Abstract
Aims
Contemporary adjuvant treatment for early breast cancer is associated with improved survival but at the cost of increased risk of cardiotoxicity and cardiac dysfunction. We tested the hypothesis that concomitant therapy with the angiotensin receptor blocker candesartan or the β-blocker metoprolol will alleviate the decline in left ventricular ejection fraction (LVEF) associated with adjuvant, anthracycline-containing regimens with or without trastuzumab and radiation.
Methods and results
In a 2 × 2 factorial, randomized, placebo-controlled, double-blind trial, we assigned 130 adult women with early breast cancer and no serious co-morbidity to the angiotensin receptor blocker candesartan cilexetil, the β-blocker metoprolol succinate, or matching placebos in parallel with adjuvant anticancer therapy. The primary outcome measure was change in LVEF by cardiac magnetic resonance imaging. A priori, a change of 5 percentage points was considered clinically important. There was no interaction between candesartan and metoprolol treatments (P = 0.530). The overall decline in LVEF was 2.6 (95% CI 1.5, 3.8) percentage points in the placebo group and 0.8 (95% CI −0.4, 1.9) in the candesartan group in the intention-to-treat analysis (P-value for between-group difference: 0.026). No effect of metoprolol on the overall decline in LVEF was observed.
Conclusion
In patients treated for early breast cancer with adjuvant anthracycline-containing regimens with or without trastuzumab and radiation, concomitant treatment with candesartan provides protection against early decline in global left ventricular function.
Keywords: Angiotensin antagonist, β-Blocker, Breast cancer, Cardiomyopathy, Imaging, Biomarkers
See page 1681 for the editorial comment on this article (doi:10.1093/eurheartj/ehw133)
Introduction
Progress in detection and treatment of breast cancer during the past two decades has led to substantial improvement in life expectancy but at the cost of increased risk of unintended side effects of cancer therapy.1 Adjuvant breast cancer treatment may encompass anthracycline-containing chemotherapy and in patients with more aggressive human epidermal growth factor receptor-2 (HER-2)-positive cancers, the use of higher doses of anthracyclines followed by taxanes and the anti-HER-2 agent trastuzumab. Both anthracyclines and trastuzumab have been associated with cardiotoxicity and increased risk of developing asymptomatic and symptomatic cardiac dysfunction.2–7 Given the increasing number of long-term survivors after breast cancer treatment, cardiotoxicity has been recognized as a major concern in oncology.1
Neuroendocrine blockade, including treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers, has proved effective in reducing mortality and morbidity in all stages of heart failure, and to prevent the transition from asymptomatic to symptomatic left ventricular dysfunction.8–10 Experimental studies in animals11 as well as observational studies12 and small-scale, randomized, open-label,13,14 single-blind,15,16 or double-blind,17 clinical trials in heterogeneous patient populations with different cancer types and treatment regimens have suggested a potential benefit from early initiation of angiotensin-converting enzyme inhibitors and β-blockers in preventing anthracycline-induced left ventricular dysfunction.12–18 However, a very recent meta-analysis identified only 79 breast cancer patients who had previously been included in randomized studies of β-blockers and 47 patients who had been included in randomized studies of angiotensin-converting enzyme inhibitors or receptor blockers,18 and currently no data are available from randomized, placebo-controlled, double-blind trials in breast cancer patients assessed with cardiac magnetic resonance imaging (MRI) and highly sensitive biochemical markers of cardiac injury. We therefore conducted a randomized, 2 × 2 factorial, placebo-controlled, double-blind clinical trial to test the hypotheses that concomitant therapy with the angiotensin receptor blocker candesartan or the β-blocker metoprolol will attenuate the decline in left ventricular ejection fraction (LVEF) associated with adjuvant, anthracycline-containing regimens with or without trastuzumab and radiation for early breast cancer.
Methods
Study design and participants
PRevention of cArdiac Dysfunction during Adjuvant breast cancer therapy (PRADA) was a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial conducted at Akershus University Hospital, Norway. The study complied with the Declaration of Helsinki. The study protocol was approved by the Regional Ethics Committee of South-Eastern Norway (2010/2890), and the trial was registered in the ClinicalTrials.gov registry (NCT01434134) prior to study initiation. All participants provided written, informed consent.
The rationale for and design of the study have been described in detail previously.19 In brief, women who after breast cancer surgery in the period between September 2011 and September 2014 were scheduled to initiate adjuvant chemotherapy with 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) and had no serious concomitant illness, prior cardiovascular disease, and indication or contraindications for the study drugs were eligible for inclusion. Detailed study inclusion and exclusion criteria are listed in Supplementary material online, Table S1.
Randomization and masking
Participants were randomly assigned on a 1:1:1:1 basis to receive one of the following treatment combinations: candesartan cilexetil 32 mg q.d. and metoprolol succinate 100 mg q.d.; candesartan cilexetil 32 mg q.d. and placebo q.d.; metoprolol succinate 100 mg q.d. and placebo q.d.; or placebo and placebo q.d. Details on patient inclusion and randomization are described in the Supplementary material online. Figure 1 summarizes patient screening and randomization. A similar figure for the per-protocol cohort is provided in the Supplementary material online, Figure S1.
Figure 1.
