GRK in the heart. Several rodent and large animal experiments have shown that expression of a peptide inhibitor of GRK2 (βARKct) can improve the contractile function of the failing myocardium.4
In contrast to detrimental outcomes demonstrated with several classes of the β-adrenergic stimulators to improve the expression of cAMP, activation of adenylyl cyclase type 6 (AC6) seems to have a unique favourable profile. Transgenic mice that over-express AC6 showed improved cardiac function upon adrenergic stimulation along with increased cAMP production in isolated cardiac myocytes. Importantly, AC6 over-expression was not associated with abnormal basal heart function or any structural heart abnormalities.5 In a pacing model of porcine HF, adenovirus-mediated AC6 gene transfer resulted in improved LV function and remodelling, together with increased cAMP generating capacity.6
Targeting Ca2+ cycling proteins
Multiple defects in Ca2-handling proteins involved in excitation–contraction coupling is a consistent finding in HF. Gwathmey et al. first reported that calcium cycling is abnormal in human HF7 and was found to be partially due to decreased sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) activity regardless of the HF aetiology. A large number of experimental models of HF have demonstrated improved cardiac contractility after SERCA2a gene transfer, including the swine volume-overload model of HF.8 Beyond their effects on enhancing contractility, SERCA2a gene transfer has been shown to restore the energetic state of the heart both in terms of energy supply and utilization,9 decrease ventricular arrhythmias,10 and enhance coronary flow through activation of eNOS in endothelial cells.11 Recently, small ubiquitin-like modifier type 1 (SUMO1) has been reported to play a major role in regulating the activity of SERCA2a in cardiomyocytes.12 SUMO1 levels were reduced in failing human ventricles, whereas increasing SUMO1 levels by adeno-associated virus type 9 (AAV9)-mediated gene transfer led to a restoration of SERCA2a levels, improved haemodynamic performance, and reduced mortality in small and large animals with HF.12
Inhibition of phospholamban (PLN) is another approach to improve Ca2+ handling. Elevated protein phosphatase-1 (PP1) activity is found in human HF, resulting in dephosphorylation of PLN. Over-expression of PP1 or ablation of its inhibitor (inhibitor-1) in murine hearts has been associated with decreased β-AR-mediated contractile responses, depressed cardiac function, and premature death consistent with HF.13 In contrast, transgenic mice expressing constitutively active inhibitor-1 (I1c) exhibited PP1 inhibition with increased phosphorylation of PLN and improved cardiac contractility.13I1c gene transfer approach to improve cardiac function was recently validated in large animal model of ischaemic HF.14
S100 belongs to a family of Ca2+-modulated proteins implicated in intracellular regulatory activities. In the heart, S100A1 is the most abundant isoform and it promotes cardiac contractile and relaxation through enhancing the activity of both ryanodine receptors and SERCA2a. Adeno-associated virus type 6-mediated long-term expression of S100A1 resulted in a sustained reversal of LV dysfunction and remodelling in a rat model of HF.15 More recently, AAV9-mediated gene transfer of S100A1 in a pre-clinical model of ischaemic cardiomyopathy induced dramatic improvements in contractile function.16
Targeting the myofilaments
The contraction of cardiac muscle cells results from the interaction between actin and myosin, this later using the energy from ATP hydrolysis to move along actin filaments. In animal studies, it was shown that replacing ATP by 2-deoxyATP improves contractility in striated muscle by increasing myosin binding to actin and cycling kinetics.17 The production of 2-deoxyATP is naturally limited but can be increased by over-expressing the enzyme ribonucleotide reductase (R1R2). R1R2 over-expression leads to increased contractility of isolated rat cardiac myocytes as well as cardiac tissue from human HF patients.18 Similarly, transgenic mice that over-express R1R2 show increased LV systolic function.17
Enhance angiogenesis
Vascular endothelial growth factors (VEGFs) have been used extensively to enhance angiogenesis in ischaemic hearts without consistent favourable clinical outcomes. It has been suggested that the prolonged over-expression of VEGF-A is associated with leaky vessel formation. Transient over-expression of VEGF-A using modified RNA enhanced formation of functional non-leaky vessels and improved survival in mouse myocardial infarction (MI) model.19 Other VEGF isoforms have also shown beneficial effects in improving angina.
