Abstract
Objective(s):
Systemic lupus erythematosus (SLE) is a multi-factorial autoimmune disease which may be characterized by T lymphocytes dysfunctions. Th17 cells have been identified as new effector cells, which play an important role in the pathogenesis. In recent years, immunomodulatory effect of vitamin D3 has been noticed. In the present experiment, the effect of vitamin D3 on the expression of IL-17, IL-23, IL-4 and IFN-γ were assessed in activated chromatin-induced mouse model for SLE.
Materials and Methods:
Five groups of mice were included in this study; Group one received active chromatin +CFA + PBS; Group 2 received vitamin D3 starting 2 weeks before disease induction; Group 3 received vitamin D3 (50 ng/day) starting with the disease establishment; Group 4 received non active chromatin +CFA + PBS; Group 5 received CFA + PBS. On day 56 splenocytes were isolated and gene expression of interleukin IL-17, IL-23, IL-4 and IFN-γ were analyzed by Real-Time PCR method. Proteinuria and serum anti-dsDNA and Th17 levels were measured using commercial kits.
Results:
The results showed that IL-17, IL-23, and IFN-γ mRNA expression, and IL-17 titers were decreased remarkably and that of IL-4 increased in mice which received vitamin D3 before SLE induction. Administration of vitamin D3 after the establishment of SLE failed to affect the IL-17 or IL-23 mRNA levels. Lastly, pre-treatment of mice with vitamin D3 decreased the anti-ds DNA antibody titer.
Conclusion:
Our findings showed that vitamin D3 supplementation in lupus induced mice through modulating the expression rate of some inflammatory cytokines diminished the inflammatory conditions in SLE.
Keywords: Cytokines, Mice, Systemic lupus - erythematosus, Th17 cells, Vitamin D
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that could affect any organ such as brain, blood and kidney (1, 2). The exact mechanisms involved in the pathogenesis of SLE remains unclear, but various immunological and environmental factors are considered important in progress of SLE (3). Animal models of SLE are useful tools in studying the disease. Spontaneous and induced established mouse models of SLE exist. Among theses, the induced models seem to be more useful for studying the impact of environmental factors in the etiopathogenesis of SLE (4). Among induced models, the one established by the use of activated chromatin most resembles human SLE (5).
Immune system cells, especially CD4+ T helper lymphocytes, play a critical role in the pathogenesis of SLE (6). Recently, a new subset of CD4+ T cells, i.e., T-helper 17 (Th17) cells, has been identified, which is assumed to be one of the key players in SLE (7, 8). Th17 cells are effector cells, which produce IL-17 and are present in the damaged organs in systemic lupus erythematosus patients (9). Increased number of Th17 cells and higher levels of interleukin IL-17 and IL-23 in the affected organs of SLE patients has been reported (6, 10).
SLE is a disease with no cure, and the current available therapeutic approaches are partially effective and mostly delay the disease progression (11-13). In recent years, a number of studies have been concerned with the immunomodulatory effects of 1,25-dihydroxyvitamin D3 (the active form of vitamin D) on autoimmune diseases (14). Molecular mechanisms of the immunomodulatory effects of vitamin D3 on SLE are under extensive investigations (15, 16). In the present study, we evaluated the effects of oral administration of vitamin D3 on the expression of IL-17, IL-23, IL-4 and IFN-γ before and after SLE induction in activated chromatin induced SLE mouse model.
Materials and Methods
Animals
Balb/c mice (female, 4-6 weeks) were obtained from Pasteur Institute (Tehran, Iran) and housed under standard laboratory conditions (25 ±2 °C and 40-70% relative humidity) with a 12 hr day/night lighting cycle throughout the experimental period. The animals were kept in large spacious polypropylene cages and were provided access to standard rodent chow and filtered water. All mice were allowed to acclimate for 2 weeks prior to the initiation of any experiment. The Ethic Council of Mashhad University of Medical Sciences (Mashhad, Iran) approved all the protocols used with these mice in the studies herein. All national guidelines for the care and use of laboratory animals were followed in this study.