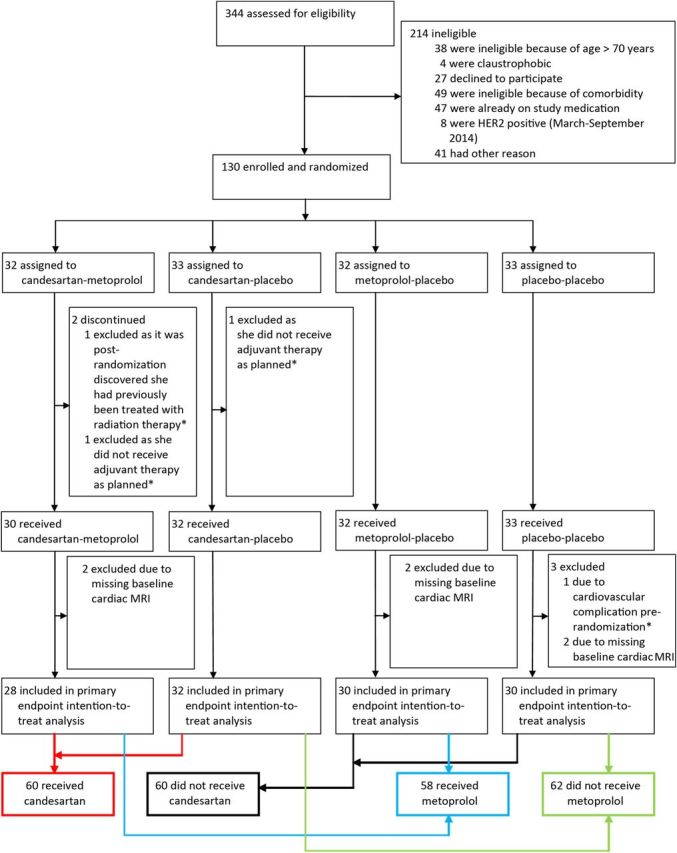
Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): screening and randomization. *Excluded from all analysis. The intention-to-treat population included all patients who had a valid measurement for the primary outcome, received chemotherapy, and had no pre-randomization cardiac complications. HER, human epidermal growth factor receptor; MRI, magnetic resonance imaging.
Procedures
Patients were examined serially with cardiac MRI, blood samples, physical examinations, and electrocardiograms at the following time points during the trial: at baseline, after completion of the first cycle of anthracycline therapy, after completion of the final cycle of anthracycline therapy, and for those concerned, at completion of trastuzumab or radiation therapy (Supplementary material online, Figure S2). Echocardiography was performed at the same time points, except for after completion of the first cycle of anthracyclines. The duration of adjuvant therapy ranged from 10 to 61 weeks depending on the anticancer regimens (Supplementary material online, Figure S2).
Initiation of intervention commenced after baseline examination and prior to initiation of chemotherapy. Dose titration is described in detail in the Supplementary material online. Starting dose for candesartan cilexetil was 8 mg and for metoprolol succinate 50 mg, target dose 32 and 100 mg, respectively. Compliance was registered by counting residual tablets on every second visit during FEC treatment and every third visit during trastuzumab treatment. In addition, the patients were given a diary to register intake of tablets.
All cardiac MRI examinations were performed on a 1.5-T MRI scanner (Achieva; Philips Medical Systems, Best, The Netherlands), using a five-element phased-array cardiac coil. Breath-hold, steady-state-free-precession sequences in contiguous, 8 mm thick short-axis images covering the entire ventricles were used to quantify ejection fraction. All image analyses were performed according to Society for Cardiovascular Magnetic Resonance guidelines20 by a single, board-certified radiologist (S.L.H.) blinded for treatment allocation and study order. Transthoracic echocardiography was performed by using a Vivid E9 (GE Vingmed, Horten, Norway). Images were digitally stored for offline analysis on custom software (EchoPAC, GE Vingmed, Horten, Norway). Left ventricular, two-dimensional peak systolic global longitudinal strain was analysed by an offline semi-automated speckle tracking imaging technique from the three standard apical views. Diastolic function was assessed by the ratio between peak early (E) transmitral velocity by pulsed Doppler and peak early tissue Doppler (E′) by averaging septal and lateral E′ at the base of septal and mitral leaflet, respectively. Analyses were performed by a board-certified physician (G.G.), who was blinded to treatment assignment and study order. Detailed descriptions of the cardiac MRI and echocardiographic analyses are provided in the Supplementary material online.
Cardiac troponin I in serum was measured by using an assay from Abbott Diagnostics: ARCHITECT STAT High Sensitive Troponin, as described previously.21 The level of detection for this assay has been reported to be 1.2 ng/L (range 0–50 000 ng/L) and the level of blank 0.8 ng/L.22 Samples with a level below or equal to the level of blank (i.e. 0.8 ng/L) were assigned a value of 0.8, whereas levels below or equal to the level of detection (i.e. 1.2 ng/L) and greater than the level of blank, were assigned a value of 1.2 ng/L. The coefficient of variation of 10% has been observed at a concentration of 3.0 ng/L. B-type natriuretic peptide (BNP) in plasma was measured by a chemiluminescent microparticle immunoassay (BNP, Abbott Diagnostics; ARCHITECT). The level of detection is 10 pg/mL. Samples with a level <10 pg/mL were assigned a concentration of 5 pg/mL.