Enhance cytoprotection
Heart failure is a progressive disease associated with continuous deterioration of cardiac function due to various stresses including oxidative stress, apoptosis, and ischaemia. Cytoprotective cardiac gene therapy approaches were recently shown to be promising in clinically relevant large animal models. Hinkel et al. demonstrated the cytoprotective role of haem oxygenase-1 over-expression against ischaemic/reperfusion injury for the first time in large animals.20 Isoforms of VEGF (B and D) have also been found to be cardioprotective, and AAV-mediated VEGF-B167 gene transfer preserved cardiac function and prevented the elevation of LV end-diastolic pressure in a dog model of tachy-pacing-induced HF.21
Enhance stem cell homing
The stem cell-derived factor 1 (SDF-1)/C-X-C chemokine receptor (CXCR) type 4 complex has been shown to promote the homing of stem cells to infarcted myocardium.22 SDF-1 is a chemokine protein that binds the G protein-coupled CXCR4. SDF-1 was originally shown to recruit bone marrow-derived stem cells to the site of myocardial injury and appears as an important regulator of endogenous tissue repair. Pre-clinical studies have shown that SDF-1 over-expression into the border zone of chronically remodelled post-MI myocardium results in improvement in cardiac function and cardiac vascular density.23
Vectors and delivery
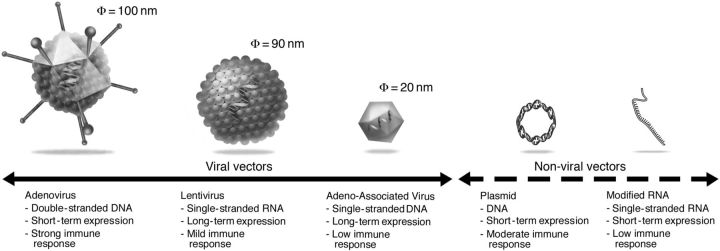
The success of cardiac gene therapy depends critically on the efficient transfer of the genetic material to the target tissue. In particular, the targeted delivery of therapeutic nucleic acids to cardiac cells remains a formidable hurdle in advanced mammalians. Broadly, cardiac gene delivery vehicles can be divided into two groups: non-viral vectors and viral vectors. Each of the vector systems used for cardiac gene transfer has its own set of advantages and disadvantages (Figure 1). We will briefly discuss the main vectors and delivery methods currently employed in cardiovascular gene therapy.
Figure 1.
Representative vectors used for cardiac gene therapy.
Non-viral gene delivery
To date, excluding oligonucleotide therapeutic modalities, non-viral gene transfer almost exclusively meant the use of naked plasmid DNA. Among the major advantages of plasmid DNA are (i) the ease of large-scale production, (ii) no DNA size limit, and (iii) the limited innate, cellular, and humoral immune response. The lack of a significant humoral immune response has the advantage of allowing repeated vector administration without loss of gene transfer efficiency. Unfortunately, the transfection efficiency of this vector remains low. Clearly, further improvements in the efficiency of transfection are needed to realize the full potential of gene transfer with plasmid DNA.
Recently, an additional non-viral method for gene transfer has emerged. This approach is based on the use of mRNA with modified nucleotides (referred as modified mRNAs or modRNAs24). ModRNAs, unlike unmodified nucleic acids, do not bind to toll-like receptors,24 which limit innate immune reaction to the transfection. As a result, modRNAs can be administered repeatedly. In addition, because mRNAs are readily translated in the cytoplasm, they do not need to be imported into the nucleus. ModRNAs trigger high-level, but relatively short-lived transgene expression, estimated to 2–6 days (Figure 1).19 Recently, Turnbull et al. have shown that modRNA mixed with nanoparticles can induce expression as fast as 20 min following delivery in rodent and pig hearts.25
Viral vectors
Viral vectors can efficiently deliver the therapeutic genetic material to the intended target cells, but each viral vector system has its own limitations. So far, the majority of cardiovascular gene therapy trials with viral vectors have been performed with adenoviral vectors. Adenoviral vectors can transduce a broad array of cell types, including cardiomyocytes, and result in high—although transient (1–4 weeks) gene expression (Figure 1). The most significant limitation of adenoviruses for cardiovascular gene therapy is the induction of a strong immune response. Even so-called gutless adenoviral vectors, which do not encode for any viral genes, still trigger a strong innate immune response against the adenoviral capsid,26 a risk that limits its use for cardiac gene therapy.