Preparation of materials for use in immunizations
Spleens from 10 naïve female Balb/c mice were isolated and transferred into cooled RPMI 1640 medium (Gibco Laboratories, Detroit, MI). Splenocytes were then isolated by needle perfusion using RPMI 1640 and red blood cells removed by addition of lyses buffer (0.84% [w/v] ammonium chloride solution) and the samples were incubated on ice for 4 min. The remaining splenocytes were then washed twice by centrifugation (400 ×g, 10 min, 4 °C) with ice-cold PBS and finally re-suspended (2 x 106 cells/ml) in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U penicillin/ml, and 100 μg streptomycin/ml (all from Gibco Laboratories, Detroit, MI). Thereafter, half of the re-suspended cells received 5 μg Concanavalin A (Sigma, St. Louis, MO) to activate the cells (to prepare ‘active’ chromatin) and the other half only received medium; all cells were then cultured for 48 hr at 37 °C in a 5% CO2 incubator.
At the end of the incubation time, ‘activated’ and ‘non-activated’ chromatins were each extracted using a genomic DNA extraction kit (BioGene, Mashhad, Iran) by using salting out method. DNA level in each sample was determined at 260 nm. Endotoxin content in the samples was confirmed to be < 0.01 U/μg by Samen Research Institute (Mashhad, Iran) using a standard Limulus Amebocyte Lysate (LAL) assay.
Induction of experimental SLE model
For induction of SLE, 5 groups of mice (female, 4-6 weeks) were immunized on “Day 0” by a subcutaneous injection of active chromatin (chromatin extracted from Concanavalin A [ConA] activated splenocytes) (≈ 50 μg in phosphate-buffered saline [PBS, pH 7.4]) combined with 200 μl complete Freund’s adjuvant (CFA, containing 10 g Bacille Calmette-Guerin [BCG]/L) into the back of the mice. After the first immunization (Day 0), these mice received booster immunizations consisted of incomplete Freund’s adjuvant and activated chromatin on Days 14 and 28. In this study, we utilized 2 negative control groups (n= 6 in each group). One group only received CFA and PBS and the other received non-activated chromatin plus CFA and PBS.
Vitamin D3 administration
Mice were divided into 5 groups to assess the potential therapeutic or preventive effects of Vitamin D3 on experimental SLE induction/progression (Table 1).
Table 1.
Different treatment and control mice groups utilized in the present study
| Group ID | Group Description | Received materials |
|---|---|---|
| 1 | Induction (positive control) | Active chromatin + CFA + PBS |
| 2 | Vitamin D (before) | Received vitamin D3 (50 ng/day) starting 2 weeks before induction |
| 3 | Vitamin D (after) | Received vitamin D3 (50 ng/day) starting with disease establishment |
| 4 | Negative control | Non active chromatin + CFA + PBS |
| 5 | Negative control | CFA + PBS |
Induction of SLE in mice was achieved by immunization with activated chromatin (see below). In this study one group of mice was used as positive control (n=6) immunized with active chromatin as aforementioned (group1).
Two protocols were used to assess vitamin D3 effects: in the preventive protocol, mice (n = 6) received 50 ng/day of the active form of vitamin D3 (1, 25 Dihydroxyvitamin D3) (Sigma, St. Louis, MO) starting at 2 weeks before immunization with the active chromatin, through the booster phase, and then for up to 2 months after the last immunization (i.e., up to week 8 post-immunizations) (group 2). Another mice group in the treatment protocol was gavaged 50 ng of vitamin D3/day for 2 months starting with the disease establishment (group 3). Treatment volume of vitamin D3 never exceeded 10 μl. Induction positive control group (group 1) received daily PBS instead of vitamin D3 over the 2-month period after the immunization.
Two other groups were used as negative controls (n=6); group 4 (received non active chromatin+ CFA & PBS) and group 5 (n=6) received CFA & PBS.
Blood samples were collected from the retro-orbital sinus immediately before the first immunization (Day 0) and then every 2 weeks over the 2-month treatment period starting after the last (Day 28) immunization (i.e., out to end of week 8 post-immunization). After the final blood sampling, all mice were euthanized by ether asphyxiation and their spleens removed aseptically and processed for further studies.