Outcome measures
The primary outcome measure of the trial was change in LVEF from baseline to the completion of adjuvant anticancer therapy, as determined by cardiac MRI. Secondary outcome measures included change in right ventricular ejection fraction, as determined by MRI, left ventricular peak systolic global longitudinal strain by two-dimensional speckle tracking imaging, diastolic function (E/E′), and concentrations of cardiac troponin I by a high-sensitivity assay. Other biomarker and echocardiographic indices of diastolic function were considered tertiary outcome measures. A Data Safety and Monitoring Board consisting of a cardiologist, an oncologist, and a statistician was constituted prior to the initiation of the study and monitored adverse events.
Statistical analysis
With α of 0.05, and power (1 − β) of 0.95, 26 patients treated with candesartan and 26 patients treated with metoprolol were required to detect an absolute between-group difference in change in LVEF of 5 ± 5% (SD) percentage points. With a dropout rate of 17%, the adjusted targeted inclusion was estimated to be a minimum of 120 patients. Out of the 120 patients included in the analysis, 28 received candesartan–metoprolol, 32 candesartan–placebo, 30 metoprolol–placebo, and 30 placebo–placebo (Figure 1).
The primary efficacy analysis was performed on an intention-to-treat sample consisting of all validly randomized patients with at least baseline MRI, and a per-protocol sample. All secondary efficacy analyses were also performed on both the intention-to-treat sample and a per-protocol sample. The per-protocol analysis excluded patients who did not have baseline and end-of-study MRI measurements, were not compliant to intervention or discontinued their study medication, withdrew consent, or did not complete adjuvant therapy.
For each continuous efficacy endpoint, we fitted a linear mixed model to all available measurements from three time points: (i) baseline, (ii) after completion of the first cycle of anthracycline therapy, and (iii) end-of-study (either after completion of the final cycle of anthracycline therapy or the completion of trastuzumab or radiation therapy). All models included fixed effects for time, candesartan treatment, metoprolol treatment, candesartan treatment × time interaction, metoprolol treatment × time interaction, age, and left-sided radiation, and a random intercept. To investigate possible interactions between the two treatments, we fitted additional models that included a candesartan × metoprolol interaction term, and applied a likelihood ratio test to the models with and without the treatment interaction term. No statistically significant treatment interactions were observed. Based on the fitted models without the treatment interaction term, we estimated baseline, end-of-study (i.e. the final visit), and change from baseline to end-of-study mean values (with 95% CI) for patients in four groups: (i) treated with candesartan, (ii) not treated with candesartan, (iii) treated with metoprolol, and (iv) not treated with metoprolol. The treatment effects were estimated as the between-group difference in change from baseline to end-of-study for the comparisons of candesartan vs. no candesartan and metoprolol vs. no metoprolol. Troponin I values were log transformed before inclusion in the linear mixed models. All terms in the linear mixed models were pre-specified in the statistical analysis plan.
A P-value of <0.05 was considered statistically significant. The reported P-values are two-sided and not adjusted for multiple comparisons. The statistical analyses were carried out with Stata 14.0 (StataCorp LP).
Results
Between September 2011 and September 2014, 120 patients with early breast cancer having surgery at Department of Surgery at Akershus University Hospital and scheduled for adjuvant therapy with the anthracycline epirubicin were enrolled in the trial and validly randomized to one of the four treatment groups (Figure 1). The four groups were well-balanced concerning patient characteristics at baseline and planned adjuvant anticancer therapy (Table 1). Details of the cancer characteristics are given in the Supplementary material online, Table S2. Adjuvant therapy was administered according to the recommendations of the Norwegian Breast Cancer Group. All patients received FEC, and if indicated taxanes (n = 100; 79.4%), trastuzumab (n = 28; 22.2%) and radiotherapy (n = 82; 65.1%). No patient developed symptomatic heart failure during the study period.
Table 1.
