Abstract
Objective(s):
Human and animal studies have shown a close relationship between obesity and asthma severity. Here, we examined the effects of diet-induced obesity (DIO) on the expression levels of IL-1β, IRAK-1 and TRAF-6 mRNA in male Wistar rats tracheal after sensitization with ovalbumin (OVA).
Materials and Methods:
Twenty male Wistar rats divided to four groups, included, control group with normal diet (C+ND), OVA-sensitized group with normal diet (S+ND), control group with high-fat diet (C+HFD), and OVA-sensitized group with high-fat diet (S+HFD). All animals fed for 8 weeks with standard pelts or high-fat diet, and then were sensitized and challenged with OVA or saline for another 4 weeks with designed regimens. At the end of study, trachea isolated and examined for expression levels of IL-1β, IRAK-1 and TRAF-6 mRNA with RT-PCR method.
Results:
Diet-induced obesity groups developed increased weight, obesity indexes and lipid profiles (P<0.05 to P<0.001). The expression levels of IL-1β mRNA in OVA-sensitization groups (S+ND and S+HFD) showed a significantly increased when compared with control group. Also in S+HFD group, expression level of TRAF-6 mRNA was higher than other groups (P<0.001). IRAK-1 expression level was high in S+HFD compared with control group.IL-1β and TRAF-6 mRNA correlated positively with obesity indexes.
Conclusion:
The results showed that DIO causes overexpression of IL-1β, IRAK-1 and TRAF-6 mRNA in an experimental model of asthma. Our results suggested that in obese-asthmatic conditions locally production and activation of pro-inflammatory agents can be increased. These findings showed that possible mechanism for obesity-asthma relationships.
Keywords: Asthma, IL-1β, IRAK-1, Obesity, Tracheal, TRAF-6
Introduction
Epidemiological studies indicate an association between obesity and asthma (1). Importantly, most people with asthma disease in the emergency department of health centers are overweight or obese subjects (2). Clinical studies have shown that asthma tends to be very severe in the obese, and they do not respond fully to treatment with standard drugs (3). The airway hyper-responsiveness, a characteristic of asthma, has been shown in obese mice, regardless of the cause of their obesity (4). In both obese humans and mice, chronic low-grade systemic inflammation is characterized by an increase in circulating leukocytes and raised serum concentrations of cytokines, chemokine, and acute phase proteins (5). The effect of obesity on lung tissue involve in the inflammatory changes in pulmonary microenvironment (6). Therefore, it is important to understand the lung inflammation differences between obese and lean subjects.
Inflammatory mediators produced by adipose tissue may participate in the immune response of pulmonary system (7). Nevertheless, the exact mechanism of asthma-obesity relationship remains uncertain. On the other hand, strong evidence in recent years had emphasis on the role of a variety of cytokines in pulmonary inflammation responses (8). Accordingly, studies have reported that serum levels of cytokines in the blood and bronchoalveolar lavage (BAL) fluid from asthmatic obese people have increased (9). Apart from the potential effects of pro-inflammatory products, the specific cytokines, particularly IL (interleukin)-1β, may also interact with altered airway responsiveness in asthma. IL-1β is a key cytokine involved in systemic and local immune responses in obesity and asthma. IL-1β is primarily produced by monocytes and macrophages, but other cells including epithelial cells, endothelial cells, B and T lymphocytes, have also been shown to release IL-1β (10, 11). Studies have shown that treatment of animals with IL-1 receptor antagonist (IL-1ra) could decrease hyper-responsiveness to histamine (12) and substance P, and also reduce lung inflammation and prevent leukocyte infiltration (13).
While many studies have emphasized the role of IL-1β in inflammatory condition, there was little attention given to the autocrine role of IL-1β in obesity associated with asthma. It has been shown that there is the synthesis of non-regulated and prolonged release of IL-1β that might involve with the pathogenesis of chronic inflammatory conditions, such as inflammatory bowel disease, psoriasis and rheumatoid arthritis (14). In addition, a lot of evidence is available that show that IL-1β modulates contraction and relaxation responses in asthmatic statue by direct effect on airways (15). In signaling pathway, IL-1β forms a ligand-induced complex with IL-1 type I receptor (IL-1RI) and IL-1 receptor accessory protein (IL-1RAcP). Then, IL-1 receptor associated kinase (IRAK)-1 attaches to receptor complex. Finally, with activation of IRAK-1, it interacts with tumor necrosis factor receptor associated factor (TRAF)-6 which is required for the activation of IL-1-induced NF-κB (16). This study was proposed to determine the expression of IL-1β, IRAK-1 and TRAF-6 mRNA in tracheal tissue and evaluate the differences of their mRNA levels in obese and lean male Wistar rats in experimental asthmatic model.