Immunoassay of anti-ds DNA antibody in blood
The total amount of anti-ds-DNA antibody was assessed in sera of mice using a commercial ELISA kit (Glory Science, Del Rio, TX). Briefly, the plate was pre-coated with mouse monoclonal ds-DNA antibodies. Then sample serum, anti-dsDNA antibody and streptavidin-horseradish peroxidase solution were added to each well. The plate was incubated for 60 min at 37 °C. Then each well was washed five times, chromogen reagent was added and the plate was incubated for a further 10 min at 37°C. After addition of stop solution (1 N H2SO4), the optical density (OD) of each well was measured at 450 nm using a Convergys ELISA reader (Convergent Technologies, Marburg, Germany). The sensitivity of the kit was 0.5 ng/ml.
Measurement of proteinuria
Proteinuria was measured by using a commercial kit according to the manufacturer’s instructions (Pierce™ BCA Protein Assay kit, USA).
Measurement of IL-17 production
To measure IL-17 produced from spleen cells, splenocytes were isolated from spleens, harvested at necropsy and cultured (2 x 106 cells/ml) for 72 hr in RPMI 1640 containing 10% fetal bovine serum (FBS; Gibco, Detroit, MI) with and without 5 ng phytohemagglutinin/ml as a stimulator (PHA; Gibco, Detroit, MI). At the end of this period, well supernatants were collected and stored at -80 °C until assessed for IL-17 levels using a mouse IL-17A ELISA kit (eBioscience, San Diego, CA; limit of detection of kit = 4 pg/ml).
Quantitative Real-Time PCR analysis
RNA was extracted from splenocytes (5 x 106/mouse) using Tripure (Roche, Germany) and cDNA was synthesized using a cDNA synthesis kit (Fermentas, Vilnius, Lithuania) according to the manufacturers’ instructions. To evaluate the expression levels of IL-17, IL-23, IL-4, and IFN-γ mRNA levels, Real-time PCR were performed using sense and anti-sense primers and probes (Table 2) using a Rotor-Gene Q real-time PCR machine by TaqMan method (QIAGEN, Hilden, Germany). A comparative Ct method (∆∆Ct) was used for the analysis of PCR data. β2-microglobulin gene was used as the internal control reference gene (Table 2). Normalization of differences between the amounts of total cDNA added to each reaction and efficiency of the Real-time PCR were performed by calculating differences between Ct values of the target gene and the internal control gene (∆Ct = Ct target - Ct internal control). To estimate ∆∆Ct, differences were calculated between the ∆Ct of each sample and the calibrator. Gene expression was ultimately calculated using a 2-∆∆Ct formula.
Table 2.
Sequences of sense and anti-sense primers and probes used for gene expression analysis of IL-17, IL-4, IL-23, IFN-γ and β2-microglobulin in the present study by using TaqMan Real-time PCR method
| Genes | Sequences | bp | |
|---|---|---|---|
| IFN-γ | F | 5’-CCAAgTTTgAggTCAACA-3’ | 18 |
| R | 5’-CTggCAgAATTATTCTTATTgg-3’ | 22 | |
| Probe | 5’-FAM CCgAATCAgCAgCgACTCCT TAMRA-3’ | 20 | |
| IL-4 | F | 5’-CTggATTCATCgATAAgC-3’ | 18 |
| R | 5’-gATgCTCTTTAggCTTTC-3’ | 18 | |
| Probe | 5’-FAM TgAATgAgTCCAAgTCCACATCACT-3’ | 25 | |
| IL-17 | F | 5’-CCTCAgACTACCTCAACC-3’ | 18 |
| R | 5’-CCAgATCACAgAgggATA-3’ | 18 | |
| Probe | 5’ FAM ACTCTCCACCgCAATgAAgACC TAMRA-3’ | 22 | |
| IL-23 | F | 5’-CgggACATATgAATCTACTAA-3’ | 21 |
| R | 5’-TgTCCTTgAgTCCTTgTg-3’ | 18 | |
| Probe | 5’-FAM CAACCATCTTCACACTggATACgg TAMRA-3’ | 24 | |
| Β2m | F | 5’-CCTgTATgCTATCCAgAA-3’ | 18 |
| R | 5’-gTAgCAgTTCAgTATgTTC-3’ | 19 | |
| Probe | 5’-FAM TATACTCACgCCACCCACCg TAMRA-3’ | 20 | |
*F: Forward Primer, **R: Reverse Primer
Statistics
SPSS software was used for all analyses (IBM Company, Armonk, NY). The normality of the data, were first examined using descriptive statistics. Comparison between variables with normal distribution was made by using Analysis of variance [ANOVA] and in variables with non-normal distribution, analysis was made using the Kruskal-Wallis.