Baseline characteristics of the study population
| Candesartan–metoprolol | Candesartan–placebo | Placebo–metoprolol | Placebo–placebo | |
|---|---|---|---|---|
| n | 30 | 32 | 32 | 32 |
| Age at recruitment (years) | 50.0 ± 8.9 | 51.7 ± 10.7 | 50.5 ± 9.1 | 50.8 ± 9.2 |
| Height (cm) | 166.8 ± 6.6 | 165.5 ± 6.8 | 167.1 ± 6.1 | 168.0 ± 5.5 |
| Weight (kg) | 70.3 ± 11.3 | 71.4 ± 14.3 | 77.7 ± 18.1 | 72.3 ± 13.7 |
| Systolic blood pressure (mmHg) | 124.7 ± 12.8 | 131.9 ± 14.1* | 134.4 ± 13.1** | 130.3 ± 12.9 |
| Diastolic blood pressure (mmHg) | 78.2 ± 11.5 | 80.5 ± 8.5 | 80.5 ± 11.3 | 80.2 ± 9.9 |
| Heart rate (b.p.m.) | 70.8 ± 11.4 | 71.7 ± 6.7 | 73.3 ± 10.1 | 68.3 ± 11.6 |
| Body mass index (kg/m²) | 25.3 ± 3.6 | 25.9 ± 4.3 | 27.8 ± 6.3 | 25.6 ± 4.5 |
| Current smokers | 6/30 (20.0%) | 7/32 (21.9%) | 5/32 (15.6%) | 7/32 (21.9%) |
| Hypertension | 1/30 (3.3%) | 5/32 (15.6%) | 2/32 (6.3%) | 0/32 (0%) |
| Diabetes | 0/30 (0%) | 1/32 (3.1%) | 1/32 (3.1%) | 0/32 (0%) |
| Serum troponin I ≥1.2 ng/L | 7/30 (23.3%) | 12/32 (37.5%) | 9/32 (28.1%) | 13/32 (40.6%) |
| Serum creatinine (mg/dL) | 0.75 ± 0.11 | 0.73 ± 0.10 | 0.79 ± 0.10 | 0.74 ± 0.10 |
| Blood haemoglobin (g/dL) | 13.2 ± 0.9 | 13.3 ± 1.0 | 13.4 ± 0.7 | 13.2 ± 0.8 |
| Baseline MRI (n) | 28 | 32 | 30 | 30 |
| Left ventricular ejection fraction (%) | 62.2 ± 4.4 | 62.3 ± 5.3 | 63.5 ± 5.0 | 63.6 ± 4.1 |
| Right ventricular ejection fraction (%) | 60.6 ± 5.2 | 60.0 ± 5.2 | 62.0 ± 4.8 | 61.2 ± 4.8 |
| Baseline peak systolic global longitudinal strain (n) | 24 | 21 | 23 | 25 |
| Peak systolic global longitudinal strain | −21.7 ± 1.6 | −21.2 ± 1.7 | −21.7 ± 2.2 | −21.6 ± 1.5 |
| Baseline E/E′ (n) | 29 | 30 | 31 | 32 |
| E/E′ | 7.3 ± 2.1 | 7.5 ± 1.9 | 6.7 ± 2.1 | 7.5 ± 1.9 |
| Additional therapy after FEC | ||||
| Trastuzumab | 7/30 (23.3%) | 7/32 (21.9%) | 7/32 (21.9%) | 7/32 (21.9%) |
| Radiation | 18/30 (60.0%) | 19/32 (59.4%) | 22/32 (68.8%) | 23/32 (71.9%) |
| Taxanes | 25/30 (83.3%) | 25/32 (78.1%) | 26/32 (81.3%) | 24/32 (75%) |
Data are expressed as mean ± SD or numbers (per cent).
MRI, magnetic resonance imaging; FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; E/E′, diastolic function.
* P < 0.05 for the comparison with candesartan–metoprolol; **P < 0.01 for the comparison with candesartan–metoprolol; there were no significant differences between the four study groups, except as noted.
There was no statistical interaction between candesartan and metoprolol treatment on the primary endpoint (P = 0.53) or on any of the secondary endpoints. Accordingly, the patients in the two groups receiving candesartan were compared with patients receiving placebo–placebo or metoprolol–placebo (Table 2). The overall decline in the primary outcome measure from baseline to the end-of-study was 2.6 (95% CI 1.5, 3.8) percentage points in the placebo group and 0.8 (95% CI −0.4, 1.9) percentage points in the candesartan group in the intention-to-treat analysis (P-value for between-group difference in linear mixed model analysis: 0.026). Corresponding values in the per-protocol analysis were 2.6 (95% CI 1.4, 3.8) percentage points in the placebo group and 0.6 (95% CI −0.6, 1.8) percentage points in the candesartan group (P = 0.021 in mixed linear model). Notably, the effect of candesartan on change in LVEF was not influenced by adjustment for change in systolic blood pressure. The effect of candesartan on LVEF was consistent across predefined subgroups with no significant interaction observed when patients were stratified according to age, current smoking, history of hypertension, body mass index, radiation, or trastuzumab (Figure 2A). No significant effect of candesartan was observed for right ventricular ejection fraction, left ventricular global longitudinal strain, E/E′, cardiac troponin I (Table 2), or BNP (Supplementary material online, Tables S3 and S4). The effect of candesartan on diastolic function indices is summarized in Supplementary material online, Table S3.
Table 2.