Lentiviral vectors are another potential vector system for cardiac gene therapy. The immune response is in general moderate,27 and lentiviral vectors are capable of transducing non-dividing cells including cardiomyocytes. Furthermore, because they integrate their genetic material into the host genome, lentiviral vectors can generate long-term expression. Like adenoviral vectors, lentiviral vectors have no specific tropism to cells of the cardiovascular system and they likely require intramyocardial injection as a vector delivery method. To date, lentiviral vectors have so far not been used in clinical cardiovascular gene therapy trials.
One of the most promising gene delivery platforms for cardiac gene therapy is based on AAVs. Adeno-associated viruses are small, non-pathogenic, non-enveloped, single-stranded DNA viruses and are members of the Parvoviridae family. Recombinant AAVs (rAAVs) can transduce both dividing and non-dividing cells. In post-mitotic tissues, such as the myocardium, rAAVs induce long-term transgene expression even in the absence of genome integration. One of the main advantages of rAAV vectors for cardiac gene therapy is that a number of AAV serotypes display natural tropism for cardiomyocytes.28 In rodent models of cardiac diseases, this allows the systemic administration of rAAVs to efficiently transduce the myocardium.9,12 Thus far, a cellular immune response has not been detected in >300 patients in the later mentioned CUPID trials.29 In contrast to the near absence of a cellular immune response against rAAVs, pre-existing neutralizing antibodies against the naturally occurring serotypes (presumably, a result of a prior infection with wild-type AAVs) rapidly emerged as a significant obstacle to the broad application of AAV gene therapy. In fact, more than half the patients (and up to 80% in certain regions such as Eastern Europe) present with high titre of neutralizing antibodies, limiting uniform application of this vector. Nonetheless, these constraints and the comparably small DNA packaging capacity (≤5 kb) notwithstanding, rAAVs are arguably the most promising vectors currently available for cardiac gene therapy.
Promoters
Constitutively active promoters such as CMV (cytomegalovirus) or RSV (respiratory syncytial virus) have been used to drive strong expression. More recently, cardiac-specific promoters such as myosin heavy chain promoter, the myosin light chain promoter, and troponin T promoter30 have been used to restrict the transgene expression to the heart. Disease-specific promoters such as ANF (atrial natriuretic factor), which are highly expressed in HF, have also been used experimentally in a dog model of HF.21 Even though these promoters do confer a higher specificity to the heart, the resulting expression is weaker compared with constitutively active promoters requiring higher doses of vectors. Inducible promoters have also been used, which can be either activated or silenced by a drug or a small molecule.31
Methods of gene transfer
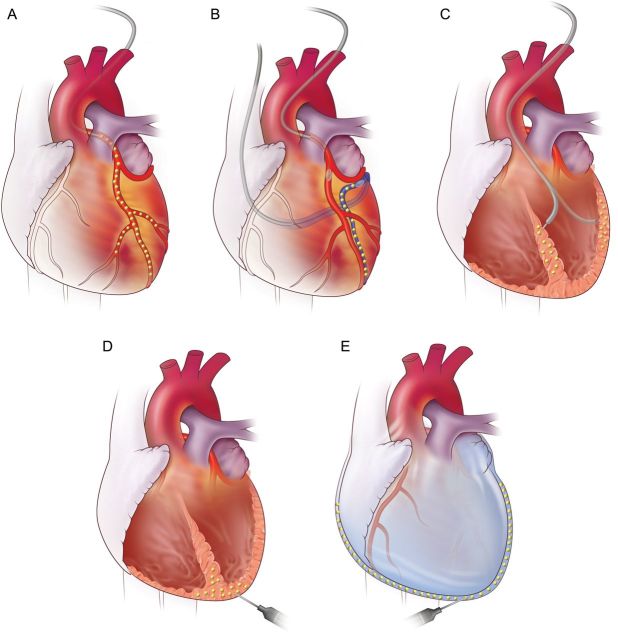