Materials and Methods
Animals and diets
Twenty male Wistar rats (8 weeks old, weighing approximately 160 g) were obtained from animal house of Tabriz University of Medical Science. All animals were placed in cages under controlled conditions with a 22 °C with 12-12 hr light-dark cycle. Food and water throughout the accommodation and experimental period was provided ad libitum.
After a week of accommodation, all rats randomly divided to four groups (5 rats in each group), included: control group with normal diet (C+ND), OVA-sensitized group with normal diet (S+ND), control group with high-fat diet (C+HFD), and OVA-sensitized with high-fat diet (S+HFD). Diet-induced obesity (DIO) model was prepared according to the method to the previous study (17). Briefly, in DIO groups (C+HFD and S+HFD), rats were fed with high fat diets (42 % energy from fat, 19% energy from protein and 39% energy from carbohydrate) and other rats (C+ND and S+ND) were fed standard rat chow (11% energy from fat, 28% energy from protein and 61% energy from carbohydrate).
After 8 weeks, sensitization of animals to ovalbumin (OVA) in S+ND and S+HFD groups was performed with following protocol. The experiment continued for four weeks for sensitized with OVA with the previous regime.
Animal sensitization
Sensitization of animals to OVA in S+ND and S+HFD groups was performed using the method described previously (18, 19). Briefly, 1 mg OA and 200 mg Al (OH)3 (Sigma) injected intraperitoneal (IP) on day 1 and 8. From day 14, the animals were placed in a closed chamber, with dimensions 30×20×20 cm, for exposing to an aerosol of 4% OVA for 18 ± 1 days for 15 min daily by using a nebulizer (CX3, Omron Health care Europe B.V., and the Netherlands). All control animals (C+ND and C+HFD) were treated similarly but saline was used instead of OVA solution. The study was approved by the Ethical Committee of Tabriz University of Medical Sciences.
Body weight
The animals were weighted weekly on a certain day (at 16.00) during the experiment. At the end of study, rats were anesthetized by 50 mg/kg ketamine and xylazine injection. Then they were weighted and naso-anal length; the distance between nose and anus of rats, measured. Final body weight, Lee index, and percentage of body fat (BF %) were also determined to calculation of obesity indexes used in rodents. All obesity indexes described previously. Briefly, Table 1 shows how to calculate all obesity indexes.
Table 1.
Obesity indexes formula
Biochemical measurements
The fasted rats after anesthetized with ketamine and xylazine, blood samples (3-5 ml per rat) were collected from the heart. The plasma total cholesterol (TC), triglycerides (TG) and HDL-C concentrations were measured using an auto blood analyzer (Bayer corp. USA). Triglycerides, total cholesterol and HDL-C kits were obtained from Pars Azmoon CO, Iran. LDL-C was evaluated using the Friedewald formula: LDL-C= TC-[HDL-C+TG/5] (20).
Lysate preparation from tracheal tissue
After anesthetized with ketamine injection, chest and neck were opened and the trachea was isolated. Trachea isolated immediately weighed and recommended amount of tissue removed. Tissue grinded homogenized tissue was provided. Then, after adding of 600 μl RNase-free water and 20 μl reconstituted proteinase K, lysate was incubated and spin 1 min. For the RNA binding to column, about 650 μl of lysate with ethanol mixture was load to column and centrifuged for 1 min. Then, DNase I treat performed followed wash step. RNA was eluted from column and the purified RNA sample kept at −70 °C for RT-PCR analysis.
Real-time PCR
RNA content and purity was measured using Nanodrop 1000 spectrophotometer (Thermo scientific, Wilmington, DE, USA). For determination of IL-1β, IRAK-1 and TRAF-6 mRNA expression levels RevertAid First-Strand cDNA Synthesis Kit (Ferment as GmBH, Leon-Rot, Germany) with aid of random hexamer primers and MMLV reverse transcriptase (as a complete system for efficient synthesis of first-strand cDNA from mRNA or total RNA templates) were used.
By using SYBR Green master mix (Exiqon) each cDNA was used as a template for separate assay for mRNA quantitative real-time PCR. Table 2 shows locked nucleic acid (LNA) forward and reverse primer sets (Exiqon) for mRNA. Real-time PCR reactions were performed on a Rotor-Gene 6000 instrument (Corbett Life Science, Australia).