Primary and secondary endpoints, estimated values from linear mixed models (intention-to-treat analysis)
| n | Baseline | EOS | Change from baseline to EOS | Between-group difference in change from baseline to EOS | P-value | |
|---|---|---|---|---|---|---|
| LVEF | ||||||
| No candesartan | 60 | 63.2 (62.0, 64.4) | 60.6 (59.4, 61.8) | −2.6 (−3.8, −1.5) | 1.9 (0.2, 3.5)a | 0.026 |
| Candesartan | 60 | 62.1 (61.0, 63.3) | 61.4 (60.2, 62.6) | −0.8 (−1.9, 0.4) | ||
| No metoprolol | 62 | 62.8 (61.6, 64.0) | 61.0 (59.8, 62.2) | −1.8 (−3.0, −0.7) | 0.2 (−1.4, 1.9) | 0.772 |
| Metoprolol | 58 | 62.5 (61.3, 63.7) | 61.0 (59.8, 62.2) | −1.6 (−2.8, −0.4) | ||
| RVEF | ||||||
| No candesartan | 60 | 61.3 (60.0, 62.5) | 58.9 (57.6, 60.1) | −2.4 (−3.7, −1.1) | 0.8 (−1.0, 2.6) | 0.370 |
| Candesartan | 60 | 60.2 (59.0, 61.4) | 58.7 (57.4, 59.9) | −1.6 (−2.8, −0.3) | ||
| No metoprolol | 62 | 60.4 (59.2, 61.6) | 58.0 (56.8, 59.3) | −2.4 (−3.7, −1.1) | 0.8 (−1.0, 2.6) | 0.377 |
| Metoprolol | 58 | 61.1 (59.8, 62.3) | 59.5 (58.3, 60.8) | −1.6 (−2.9, −0.3) | ||
| LV GLS | ||||||
| No candesartan | 48 | −21.6 (−22.1, −21.1) | −21.0 (−21.5, −20.5) | 0.6 (0.1, 1.1) | −0.7 (−1.4, 0.1) | 0.076 |
| Candesartan | 45 | −21.3 (−21.8, −20.7) | −21.3 (−21.9, −20.8) | −0.1 (−0.6, 0.5) | ||
| No metoprolol | 46 | −21.4 (−21.9, −20.8) | −21.0 (−21.6, −20.5) | 0.3 (−0.2, 0.8) | −0.1 (−0.8, 0.7) | 0.824 |
| Metoprolol | 47 | −21.5 (−22.0, −21.0) | −21.3 (−21.8, −20.7) | 0.2 (−0.3, 0.7) | ||
| E/E′ | ||||||
| No candesartan | 63 | 7.1 (6.6, 7.6) | 7.2 (6.7, 7.7) | 0.1 (−0.4, 0.5) | 0.1 (−0.5, 0.8) | 0.688 |
| Candesartan | 59 | 7.4 (6.9, 7.9) | 7.6 (7.1, 8.1) | 0.2 (−0.2, 0.7) | ||
| No metoprolol | 62 | 7.4 (7.0, 7.9) | 7.2 (6.7, 7.7) | −0.3 (−0.7, 0.2) | 0.8 (0.2, 1.5) | 0.009 |
| Metoprolol | 60 | 7.1 (6.6, 7.5) | 7.6 (7.1, 8.1) | 0.6 (0.1, 1.0) | ||
| Troponin Ib | ||||||
| No candesartan | 64 | 1.1 (0.9, 1.2) | 2.7 (2.3, 3.1) | 2.5 (2.0, 3.1) | 1.1 (0.8, 1.4) | 0.666 |
| Candesartan | 62 | 1.0 (0.8, 1.1) | 2.5 (2.2, 2.9) | 2.6 (2.2, 3.2) | ||
| No metoprolol | 64 | 1.1 (0.9, 1.3) | 2.8 (2.4, 3.3) | 2.6 (2.1, 3.2) | 1.0 (0.7, 1.3) | 0.831 |
| Metoprolol | 62 | 0.9 (0.8, 1.1) | 2.4 (2.0, 2.8) | 2.5 (2.0, 3.1) | ||
Data are expressed as mean (95% CI).
LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction; LV GLS, left ventricular peak systolic global longitudinal strain; EOS, end-of-study; E/E′, diastolic function.
aRounding effect.
bGeometric means.
Figure 2.
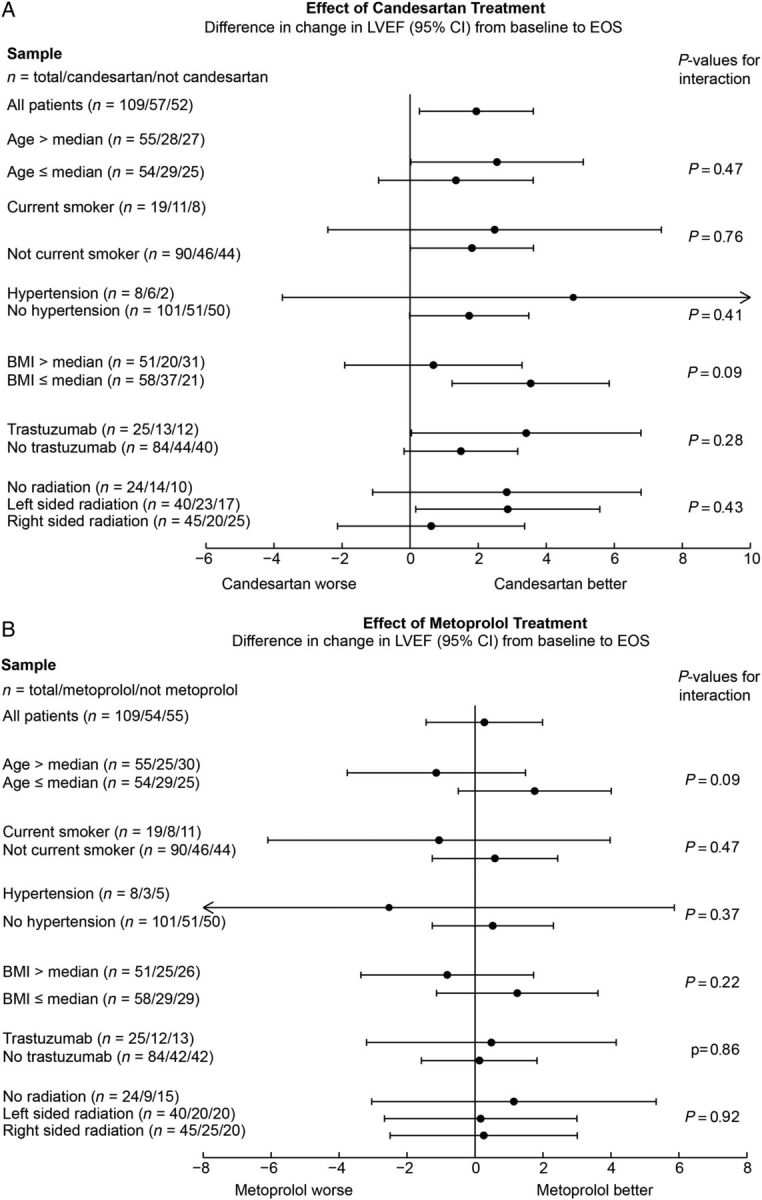
Effect of candesartan and metoprolol on left ventricular ejection fraction during adjuvant therapy for early breast cancer. Shown are the changes in left ventricular ejection fraction expressed in percentage points with 95% confidence intervals. Concomitant therapy with candesartan alleviated the decline in left ventricular ejection fraction observed in the placebo group. This effect was consistent across subgroups with no formal interaction observed when patients were stratified according to age, current smoking, history of hypertension, body mass index, trastuzumab, or radiation (A). No effect of metoprolol on the mean left ventricular ejection fraction was observed (B). Median age at baseline was 49 years, and median body mass index at baseline was 25.6 kg/m². EOS, end-of-study; LVEF, left ventricular ejection fraction by magnetic resonance imaging; BMI, body mass index.