Results
Body weight
Mice body weights were measured on day 0 and 2 months after the last set of immunizations (i.e., week 8 post-immunization protocol). In all groups, body weights increased significantly compared to the first measurement (Figure 1).
Figure 1.
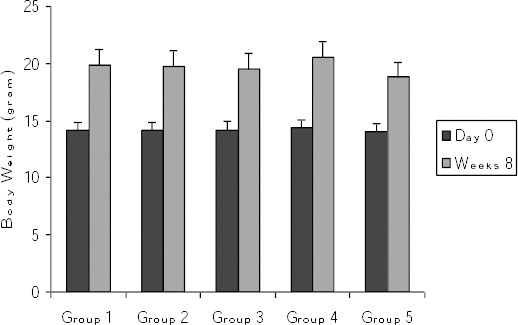
Comparison of body weights at start of protocols and at week 8 post-immunization Values are shown as mean ± SE. At all groups body weights at week 8 significantly increased compared to day 0. Group 1 = induction group (positive control); group 2= received vitamin D3 (50 ng/day) starting 2 weeks before disease induction; group 3= received vitamin D3 (50 ng/day) starting with the disease establishment; group 4 = negative control (non active chromatin+ CFA + PBS); group 5 = negative control (PBS + CFA)
Effects of vitamin D3 on anti-dsDNA antibody levels
At day 0 and at week 8 after the last immunization, serum anti-dsDNA antibodies titers were evaluated. In the induction group (Group 1), the titer of anti-dsDNA antibody increased significantly (P=0.01) compared to day zero (Figure 2). However, in groups 2 and 3 (which received vitamin D3 before or after disease induction) no significant difference was seen in the levels of anti-ds DNA antibodies compared to day 0.
Figure 2.
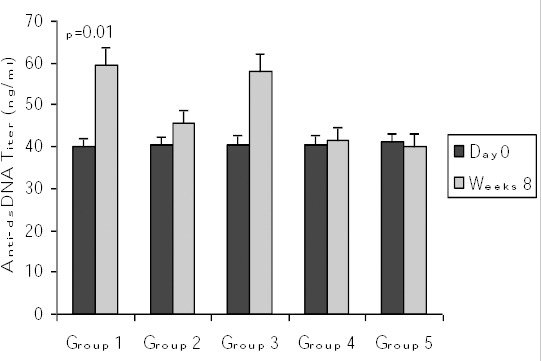
Comparison of serum anti-dsDNA titers at start of protocols and at week 8 post-immunization. Values are shown as mean ± SE. oup 1 = induction group (positive control); group 2= received vitamin D3 (50 ng/day) starting 2 weeks before disease induction; group 3= received vitamin D3 (50 ng/day) starting with the disease establishment; group 4 = negative control (non active chromatin+ CFA + PBS); group 5 = negative control (PBS + CFA)
In studying the effects of the timing of the initiation of vitamin D3 treatments on the titer of anti-dsDNA, we realized that in group 2 the titer of anti-dsDNA at Week 8 decreased (45.51±5.94 ng/ml) compared to group 1 (59.35±5.11 ng/ml) (although not significantly). In group 3 mice that received vitamin D3 starting with disease induction the titer of anti-dsDNA was higher than group 2.
Effects of vitamin D3 on proteinuria
There was no significant difference in the proteinuria level between different groups at day 0 (p=0.23). However, in group 1 (induction group) the proteinuria level increased considerably at week eight after the final immunization in comparison to day zero (p=0.004). In groups 2 and 3 (received vitamin D3 before or after disease induction) there was no significant increase in the proteinuria level at week eight in comparison to day 0 (Figure 3).
Figure 3.