Table 2.
Primer set list for mRNAs
| Gene name | Accession number | Primer sequence a | |
|---|---|---|---|
| IL-1β | NM_031512 | Forward primer 5’-AGA GTG TGG ATC CCA AAC AA-3’ | |
| Reverse primer 5’-AGT CAA CTA TGT CCC GAC CA -3’ | |||
| TRAF-6 | NM_001107754.2 | Sense: 5’-CAG TCC CCT GCA CATT-3’ | |
| Antisense 3’-GAG GAG GCA TCG CAT-5’ | |||
| IRAK-1 | NM_001127555 | Forward primer 5’-GAG AGT GTT CCT GGC CTC TC -3’ | |
| Reverse primer 5’-GCT GGG TTG ATG ATG ATC TG -3’ | |||
| Beta Gusp | NM_017015 | Forward primer 5’-GTGGGGATAATGACTTGCAG -3’ | |
| Reverse primer 5’-GGAACCCCTGGTAGAACAGT-3’ | |||
Sequences were derived from NCBI (www.ncbi.nlm.nih.gov)
The amount of PCR products was normalized with housekeeping beta-glucuronidase gene for mRNA samples (23). The 2-(ΔΔ Ct) method was used to determine relative quantitative levels of IL-1β, IRAK-1 and TRAF-6 mRNA. The results were expressed as fold change versus the relevant controls (24).
Statistical analysis
Results were given are mean ±SD. The data between different groups were compared using one way analysis of variance (ANOVA) with Tukey-Kramer post-test. P less than 0.05 were considered significant. The correlation between IL-1β, IRAK-1 and TRAF-6 mRNA levels with weight was assessed with Pearson’s correlation coefficient.
Results
Body weight
Mean (±SD) initial body weight, final body weight, Lee index, and percentage of body fat for all groups are given in Table 3. Results showed that final body weight, Lee index, and percentage of body fat in C+HFD and S+HFD animals were significantly higher than C+ND and S+ND animals (P<0.05 to P<0.001). But, there were no significant differences in final body weight, Lee index, and percentage of body fat between the C+ND versus S+ND and C+HFD versus S+HFD.
Table 3.
Weight changes and obesity indexes in experimental groups
| variables | C+ND | S+ND | C+HFD | S+HFD |
|---|---|---|---|---|
| Initial body weight (g) | 158±6.67 | 153.20±11.38 | 155.20±5.40 | 152±10.78 |
| Final body weight (g) | 318.20±23.49 | 314.80±23.63 | 353.20±12.67 | 367±36.34* + |
| Percentage of body weight change (%) | 101.18±6.34 | 105.96±7.73 | 127.81±11.89** + | 140.40±16.10*** ++ |
| Lee index (mg/mm) | 304.56±7.62 | 306.24±3.54 | 319.93±2.79*** ++ | 321.33±3.15*** ++ |
| Percentage of body fat (%) | 19.89±5.45 | 18.57±2.58 | 28.55±2.03** ++ | 29.58±2.30** ++ |
Values are represent as mean±SD. C+ND; control with normal diet, S+ND; OVA-sensitized with normal diet, C+HFD; control with high-fat diet, S+HFD; OVA-sensitized with high-fat diet. Differences between the results of C+ND with those of other groups;
; P<0.05,
; P<0.01,
; P<0.001.
Differences between the results of S+ND with those of control high fat diet and sensitized high fat diet;
; P<0.05,
; P<0.01,
+++; P<0.001.
For each group, n=5
Serum lipid profile
As seen in Figure 1, lipid profile (TC, TG, HDL-C and LDL-C) in obese groups (C+HFD and S+HFD) were significantly higher than the control groups (P<0.05 to P<0.001). With the exception of differences on HDL between C+ND with S+ND (P<0.05), a significant differences between the control groups together (C+ND versus S+ND) and obese groups together (C+HFD versus S+HFD) were not observed.
Figure 1.