In the two groups that were assigned to metoprolol, the mean LVEF reduction was 1.6 (95% CI 0.4, 2.8) percentage points from baseline to the end-of-study, whereas in the two groups assigned to placebo the corresponding decrease was 1.8 (95% CI 0.7, 3.0). This between-group difference was not statistically significant (P = 0.77) (Figure 2B). There were small but statistically significant increases in E/E′ and BNP levels in the group that received metoprolol compared with the group that did not receive metoprolol. Otherwise, no effect of metoprolol was observed for the secondary outcome measures listed in Table 2 or the diastolic function indices listed in Supplementary material online, Table S3. The effect of metoprolol on heart rate is shown in Supplementary material online, Figure S3.
When considering the four randomization groups separately and using the placebo–placebo group as the reference [−2.8 (95% CI −4.3, −1.3)], the reduction in LVEF was significantly less in the candesartan–placebo group than in the placebo–placebo group [−0.9 (95% CI −2.3, 0.4); P = 0.025] but not significantly less in the candesartan–metoprolol group than in the placebo–placebo group [−0.6 (95% CI −2.1, 0.8); P = 0.075]. No significant difference was observed between the placebo–placebo group and the metoprolol–placebo group [−2.5 (95% CI −3.9, −1.1); P = 0.71] (Supplementary material online, Table S5).
Compliance, side effects, and serious adverse events
Compliance with study drugs was generally excellent. Two, one, three, and three patients did not adhere to the assigned candesartan, candesartan–placebo, metoprolol, and metoprolol–placebo, respectively, at completion of adjuvant therapy. The mean daily study drug dose at completion of adjuvant therapy was 23 ± 11 mg for candesartan, 26 ± 9 mg for candesartan–placebo, 68 ± 34 mg for metoprolol, and 78 ± 32 mg for metoprolol–placebo. There were no unexpected serious adverse events, the intervention was well tolerated, and no patient in the intention-to-treat analysis was withdrawn because of adverse events. Details concerning the serious adverse events are summarized in Supplementary material online, Table S6.
Discussion
This randomized, placebo-controlled, double-blind clinical trial demonstrates that in patients with early breast cancer, contemporary anthracycline-containing adjuvant regimens are associated with a numerically modest absolute reduction in left ventricular systolic function and that concomitant administration of the angiotensin receptor blocker candesartan significantly alleviates the decline in LVEF that occurs during adjuvant therapy. Importantly, the effect seemed independent of a direct haemodynamic effect of candesartan as adjustment for change in systolic blood pressure did not impact on the results. No significant beneficial effect of candesartan was observed for the secondary endpoints right ventricular ejection fraction, left ventricular global longitudinal strain, and E/E′, probably reflecting the higher methodological variability of these measurements compared with MRI assessment of LVEF.23 Candesartan was also ineffective in reducing the increase in circulating cardiac troponin I associated with anthracycline-containing adjuvant therapy, suggesting that angiotensin receptor blockade may not interfere with the direct cardiotoxic effect of anthracyclines, but rather plays a role in the myocardial remodelling process that occurs after cardiac injury.24
In contrast to the attenuation of the reduction in left ventricular function observed for candesartan, no short-term beneficial effect was observed for the β-blocker metoprolol. This is in contrast to findings in some prior, small-scale, randomized studies.14,15,17 Potential reasons for this apparent discrepancy include that patients in prior studies may have received higher doses of anthracyclines and had a higher prevalence of cardiovascular co-morbidities, which could contribute to a favourable effect of β-blockade. Moreover, given that the reduction in LVEF in the placebo–placebo group in our study was less than originally anticipated, the power of the study to detect between-group differences was reduced. Accordingly, the apparent lack of effect of metoprolol on LVEF may also be due to inadequate statistical power and does not rule out a beneficial effect of β-blockade. Finally, we cannot rule out the possibility that an alternative β-blocker or a higher dose would have proved effective.