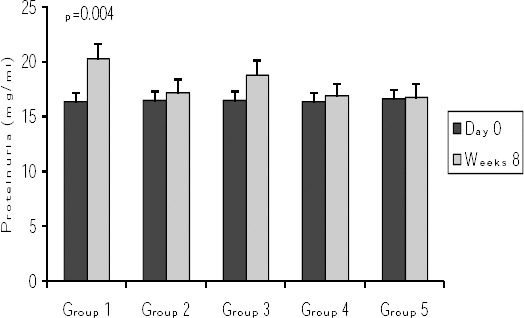
Comparison of proteinuria at start of protocols and at week 8 post-immunization. Values are shown as mean ± SE. Group 1 = induction group (positive control); group 2= received vitamin D3 (50 ng/day) starting 2 weeks before disease induction; group 3= received vitamin D3 (50 ng/day) starting with the disease establishment; group 4 = negative control (non active chromatin+ CFA + PBS); group 5 = negative control (PBS + CFA)
At week 8 the level of proteinuria in group 2 was lower than group 1 but this difference was not significant.
Effects of vitamin D3 on PHA-induced IL-17 production by splenocyte (ex vivo)
In SLE induced mice (group 1 which received activated chromatin), the production levels of IL-17 increased (although not significantly) compared to negative control groups (groups 4 and 5) (Figure 4). In mice received vitamin D3 prior to the disease induction (group 2), the production levels of IL-17 reduced (albeit not significantly so) compared to group 1.
Figure 4.
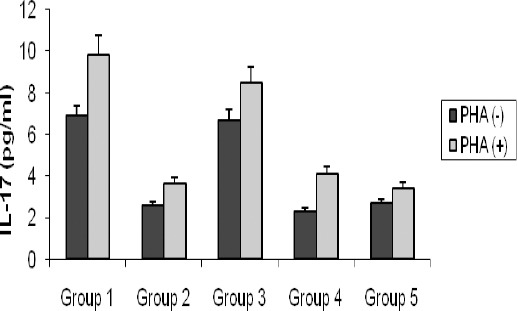
Comparison of IL-17 titers at weeks 8 post-immunization in splenocytes of each group in presence (PHA+) and absence (PHA-) of PHA. Values are shown as mean±SE
Group 1 = induction group (positive control); group 2= received vitamin D3 (50 ng/day) starting 2 weeks before disease induction; group 3= received vitamin D3 (50 ng/day) starting with the disease establishment; group 4 = negative control (non active chromatin+ CFA + PBS); group 5 = negative control (PBS + CFA)
Effects of vitamin D3 on the expression of IL-17, IL-23, IFN-γ and IL-4 mRNA
In SLE induced mice group (group 1 which received activated chromatin), the expression levels of IL-17, IL-23, and IFN-γ increased (although not significantly) compared to negative control groups (groups 4 and 5) (Figure 5). In mice received vitamin D3 prior to immunization (group 2), the expression levels of IL-17, IL-23, and IFN-γ reduced (albeit not significantly so) in comparison to disease induced group (group1). In mice received vitamin D3 after the immunizations (group 3) the expression level of IL-17 and IL-23 was not different compared to group 1, but IFN-γ expression decreased.
Figure 5.
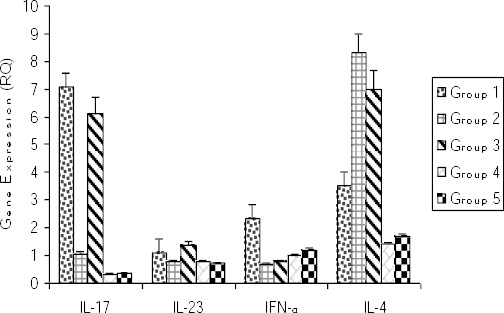
Gene expression of IL-17, IL-23, IFN-γ, and IL-4 in Splenocytes of each group at weeks 8 post-immunization
Group 1 = induction group (positive control); group 2= received vitamin D3 (50 ng/day) starting 2 weeks before disease induction; group 3= received vitamin D3 (50 ng/day) starting with the disease establishment; group group 4 = negative control (non active chromatin+ CFA + PBS); group 5 = negative control (PBS + CFA)
IL-4 mRNA expression in group 1 mice (induction group) increased in comparison to negative control groups (Figure 4). In mice that received vitamin D3 either before or after the induction of SLE (groups 2 and 3), the expression level of IL-4 increased in comparison to group 1, though in neither cases the difference was significant.