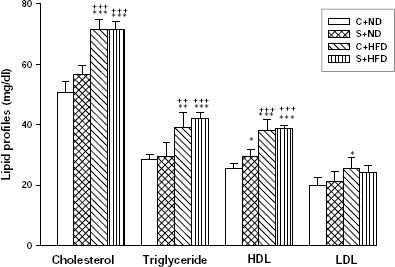
The serum levels of total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL-C) and low density lipoprotein (LDL-C) in experimental groups. Values are depicted as mean±SD. C+ND; control with normal diet, S+ND; OVA-sensitized with normal diet, C+HFD; control with high-fat diet, S+HFD; OVA-sensitized with high-fat diet. Statistical differences between the control and other groups; *; P<0.05, **; P<0.01, ***; P<0.001. Statistical differences between the sensitized normal diet with obese groups; +; P<0.05, ++; P<0.01, +++; P<0.001. For each group, n=5
Relationship between lipid profile with IL-1β, IRAK-1, and TRAF-6 mRNA expression
Pearson correlation analysis of lipid profile with IL-1 β, IRAK-1 and TRAF-6 was shown in Table 4. As shown, IL-1 β mRNA with TG and HDL (P<0.05), IRAK-1 borderline with HDL (P=0.073), and TRAF-6 with cholesterol, TG, and HDL (P<0.05), a significant positive correlation was found.
Table 4.
Pearson correlation analysis of lipid profile with IL-1 β, IRAK-1 and TRAF-6
| Lipid profile | IL-1 β | IRAK-1 | TRAF-6 | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Cholesterol | 0.365 | 0.114 | 0.332 | 0.152 | 0.448 | 0.048 |
| Triglyceride | 0.438 | 0.054 | 0.309 | 0.186 | 0.540 | 0.014 |
| HDL | 0.460 | 0.041 | 0.409 | 0.073 | 0.482 | 0.031 |
| LDL | 0.032 | 0.893 | 0.081 | 0.734 | 0.178 | 0.452 |
IL-1β mRNA expression in tracheal tissue
Figure 2 presents the IL-1β mRNA expressions in rat tracheal tissue. OVA-sensitization caused a significant increased in IL-1β mRNA expression in S+HFD group (P<0.01) and S+ND group (P<0.05) when compared with C+ND group, but with C+HFD group was not significant. Although the amount of IL-1β mRNA was higher in S+HFD (2.17±0.44) group than S+ND (1.84±0.37) group, but not significant differences were found between those groups. There was not any significant difference between C+ND and C+HFD groups.
Figure 2.
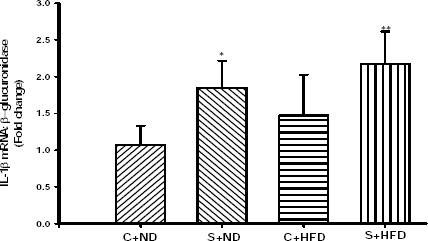
Expression level of IL-1β mRNA in the tracheal tissue of experimental groups. Values are depicted as mean±SD. C+ND; control with normal diet, S+ND; OVA-sensitized with normal diet, C+HFD; control with high-fat diet, S+HFD; OVA-sensitized with high-fat diet. Statistical differences between the control and other groups; *; P<0.05, **; P<0.01. For each group, n=5
Relationship between IL-1β mRNA expression and obesity indexes
A significant positive correlation was found between IL-1β mRNA expression and percentage of weight changes (r=0.607, P<0.005; Figure 3). This correlation was not significant with other obesity indexes, Lee index (r=0.428, P=0.060) and percentage of body fat (r=0.344, P=0.138).
Figure 3.

Pearson correlation analysis of IL-1β mRNA with percentage of body weight changes in the study groups. There was a significant positive correlation between IL-1 mRNA expression and percentage of body weight changes (correlation coefficient =0.434, P<0.05)
IRAK-1 mRNA expression in tracheal tissue
In Figure 4, IRAK-1 mRNA expressions in tracheal tissue in different groups are shown. The IRAK-1 mRNA expressions in group S+HFD increased significantly compared to C+ND (P<0.01) and C+HFD (P<0.05) groups, but was not seen any significant difference with S+ND group. Also, IRAK-1 mRNA expression in normal diet sensitized group increased significantly compared with C+ND (P<0.05), but no difference greater than C+HFD group was observed. There was not any significant difference between C+ND with C+HFD.
Figure 4.

Expression level of IRAK-1 mRNA in the tracheal tissue of experimental groups. Values are depicted as mean±SD. C+ND; control with normal diet, S+ND; OVA-sensitized with normal diet, C+HFD; control with high-fat diet, S+HFD; OVA-sensitized with high-fat diet, TRAF-6; TNF receptor associated factor-6. Differences between the results of normal diet with those of other groups; *; P<0.05, **; P<0.01. Differences between the results of control high fat diet with those of sensitized high fat diet; #; P<0.05. For each group, n=5
Relationship between IRAK-1 mRNA expression and obesity indexes
There was not significant correlation between IRAK-1 mRNA expression with obesity indexes, percentage of weight changes (r=0.398, P<0.082), Lee index (r=0.178, P=0.453) and percentage of body fat (r=0.090, P=0.707).