Although some studies have indicated that echocardiographic indices of diastolic function may detect subclinical changes in cardiac function during cancer treatment, their predictive value remains unproven.23,25 Moreover, in the oncological setting, changes in diastolic indices such as the E/E′ ratio could be the result of changes in loading conditions secondary to the nausea and vomiting commonly associated with chemotherapy.25 We observed a small increase in E/E′, an index closely associated with left ventricular filling, in the metoprolol but not in the no-metoprolol group. This increase is likely associated with a direct haemodynamic effect of β-blockade.26 Similarly, BNP concentrations increased in the metoprolol group but remained unchanged in the candesartan group during adjuvant chemotherapy. It is well documented that β-blockade, via its effects on heart rate and stroke volume, causes increased release of natriuretic peptides.27 Accordingly, in the absence of development of symptomatic ventricular dysfunction, it is not surprising that metoprolol is associated with an increase in BNP levels in the current study. The lack of effect of candesartan on BNP can probably be accounted for by its relatively high intra- and inter-individual variability28 and is in accordance with other recent studies examining the effect of anthracycline therapy on BNP.29
The current results may have potential important implications. A reduction in LVEF is commonly considered a late-occurring phenomenon in the cardiotoxic process, manifesting itself first after myocardial reserves are exhausted.1 This study, using the reference method for assessment of left ventricular function, demonstrates that low-to-moderate doses of anthracyclines with or without trastuzumab or radiation are associated with a numerically modest, but significant reduction in LVEF that was somewhat less than that we a priori had defined as a clinically important difference. This observation is in accordance with another recent, smaller (n = 58 with cardiac MRI imaging) randomized, controlled, but non-blinded trial of malignant haemopathies receiving anthracycline-based chemotherapy that found an absolute reduction of LVEF of 3.0 percentage points in the placebo group.14 Moreover, our findings are in accordance with those of an observational study using cardiac MRI in a more heterogeneous population of cancer patients (n = 53) treated with low-to-moderate dose anthracycline-based chemotherapy.2 Although the latter study included patients with prior coronary artery disease and a high proportion of patients had hypertension (40%) and other cardiovascular risk factors, the absolute reduction in LVEF was only moderately higher than in the current, all-female previously healthy study population. Taken together, these studies consistently show that contemporary doses of anthracycline-containing chemotherapy regimens are associated with a modest, but highly statistically significant reduction of LVEF, but that development of severe ventricular dysfunction is a rare-occurring event in the short term.
A crucial question, however, is whether these numerically modest early changes in LVEF and the prevention of early decline in ventricular function may have any consequences for the long-term risk of developing more severe asymptomatic or symptomatic ventricular dysfunction. As imaging methods used in the past may have lacked the precision to identify minor LVEF changes, the long-term implications of reduction in LVEF following the exposure to cardiotoxic agents are not yet fully known, but it is well documented that the process of left ventricular dysfunction after other types of myocardial injury is progressive and early intervention is crucial to prevent deterioration in the long term. The notion of the importance of early intervention is also supported by observational data, suggesting that the duration from completion of high-dose anthracycline therapy to initiation of angiotensin-converting enzyme inhibition is a key determinant of the magnitude of the beneficial effect.30 This was recently highlighted by Cardinale et al., who in a prospective study of 2625 anthracycline-receiving patients reported an association between end-of-chemotherapy LVEF and cardiotoxicity development.6 In our study, concomitant treatment with candesartan prevented the early LVEF decline associated with adjuvant therapy for breast cancer. Accordingly, it seems likely that this attenuation of the early decline in ventricular function may have beneficial long-term consequences concerning the risk of developing asymptomatic or symptomatic ventricular dysfunction.
Strengths of the current study include the 2 × 2 factorial design, permitting a head-to-head comparison of two different drugs, the use of serial cardiac MRI investigations in a homogeneous cohort of patients with breast cancer treated with contemporary adjuvant therapy, including low-to-moderate doses of epirubicin. Accordingly, our results are generalizable to a large number of women with early breast cancer. Using a method with low variability, the current trial had a high likelihood to detect even modest differences between groups. Limitations of the current report include the lack of follow-up information beyond the adjuvant therapy period, but long-term follow-up of the participants with repeat cardiac MRI investigations is planned. We excluded some patients at high cardiovascular risk, but many of these, including those with prior cardiovascular disease, had indications for treatment with β-blockers or inhibitors of the renin–angiotensin system. The dose of metoprolol attained was moderately high, but resulted in a significant reduction in heart rate compared with the placebo group, suggesting good compliance and adequate β-blockade. Although predefined subgroup analyses showed a consistent effect across subgroups, including those who received higher dose anthracyclines and trastuzumab, the statistical power to conduct subgroup analyses in this study is limited, and this observation must be verified in adequately powered trials with long-term follow-up.
In conclusion, using cardiac MRI we found that adjuvant breast cancer treatment is associated with a decline in LVEF that is alleviated by concomitant neurohormonal blockade with candesartan. Long-term follow-up of these patients will document whether the beneficial effect of candesartan is sustained and will translate into reduced incidence of left ventricular dysfunction.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors' contributions
M.W.F. performed statistical analysis. T.O., J.G., K.S., P.H., T.H.S., J.S.-M., and F.K.-B. handled funding and supervision. G.G., S.L.H., B.G., Å.B., and H.R. acquired the data. T.O., A.H.R., and J.G. conceived and designed the research. T.O., G.G., and S.L.H. drafted the manuscript. A.H.R., P.H., J.S.-M., M.W.F., B.G., F.K.-B., Å.B., T.H.S., T.-A.H., H.R., K.S., and J.G. made critical revision of the manuscript for key intellectual content. T.-A.H. was responsible for the biochemical analysis.