Choice of gene delivery is another important factor that determines the efficacy of gene transfer. Unfortunately, current vector technologies do not sufficiently transduce myocardium using the intravenous route in advanced mammalians, thus cardiac-specific targeting is required. Figure 2A–E illustrates the different gene delivery methods for targeting the heart. The optimal delivery technique differs depending on the vector used, targeted gene, treated disease, and patients' background. For example, surgical delivery may be appropriate when the heart is readily accessible in patients undergoing bypass or valve replacement surgeries. Meanwhile, gene distribution differs significantly from one method to another and therapeutics targeting the infarct border or biological pacemaker may benefit more from local gene over-expression.
Figure 2.
Methods of myocardial targeted gene delivery. (A) Antegrade intracoronary injection. (B) Retrograde injection through the coronary sinus with simultaneous blockade of the antegrade flow. (C and D) Direct myocardial injections through the left ventricle using catheter-based and surgical approach. (E) Intrapericardial injection.
So far, intramyocardium and intravascular delivery have been the methods of choice as the most clinically applicable approaches. With the intramyocardial delivery technique, transgene expression pattern is local, usually within 5–10 mm range around the needle track. Surgical approach is superior in manipulability, whereas endovascular approach offers less invasive advantage. Unfortunately, systemic leak of the vectors cannot be prevented even though the vectors are directly injected into the myocardium. The vectors leave the heart through venous drainage, lymph system, and from the injection needle holes.
Antegrade coronary artery delivery uses similar devices and techniques employed in percutaneous coronary interventions. The vectors go through the physiological route, thus homogeneous distribution is attained. However, simple bolus injection of vectors into the coronary artery results in very low transgene expression. The factors that influence myocardial uptake in the heart include higher coronary flow, longer virus exposure time, and higher virus concentrations. Higher transduction efficacy has been reported using brief coronary occlusions; however, this can cause stunning of the myocardium due to short ischaemia, which can be a problem when targeting advanced HF patients.
Another route to target the coronary system is a retrograde injection into the coronary sinus. This approach has been shown to increase the transduction efficacy compared with antegrade delivery with epicardial dominant expression.32 The downside of this approach is that it requires balloon occlusion of both the coronary artery and the sinus to prevent rapid washout of the vectors. It is anticipated that higher gene expression is achieved with the longer occlusion time; however, the benefit of achieving higher efficiency needs to be balanced with the risk of causing delivery-related complications. In addition to these mechanical factors, co-administration of permeability increasing agents increases the transduction efficacy.21
Clinical trials
There are several completed or ongoing clinical trials targeting HF, which are listed and summarized in Table 1. The details of completed and reported studies are discussed in the following sections.
Table 1.