TRAF-6 mRNA expression in tracheal tissue
Figure 5 shows that TRAF-6 mRNA expressions in group S+HFD increased significantly compared to C+ND, C+HFD, and S+ND groups (P<0.001). Despite high TRAF-6 mRNA expression in lean sensitized group (1.49±0.50), this increase was not statistically significant compared with lean and obese non-sensitized groups. There was not any significant difference between C+ND with C+HFD.
Figure 5.
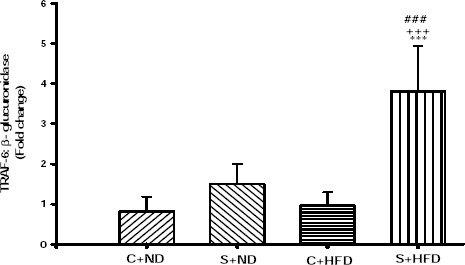
Expression level of TRAF-6 mRNA in the tracheal tissue of experimental groups. Values are depicted as mean±SD. C+ND; control with normal diet, S+ND; OVA-sensitized with normal diet, C+HFD; control with high-fat diet, S+HFD; OVA-sensitized with high-fat diet, TRAF-6; TNF receptor associated factor-6. Differences between the results of normal diet with those of other groups; ***; P<0.001. Differences between the results of sensitized normal diet with those of control high fat diet and sensitized high fat diet; +++; P<0.001. Differences between the results of control high fat diet with those of sensitized high fat diet; ###; P<0.001. For each group, n=5
Relationship between TRAF-6 mRNA expression and obesity indexes
There was a positive correlation between TRAF-6 mRNA expression and percentage of weight changes (r= 0.704, P<0.001; Figure 6). But this correlation was not significant when compared with Lee index (r= 0.443, P=0.050) and percentage of body fat (r= 0.413, P=0.71).
Figure 6.
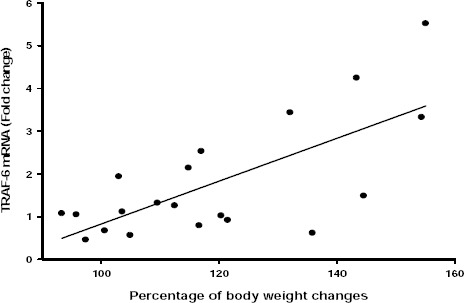
Pearson correlation analysis of TRAF-6 mRNA with percentage of body weight changes in the study groups. There was a significant positive correlation between IL-1 mRNA expression and percentage of body weight changes (correlation coefficient =0.686, P<0.001)
Discussion
In the present study the effect of high fat diet on IL-1 β, IRAK-1 and TRAF-6 mRNA expression in trachea of OVA-sensitized and non-sensitized male Wistar rats were examined.
The present study indicated that enhanced IL-1 β mRNA expression in trachea in sensitized lean and obese rats compared with non sensitized obese and lean control groups. Although, the most increase was seen in obese sensitized group, but the difference was not statistically significant with non-obese sensitized group. Although a significant positive correlation was seen between IL-1β mRNA expression and the value of the percent weight changes, this correlation was not significant with other obesity indexes (Lee and fat percent) in rodent. After diet-induced obesity and sensitized with OVA, TRAF-6 mRNA expression in trachea was highly significant in obese sensitized rats compared with other groups. The correlation of TRAF-6 mRNA expression in trachea with percentage weight changes also was positively significant, but there were not significant correlation with other obesity indexes. Moreover, IRAK-1 mRNA expression was high in obese sensitized rats when compared with control and obese non sensitized groups. There was not correlation between IRAK-1 mRNA and obesity indexes in rodent.
Obesity in humans is mostly derived from the interaction of consumption high fat diet and low expenditure of energy (25). The high prevalence of asthma in overweight and obesity in adults and children has been shown by several clinical trials (26). Different kinds of genetically engineered animals were used to investigate the mechanisms responsible to this possible relationship between obesity and asthma. The genetically obese mice have some complications by comorbid conditions in their studies designs such as leptin deficiency (ob/ob mouse) and leptin receptor deficiency (db/db mouse) which may affect on defective innate and adaptive immune responses in airways (27). So, the model of obesity caused by high fat diet was used in this study. Wistar rats showed increased body weight and obesity index (percentage of weight changes, Lee index and fat percent) after eating high fat diet for 12 weeks which were seen in previous studies using the same method of diet-induced obesity (17). In addition, analysis of lipid profiles (cholesterol, triglyceride, HDL-C and LDL-C) in this study showed that diet-induced obesity caused a significant increased in lipid profiles.