Funding
This work was supported by the University of Oslo, The Extra Foundation for Health and Rehabilitation, The Norwegian Cancer Society, Akershus University Hospital, Abbott Diagnostics, and AstraZeneca. Study medications and matching placebos were provided free of charge by AstraZeneca (Mölndal, Sweden). Reagents for the analysis of high-sensitivity cardiac troponin I were provided free of charge by Abbott Diagnostics (Abbott Park, IL, USA). The funders of the study played no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. Funding to pay the Open Access publication charges for this article was provided by the University of Oslo.
Conflict of interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. T.O. has served on advisory boards for Abbott Diagnostics and Novartis, has received research support from AstraZeneca and Abbott Diagnostics via Akershus University Hospital, and speakers’ honoraria from Roche Diagnostics. No other disclosure were reported.
Supplementary Material
Acknowledgements
We gratefully acknowledge the important work of the Data and Safety Monitoring Board. We are indebted to the staff of the Clinical Research Unit, Division of Medicine, Akershus University Hospital for skilful assistance with all aspects of the trial execution, and would particularly thank Annika Lorentzen, Vigdis Bakkelund, Marit Holmefjord Pedersen, and Mohammad Osman Pervez for their contributions. We also thank the radiographer staff at the cardiac MRI unit of the Department of Radiology at Akershus University Hospital. The expert assistance of Dominic Anthony Hoff, Senior Adviser, Department for Research Administration & Biobanking, Oslo University Hospital for registry support, is also appreciated.
References
- 1. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015;12:547–558. [DOI] [PubMed] [Google Scholar]
- 2. Drafts BC, Twomley KM, D'Agostino R, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chien KR. Herceptin and the heart—a molecular modifier of cardiac failure. N Engl J Med 2006;354:789–790. [DOI] [PubMed] [Google Scholar]
- 4. Tan TC, Neilan TG, Francis S, Plana JC, Scherrer-Crosbie M. Anthracycline-induced cardiomyopathy in adults. Compr Physiol 2015;5:1517–1540. [DOI] [PubMed] [Google Scholar]
- 5. Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014;64:938–945. [DOI] [PubMed] [Google Scholar]
- 6. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 7. Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013;34:1102–1111. [DOI] [PubMed] [Google Scholar]
- 8. The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–691. [DOI] [PubMed] [Google Scholar]
- 9. The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 10. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 11. Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc 2014;3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail 2013;6:420–426. [DOI] [PubMed] [Google Scholar]
- 13. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474–2481. [DOI] [PubMed] [Google Scholar]
- 14. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, Morales-Ruiz M, Perea RJ, Monzo M, Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013;61:2355–2362. [DOI] [PubMed] [Google Scholar]
- 15. Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006;48:2258–2262. [DOI] [PubMed] [Google Scholar]
- 16. Dessi M, Piras A, Madeddu C, Cadeddu C, Deidda M, Massa E, Antoni G, Mantovani G, Mercuro G. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubucin-induced inflammation, oxidative stress and myocardial dysfunction. Exper Therapeutic Med 2011;2:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, Berk V, Ardic I, Ergin A, Lam YY. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 2013;167:2306–2310. [DOI] [PubMed] [Google Scholar]
- 18. Yun S, Vincelette ND, Abraham I. Cardioprotective role of beta-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: a systematic review and meta-analysis. Postgrad Med J 2015;91:627–633. [DOI] [PubMed] [Google Scholar]
- 19. Heck SL, Gulati G, Ree AH, Schulz-Menger J, Gravdehaug B, Rosjo H, Steine K, Bratland A, Hoffmann P, Geisler J, Omland T. Rationale and design of the prevention of cardiac dysfunction during an adjuvant breast cancer therapy (PRADA) trial. Cardiology 2012;123:240–247. [DOI] [PubMed] [Google Scholar]
- 20. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 22. Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- 23. Altena R, Perik PJ, van Veldhuisen DJ, de Vries EGE, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol 2009;10:391–399. [DOI] [PubMed] [Google Scholar]
- 24. Nikitovic D, Juranek I, Wilks MF, Tzardi M, Tsatsakis A, Tzanakakis GN. Anthracycline-dependent cardiotoxicity and extracellular matrix remodeling. Chest 2014;146:1123–1130. [DOI] [PubMed] [Google Scholar]
- 25. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Silke B, Verma SP, Midtbo KA, Muller P, Frais MA, Reynolds G, Taylor SH. A haemodynamic study of the effects of combined slow-calcium channel blockade (nisoldipine) and beta-blockade (metoprolol) in coronary heart disease. Int J Cardiol 1986;13:231–241. [DOI] [PubMed] [Google Scholar]
- 27. Luchner A, Burnett JJC, Jougasaki M, Hense H-W, Riegger GAJ, Schunkert H. Augmentation of the cardiac natriuretic peptides by beta-receptor antagonism: evidence from a population-based study. J Am Coll Cardiol 1998;32:1839–1844. [DOI] [PubMed] [Google Scholar]
- 28. Wu AHB. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J 2006;152:828–834. [DOI] [PubMed] [Google Scholar]
- 29. Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem 2015;61:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