List of clinical cardiac gene therapy trials targeting heart failure
| Trial | Phase | Vector | Gene | Delivery | Study design | Target patients | No. of patients | Primary outcome | Study locations | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| CUPID | I/II | AAV-1 | SERCA2a | Intracoronary | Phase I: open label; Phase II: randomized, double blinded | NYHA class III/IV, LVEF ≤35% | Phase I: 12; Phase II: 39 | Composite endpoint at 6 months: NYHA, 6MWT, VO2 max, NT-proBNP, QOL, echocardiographic function | USA | Results published |
| IIb | AAV-1 | SERCA2a | Intracoronary | Randomized, double blinded | NYHA class II–IV, LVEF ≤35% | 250 | Time to recurrent cardiovascular events (12 months) | International | Results published | |
| SERCA-LVAD | II | AAV-1 | SERCA2a | Intracoronary | Randomized, double blinded | Chronic HF patients on LVAD | 24 | Safety and feasibility | UK | Not recruiting |
| AGENT-HF | II | AAV-1 | SERCA2a | Intracoronary | Randomized, double blinded | NYHA class III/IV, LVEF ≤35% | 44 | Changes in left ventricular end-systolic volume (6 months) | France | Not recruiting |
| STOP-HF | I | Plasmid | SDF-1 | Endomyocardial | Open label | NYHA class III, LVEF ≤40% | 17 | Major adverse cardiac events (30 days) | USA | Results published |
| II | Plasmid | SDF-1 | Endomyocardial | Randomized, double blinded | Ischaemic cardiomyopathy, LVEF ≤40% | 93 | 6MWT (4 months) | USA | Results published | |
| RETRO-HF | I/II | Plasmid | SDF-1 | Retrograde | Phase I: open label; Phase II: randomized, double blinded | Ischaemic cardiomyopathy, LVEF ≤40% | Phase I: 12; Phase 2: 40 | 6MWT (4 months) | USA | Completed recruitment |
| AC6 Gene Transfer for CHF | I/II | Adenovirus | hAC6 | Intracoronary | Randomized, double blinded | Chronic HF, LVEF ≤40% | 56 | Combined (i) exercise time, (ii) cardiac function before and during dobutamine | USA | Completed recruitment |
6MWT, 6 min walk test; AAV, adeno-associated virus; hAC6, human adenylyl cyclase type 6; HF, heart failure; LVEF, left ventricular ejection fraction; LVAD, left ventricular assist device; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; QOL, quality of life; SDF-1, stem cell-derived factor 1; SERCA2a, sarcoplasmic reticulum Ca2+ ATPase; VO2 max, maximal oxygen consumption.
The SERCA2a gene transfer clinical programmes (CUPID, AGENT-HF, SERCA-LVAD)
There have been three clinical trials initiated using AAV-1.SERCA2a. The Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Phase 1/2 clinical trial was a first-in-human study that investigated the clinical impact of restoring SERCA2a expression levels in patients with advanced HF. The SERCA2a gene was administered using an AAV1 in a single intracoronary infusion. Following the re-assuring results of the Phase 1 portion of the study, the Phase 2 portion of the CUPID programme was conducted.29 The primary endpoint was defined as a concordant improvement of several efficacy parameters over a 6-month follow-up period. A total of 39 patients with advanced HF with undetectable neutralizing antibodies against AAV1 were randomized to the intracoronary infusion of AAV1.SERCA2a [n = 8 in low-dose (6 × 1011 DNase-resistant particles (DRP)), n = 8 in mid-dose (3 × 1012 DRP), and n = 9 in high-dose (1 × 1013 DRP)] or to the placebo (n = 14). There was a trend for a reduction of clinical events in AAV1.SERCA2a-treated patients.29 This trend was further confirmed in the long-term follow-up of patients.33 The number of recurrent cardiovascular events was significantly lower in the high-dose AAV1.SERCA2a group when compared with placebo.33 The assessment of SERCA2a transgene expression in the subset of patients with collected cardiac tissues further demonstrated the long-term persistence of AAV (up to 31 months in a patient).33
Subsequently, a larger CUPID2 study was designed to assess the effects of intracoronary AAV1.SERCA2a therapy at a dose of 1 × 1013 DRP on clinical outcomes in patients with advanced HF.34 CUPID2 was a Phase 2b, double-blind, placebo-controlled, multinational, multicentre randomized study of patients with stable New York Heart Association (NYHA) II–IV ischaemic or non-ischaemic HF and left ventricular ejection fraction (LVEF) <35%. The study included 250 patients randomized 1:1 in >50 centres in the USA and in Europe. The primary endpoint was time to recurrent HF-related events and was not statistically different between both treatment groups [hazard ratio: 0.93, 95% CI (0.53–1.65), P = 0.81].34 The secondary endpoints, including time to first terminal events, were also non-statistically different between both groups.34 Overall, the CUPID2 study was neutral and failed to demonstrate the efficacy of AAV1.SERCA2a gene transfer at a dose of 1.0 × 1013 DRP in improving clinical outcomes of patients with advanced HF.34