Obesity is a low-grade chronic pro-inflammatory state that affects immune system responses of obese asthmatic individuals, causes inflammation of airways and consequently can be lead to deterioration of asthma (28). It seems that the measurement of the effects of inflammation in the airways shows a better understanding of the role of inflammation in obese asthmatic patients than its measurement systemically. Local inflammation in arthrosclerosis plaque has the main role in the pathogenesis of coronary arterial disease and systemic inflammation is thought to interact with this process (29). Perhaps such a similar theory is also associated in the obese asthmatic conditions.
There is strong evidence in recent years that have emphasized on a variety of cytokines in pulmonary inflammatory response in developing asthma (30). Specific cytokines, particularly IL-1β, may have an interaction with the change of airway responsiveness in asthma (14). It has been demonstrated that IL-1β caused airway hyper-responsiveness and impairment of beta adrenergic relaxation in experimentally induced asthma model (31). Accordingly, studies have shown that treatment with IL-1 antagonist prevented the hyper-responsiveness and decreased relaxation response in sensitized airways (32). In addition, the new evidence shows IL-1β-inducing expression by its airway smooth muscle (ASM), and strongly implying to function and releasing autocrine IL-1β by ASM in autologous manner (33).
In this study, the increased expression of IL-1β mRNA in trachea was seen in experimental induced asthma, which was consistent with previous findings (34). Interestingly, the diet-induced obesity in obese sensitized group enhanced the IL-1β mRNA expression in trachea that was the same with the findings of Lu et al in lung tissue (35). Indeed, it has found that the expression of IL-1β mRNA in obese asthmatic group was associated with the elaborating IL-1β into the trachea tissue. These findings indicated that diet-induced obesity in sensitized rats could induce autologous expression of IL-1β mRNA, and this made an autocrine effects on airway cell that was along with the release of extracellular IL-1β protein. Subsequently, IL-1β would increase the synthesis and release of other mediators which could lead to exacerbation of lung inflammation (13). Although the direct effects of IL-1β were not determined in the current study, the autocrine mechanisms may be involved in pathophysiology of obesity associated with asthma. The relative interaction of gene transcription of IL-1β versus regulation of post translation of IL-1β mRNA expression has not revealed yet.
In an effort to further understanding of the IL-1β’s role in relationship between obesity and asthma, the IRAK-1 and TRAF-6 mRNA expression in trachea was tested in study groups. IL-1 signal transduction pathways are interaction with TNF receptor associated (TRAF) adaptor proteins, especially TRAF-6. TRAF-6 interacts with IL-1 receptor associated kinase (IRAK) which is recruited to and activated by the IL-1 receptor complex (36).
Our results showed that diet-induced obesity caused overexpression of IRAK-1 and TRAF-6 mRNA in obese sensitized group. The expression levels of IRAK-1 mRNA in OVA-sensitized groups, lead to increased expression. On the other hand, although the TRAF-6 mRNA expression was high in lean sensitized group, however, there was not any statistically significance with non-sensitized lean and obese groups. Obvious studies have been shown increased IRAK-1 and TRAF-6 mRNA expression in animal allergic and sensitized asthma model which similar to current study. Our results also showed that the expression of IRAK-1 and TRAF-6 mRNA more increased in obesity associated with asthma. IL-1β promotes most its function by the activation of transcription of gene coding of chemokine, cytokines, acute-phase proteins, and adhesion molecules. As a result expose cell with cytokines and other extracellular stimuli, IL-1β associates with its receptors and recruitment of IRAK1-TRAF6 signaling pathways to exert of activation transcription factors, particularly NF-κB. Overexpression of IRAK-1 and TRAF-6 mRNA in obese sensitized group, at least in part, might explain the possible role of IL-1β in this condition, however, the role of other factors such as role of Toll-like receptors and free fatty acids, cannot be ruled out in IRAK-1/TRAF-6 activation (37). Increased the expression of IRAK-1 and TRAF-6 mRNA, and on the other hand, increased expression of IL-1β mRNA in trachea in obese sensitized condition, maybe indicate their interaction.
It should be noted that the complexity of the mechanisms responsible for exert IL-1β autocrine effects is difficult to explain in obesity with asthma. In fact, other members of IL-1 axis should be considered for better understanding of the role of IL-1β. Studies have shown that IL-1 exerts its effects through the IL-1 type I receptor (IL-1RI), but type 2 receptors; both IL-1RII and solution form of it (sIL-1RII) and solution form of IL-1RI (sIL-1RI), act as decoy receptors that are involved as damping effect for IL-1 (38). Also, IL-1ra, an endogen IL-1 antagonist, should be considered in this axis. Based on available evidence, it seems that under normal conditions, IL-1β and other family members of IL-1 are involved to prevent excessive immune response in all cell type. On the other hand, in certain disease conditions, there is an imbalance in the production of the IL-1β and other molecules in IL-1 axis, this phenomenon might occur in obese with asthma. Although the expression of IL-1β mRNA increased in the current study, the role of other molecules involved in IL-1axis cannot be ruled out. Perhaps the expression or activation of other molecules reduced or activated in obesity associated with asthma that required further studies. So, the ratio of active (IL-1RI) to inactive (sIL-1RI, IL-1RII, and sIL-1RII) receptors may determine the amount of inflammation.
This study examined the correlation between the expression of IL-1β, IRAK-1 and TRAF-6 mRNA with obesity indexes in rodent. The result showed that there was a positive correlation between the expression of IL-1β and TRAF-6 mRNA with percentage of weight changes in study groups, however, this correlation was weak. As noted above, despite of exit positive correlation in different groups; there was no significant correlation between IL-1β, IRAK-1 and TRAF-6 mRNA with Lee index and percentage of body fat. Interestingly, the analysis was done for different groups separately in order to determine the relationship between obesity indexes and related gene expression in this study, despite the positive linear correlation in most groups, there was no significant difference (data not published). The reason for this lack of significance may be limited samples. However, this study showed that enhance in weight increases expression of inflammatory cytokines, IL-1β. Studies have shown that conventional biomarkers in asthma, eosinophil and eNO, do not increase substantially with increasing body mass index (BMI). The study suggests that obesity may impact the severity of asthma through the other mechanisms (39). A novel adipokine visfatin (a pro-inflammatory factor) plays a role in several diseases related with inflammation such as asthma (40). However, very little is known about the role of visfatin in asthma and need further research. Based on the above observation, it is conceivable that, asthma phenotype might changes in obesity condition.
Conclusion
The results of this investigation showed that diet-induced obesity caused an increased expression of IL-1β, IRAK-1 and TRAF-6 mRNA in trachea of male OVA-sensitized rats. IL-1β is a cytokine which has pro-inflammatory effects. Elevated of IL-1β mRNA expression in obesity condition may be result in exacerbation of inflammation of airways in asthma. IL-1β has local and systemic effects. Increased of IL-1β in circulating, with its autocrine effect may involve in increase of inflammation in obesity associated with asthma in airways. Also, IL-1 exerts its effects through the IRAK-1 and TRAF-6 after the band with its receptor. Increased expressions of IRAK-1 and TRAF-6 mRNA in this study, to some extent, can show activation of IL-1 signaling pathways. On the other hand, increased expression of IL-1β, at least in part, may explain decrease the response of trachea to relaxant drugs in obesity with asthma.
Acknowledgment
This study was financially supported by Tuberculosis and Lung Diseases Research Center of Tabriz University of Medical Sciences, Tabriz, Iran. The results described in this paper were part of student thesis of Mohammad Reza Aslani, (thesis serial number: 92/4-6/2).
Conflict of interest
The authors have declared that there is no conflict of interest.