Two other clinical trials with AAV1.SERCA2a have been initiated. The AAV1-CMV-SERCA2a Gene Therapy trial in Heart failure (AGENT-HF; NCT01966887) is a Phase 2, monocentre, double-blind, randomized, placebo-controlled study that is investigating the effect of AAV1.SERCA2a gene transfer on cardiac volumes and function using multimodality cardiac imaging. A 256-slice CT-scan technique was used to determine the changes of LV end-systolic volumes over 6 months after AAV1.SERCA2a gene transfer. The other study called SERCA-LVAD (NCT00534703) is testing AAV1.SERCA2a gene transfer in HF patients who have received a LV assistance device. In addition to myocardial tissue sampling, the study is evaluating the recovery of contractile function during attempts to wean patients from the left ventricular assist device. Recruitment was, however, suspended in both studies following the CUPID2 neutral results. Detailed analysis of CUPID2 as well as data from patients included in other two programmes is awaited to fully determine the reason for neutral results.
Clinical trials with SDF-1 (STOP-HF, RETRO-HF)
Following the results of a pilot Phase 1 study using different doses of SDF-1 plasmid treatment for patients with HF, the Phase 2 trial (STOP-HF) evaluated the safety and efficacy of single treatment of plasmid SDF-1 in patients with ischaemic HF and LVEF ≤40%.35SDF-1 was administered via 15 endomyocardial injections. The study was randomized, double blind, and placebo controlled. Two different doses of pSDF-1 were tested (15 and 30 mg) and compared with a placebo in a total of 93 patients. The primary efficacy endpoint was the difference in change of a composite endpoint consisting of the 6 min walking distance and MLWHFQ in the treated patients compared with placebo at 4 months. The primary endpoint was not different between the two treated groups and the placebo group (P = 0.89). In patients with the lowest tertile of baseline LVEF (<26%), pSDF-1 at the dose of 30 mg induced a significant increase in LVEF by 11% (P = 0.01) when compared with placebo. This effect was, however, observed 1 year after pSDF-1 administration while pSDF-1 vector is expressed for less than a month in pre-clinical models of ischaemic HF. Retrograde infusion of pSDF-1 is currently being examined in a similar study design as STOP-HF (RETRO-HF). If RETRO-HF similarly presents better improvement in low LVEF patients, it may support the sub-analysis results of STOP-HF.
Ongoing and upcoming cardiovascular gene therapy clinical trials
Following positive results in pre-clinical large animal studies,16 AAV9-S100A1 is being developed as a gene therapy product to over-express S100A1. The first human clinical trial for S100A1 gene therapy is expected to start in the next few years.
Another clinical gene therapy trial is being planned involving the re-engineered AAV vector (AAV2i8) with high cardiac tropism that de-targets the liver.14I1c is the target gene in this clinical trial, and it will first assess safety at four doses (3 × 1012, 1013, 3 × 1013, and 1014 vg) in 12 patients with advanced HF.
The effects of over-expressing the human AC6 are currently tested in a dose escalation, randomized, placebo-controlled, Phase 1/2 clinical trial (AC6 gene transfer for CHF, NCT00787059) administered via intracoronary delivery. Efficacy and safety parameters are being evaluated 4 and 12 weeks after administration. The study is no longer recruiting patients, and the results are expected to be available soon.
Current challenges and future opportunities
Despite encouraging results from adequate pre-clinical models in large animals and from small-sized Phase 1 and early Phase 2 studies, none of the larger Phase 2 studies conducted so far have provided convincing evidence for a benefit of gene therapy in HF patients. Several factors could account for the overall negative results of these HF gene therapy trials, but some should be emphasized.