Reference
- 1.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma:a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson CC, Clark S, Camargo CA. Body mass index and asthma severity among adults presenting to the emergency department. CHEST. 2003;124:795–802. doi: 10.1378/chest.124.3.795. [DOI] [PubMed] [Google Scholar]
- 3.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol. 2010;108:729–734. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE. Adipose tissue from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 6.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–927. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 7.Beuther DA. Recent insight into obesity and asthma. Curr Opin Pulm Med. 2010;16:64–70. doi: 10.1097/MCP.0b013e3283338fa7. [DOI] [PubMed] [Google Scholar]
- 8.Krause K, Metz M, Makris M, Zuberbier T, Maurer M. The role of interleukin-1 in allergy-related disorders. Curr Opin Allergy Clin Immunol. 2012;12:477–484. doi: 10.1097/ACI.0b013e3283574d0c. [DOI] [PubMed] [Google Scholar]
- 9.Hakonarson H, Grunstein MM. Autocrine regulation of airway smooth muscle responsiveness. Respir Physiol Neurobiol. 2003;137:263–276. doi: 10.1016/s1569-9048(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 10.Martin M, Resch K. Interleukin 1:more than a mediator between leukocytes. Trends Pharmacol Sci. 1988;9:171–177. doi: 10.1016/0165-6147(88)90033-8. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selig W, Tocker J. Effect of interleukin-1 receptor antagonist on antigen-induced pulmonary responses in guinea pigs. Eur J Pharmacol. 1992;213:331–336. doi: 10.1016/0014-2999(92)90621-a. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 15.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992;149:3078–3082. [PubMed] [Google Scholar]
- 16.Ludigs K, Parfenov V, Du Pasquier RA, Guarda G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cell Mol Life Sci. 2012;69:3395–3418. doi: 10.1007/s00018-012-0989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets:Modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 18.Boskabady MH, Byrami G, Feizpour A. The effect of safranal, a constituent of Crocus sativus (saffron), on tracheal responsiveness, serum levels of cytokines, total NO and nitrite in sensitized guinea pigs. Pharmacol Rep. 2014;66:56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Le J, Duan X, Du W, Liu B, Wu J, et al. Molecular mechanism of icariin on rat asthmatic model. Chin Med J. 2011;124:2899–2906. [PubMed] [Google Scholar]
- 20.Otunola GA, Oloyede OB, Oladiji AT, Afolayan AA. Effects of diet-induced hypercholesterolemia on the lipid profile and some enzyme activities in female Wistar rats. Afr J Biochem Res. 2010;4:149–154. [Google Scholar]
- 21.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 22.Simson EL, Gold RM. The Lee obesity index vindicated? Physiol Behav. 1982;29:371–376. doi: 10.1016/0031-9384(82)90028-2. [DOI] [PubMed] [Google Scholar]
- 23.Alipour MR, Khamaneh AM, Yousefzadeh N, Mohammad-nejad D, Soufi FG. Upregulation of microRNA-146a was not accompanied by downregulation of pro-inflammatory markers in diabetic kidney. Mol Biol Rep. 2013;40:6477–6483. doi: 10.1007/s11033-013-2763-4. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32:S109–S119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farah CS, Salome CM. Asthma and obesity:a known association but unknown mechanism. Respirology. 2012;17:412–421. doi: 10.1111/j.1440-1843.2011.02080.x. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso P. Obesity and lung inflammation. J Appl Physiol. 2010;108:722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore SA. Obesity and asthma:possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. The pathogenesis of atherosclerosis:a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 30.Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116:851–858. doi: 10.1016/j.jaci.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:859–866. doi: 10.1046/j.1440-1681.2002.03756.x. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Lu W-L, Hong H-Y, Yao Y-S, Han P, Li Z-K, et al. Pharmacokinetics and anti-asthmatic potential of non-parenterally administered recombinant human interleukin-1 receptor antagonist in animal models. J Pharmacol Sci. 2006;102:321–330. doi: 10.1254/jphs.fpj06007x. [DOI] [PubMed] [Google Scholar]
- 33.Hakonarson H, Herrick DJ, Serrano PG, Grunstein MM. Autocrine role of interleukin 1beta in altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1997;99:117. doi: 10.1172/JCI119122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelan R, Kim C, Chen M, Leiter J, Grunstein M, Hakonarson H. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. Eur Respir J. 2004;24:559–567. doi: 10.1183/09031936.04.00133803. [DOI] [PubMed] [Google Scholar]
- 35.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 36.Lee J-H, Wang L-C, Yu H-H, Lin Y-T, Yang Y-H, Chiang B-L. Type I IL-1 receptor (IL-1RI) as potential new therapeutic target for bronchial asthma. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/567351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88:an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 38.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 39.McLachlan CR, Poulton R, Car G, Cowan J, Filsell S, Greene JM, et al. Adiposity, asthma, and airway inflammation. J Allergy Clin Immunol. 2007;119:634–639. doi: 10.1016/j.jaci.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Toru Ü, Ayada C, Genç O, şahin S, Arık Ö, Acat M, et al. Visfatin and ghrelin:can they be forthcoming biomarkers or new drug targets for asthma? Int J Clin Exp Med. 2015;8:6257–6261. [PMC free article] [PubMed] [Google Scholar]