In the CUPID trial, the viral uptake within the myocardium ranged from <20 to 561 copies of vector per μg of DNA. These results are much lower than the viral uptake observed in animal models (∼20 000–350 000 copies of vector per μg of DNA) and correspond to <1% of cardiomyocytes being infected. This level of infectivity is unlikely to induce any changes in global ventricular function. The requirement for higher doses (>1.0 × 1013 vg) may be necessary; however, T cell responses can be elicited at doses close to 1.0 × 1014 vg and T cell activation will need to be carefully monitored.
In CUPID2, all patients with HF were included and their profile was very similar to CUPID1 trial; however, certain patient subpopulation may benefit more than others from a specific intervention. Finally, the data in large animals from pigs, sheep, dogs and even primates may not perfectly predict results in humans. This discrepancy is due to the AAV infectivity, diverse and complex disease background in humans, and differences in study endpoints. It may be necessary to target more specifically patients with HF on the basis of ischaemic disease, severity of HF, or presence of implantable devices. More detailed analysis of CUPID2 is expected to provide better clues for improving our gene therapy approaches. New gene targets in upcoming clinical trials have shown promising results in large animals, and these trials will employ more cardiotropic AAV vectors than that was used in CUPID. Additionally, more efficient cardiac-targeted vector delivery methods have been validated in large animals, and can be combined with these newer vectors and gene targets. Strategies to overcome humoral immunity to AAV will also be critical to develop since a significant percentage of patients has neutralizing antibodies to all AAV serotypes. These include plasmapheresis before injection, using empty capsids to adsorb anti-AAV antibodies, and novel technology to develop AAV vectors to escape neutralizing antibodies. The optimal combination of vector, delivery method, gene, and targeted disease needs to be thoroughly examined in pre-clinical studies, and appropriate clinical endpoint should be pondered for future cardiac gene therapy trials.
Conclusion
Despite the disappointing results of CUPID2, the field of cardiac gene therapy is moving forward. The field has gained valuable knowledge that AAV gene delivery can transduce the heart and is safe in advanced HF population. It has become clear that novel re-engineered vectors that will escape innate immunity and have higher tropism to the heart will be needed. High transduction efficiency is paramount in obtaining meaningful clinical improvements. With improved vectors, novel targets, enhanced gene delivery methodologies, and specific populations, the therapeutic benefits of gene therapy will be forthcoming in the next few years.
Authors' contributions
R.J.H. handled funding and supervision. R.J.H., J.-S.H., and K.I. conceived and designed the research, drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Funding
This work is supported by National Institutes of Health grants to R.J.H. (R01 HL117505, HL119046, HL128072, HL129814, P50 HL112324, T32HL007824) and to J.-S.H. (R01 HL113497), and a Transatlantic Fondation Leducq grant to R.J.H. and J.-S.H.
Conflict of interest: R.J.H. is a scientific co-founder of Celladon Corp. and Nanocor Corp.
References
- 1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 3. Ishikawa K, Tilemann L, Ladage D, Aguero J, Leonardson L, Fish K, Kawase Y. Cardiac gene therapy in large animals: bridge from bench to bedside. Gene Ther 2012;19:670–677. [DOI] [PubMed] [Google Scholar]
- 4. Reinkober J, Tscheschner H, Pleger ST, Most P, Katus HA, Koch WJ, Raake PW. Targeting GRK2 by gene therapy for heart failure: benefits above beta-blockade. Gene Ther 2012;19:686–693. [DOI] [PubMed] [Google Scholar]
- 5. Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation 1999;99:1618–1622. [DOI] [PubMed] [Google Scholar]
- 6. Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation 2004;110:330–336. [DOI] [PubMed] [Google Scholar]
- 7. Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circulation Research 1987;61:70–76. [DOI] [PubMed] [Google Scholar]
- 8. Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 2008;51:1112–1119. [DOI] [PubMed] [Google Scholar]
- 9. Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol 2007;42:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, Hajjar RJ, Del Monte F. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation 2008;118:614–624. [DOI] [PubMed] [Google Scholar]
- 11. Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, Lebeche D, Kranias EG, Leopold JA, Lompré A-M, Lipskaia L, Hajjar RJ. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther 2010;18:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 2011;477:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res 2005;96:756–766. [DOI] [PubMed] [Google Scholar]
- 14. Ishikawa K, Fish KM, Tilemann L, Rapti K, Aguero J, Santos-Gallego CG, Lee A, Karakikes I, Xie C, Akar FG, Shimada YJ, Gwathmey JK, Asokan A, McPhee S, Samulski J, Samulski RJ, Sigg DC, Weber T, Kranias EG, Hajjar RJ. Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure. Mol Ther 2014;22:2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 2007;115:2506–2515. [DOI] [PubMed] [Google Scholar]
- 16. Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med 2011;3:92ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nowakowski SG, Kolwicz SC, Korte FS, Luo Z, Robinson-Hamm JN, Page JL, Brozovich F, Weiss RS, Tian R, Murry CE, Regnier M. Transgenic overexpression of ribonucleotide reductase improves cardiac performance. Proc Natl Acad Sci USA 2013;110:6187–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moussavi-Harami F, Razumova MV, Racca AW, Cheng Y, Stempien-Otero A, Regnier M. 2-Deoxy adenosine triphosphate improves contraction in human end-stage heart failure. J Mol Cell Cardiol 2015;79:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe DM, Li RA, Wagers AJ, Rossi DJ, Pu WT, Chien KR. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol 2013;31:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinkel R, Lange P, Petersen B, Gottlieb E, Ng JK, Finger S, Horstkotte J, Lee S, Thormann M, Knorr M, El-Aouni C, Boekstegers P, Reichart B, Wenzel P, Niemann H, Kupatt C. Heme oxygenase-1 gene therapy provides cardioprotection via control of post-ischemic inflammation: an experimental study in a pre-clinical pig model. J Am Coll Cardiol 2015;66:154–165. [DOI] [PubMed] [Google Scholar]
- 21. Woitek F, Zentilin L, Hoffman NE, Powers JC, Ottiger I, Parikh S, Kulczycki AM, Hurst M, Ring N, Wang T, Shaikh F, Gross P, Singh H, Kolpakov MA, Linke A, Houser SR, Rizzo V, Sabri A, Madesh M, Giacca M, Recchia FA. Intracoronary cytoprotective gene therapy: a study of VEGF-B167 in a pre-clinical animal model of dilated cardiomyopathy. J Am Coll Cardiol 2015;66:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther 2011;129:97–108. [DOI] [PubMed] [Google Scholar]
- 23. Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther 2011;18:867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005;23:165–175. [DOI] [PubMed] [Google Scholar]
- 25. Turnbull I, Eltoukhy A, Fish K, M, Nonnenmacher M, Ishikawa K, Chen J, Hajjar RJ, Anderson DG, Costa KD. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol Ther 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alba R, Bosch A, Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther 2005;12(Suppl. 1):S18–S27. [DOI] [PubMed] [Google Scholar]
- 27. Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV Serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080. [DOI] [PubMed] [Google Scholar]
- 29. Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011;124:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pacak CA, Sakai Y, Thattaliyath BD, Mah CS, Byrne BJ. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther 2008;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen SJ, Johnston J, Sandhu A, Bish LT, Hovhannisyan R, Jno-Charles O, Sweeney HL, Wilson JM. Enhancing the utility of adeno-associated virus gene transfer through inducible tissue-specific expression. Hum Gene Ther Methods 2013;24:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, Steinbeck G, Baretton G, Middeler G, Katus H, Franz WM. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther 2000;7:232–240. [DOI] [PubMed] [Google Scholar]
- 33. Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 2014;114:101–108. [DOI] [PubMed] [Google Scholar]
- 34. Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016. [DOI] [PubMed] [Google Scholar]
- 35. Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, Silver KH, Shin J, Ewald G, Farr MJ, Anwaruddin S, Plat F, Fisher SJ, AuWerter AT, Pastore JM, Aras R, Penn MS. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur Heart J 2015;36:2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]