Discussion
Because of limited access to human tissues and samples and the diversity of clinical manifestations, definitive studies of the pathogenesis of human SLE have been somewhat hampered. Genetically prone mouse models of lupus carry unique set of susceptibility genes that limit understanding of the other mechanisms underlying the development of SLE. Induction of SLE in mice with normal genetic backgrounds provides a useful tool for better assessment of environmental factors that impact on the development and/or the progression of SLE (5, 17). In the present experiment, induction of SLE in mice by using activated chromatin led to the significant increase in the serum anti-dsDNA titer and proteinuria levels (hallmarks of SLE). SLE induction increased the mRNA expression levels of IL-17, IL-23, IFN-γ, and IL-4 compared to negative groups (mice which received non-activated chromatin, and mice that received CFA+PBS). Increased expression of IL-17 and IL-23 in disease induced mice was in line with studies which suggested an important likely role for abnormal IL-17/IL-23 axis in SLE (18). Enhanced IL-17 mediated tissue damage was reported in MRL/lpr mice (19) and in autoimmunity-prone mice (20). Some authors showed up-regulated number of IL-17/IL-17 R producing CD3+CD4-CD8- T-cells in MRL/lpr mice, as their lupus progressed (21), a deficiency in IL-23R in lupus-prone C57BL/6-lpr/lpr mice led to the reduced numbers of IL-17 producing cells and decreased circulating anti-DNA antibodies levels (22).
Our findings about the induction of SLE in a mouse model and involvement of IL-17/IL-23 axis in the pathogenesis of disease are considerable, but the main goal of the current study was to investigate the potential effects of vitamin D3 on the inducibility /severity of the disease and on the expression of some cytokines in these mice. Some features of the modulatory effects of vitamin D3 on SLE have previously been studied in human and murine models (23-25). In MRL/lpr mice, administration of vitamin D3 decreased the serum ss-DNA antibody and proteinuria levels (26), prevented pathological renal disease and prolonged host survival (27). Some studies showed inhibitory effects of vitamin D3 on any SLE progression in MRL/lpr mice (28), while in others administration of vitamin D3 to NZB/NZW mice did not alter disease progression (29). In an in vitro study in human, vitamin D3 diminished Th17 cells and their related molecules (30). In the present study, in mice that received vitamin D3 before disease induction, the titer of anti-dsDNA antibodies and the level of proteinuria decreased more over the mice group that received vitamin D3 post immunization. Vitamin D3 consumption decreased the expression levels of IL-17, IL-23 and IFN-γ, while the expression of IL-4 was increased. IL-17 and IL-23 are the main cytokines of Th17 cell lineage. IL-17 has a critical role in tissue injury in inflammatory autoimmune diseases and promotes inflammatory cells recruitment to the affected organs (9, 31), while IL-23 is implicated in the maintenance of Th17 cells (32), and enhances the immune responses in Th17 and Th1 cells (33). The results of our study imply that vitamin D3 can inhibit Th17 cells activation and reduce the likelihood of tissue damage in SLE by decreasing the expression of IL-17 and IL-23. In accordance with our findings some studies showed beneficial effects of vitamin D3 on SLE as well as upon IL-17 and IL-23 in other pathological inflammatory states (34, 35). Our results showed that in mice which received vitamin D3 before SLE induction, the expression of IL-17 and IL-23 decreased more than it did in the case of the mice which received vitamin D3 after disease induction. A full explanation for this differential response remains elusive and needs to be further explored. However, it could signal the preventive effect of vitamin D3 as an environmental factor in the initiation of systemic lupus erythematosus.
Most published literature implies that the net effect of vitamin D3 in SLE is to shift immune responses toward a more anti-inflammatory phenotype (39), to help limit the potential tissue damage (36-38). In accordance with other researchers (40-41) in the present study, treatment with vitamin D3 led to the increased expression of IL-4, which consecutively may suppress the inflammatory T cell activity. Diminished expression of IFN-γ, IL-17, IL-23 and enhanced production of IL-4 in our study illustrates the anti-inflammatory effects of vitamin D3. Taken together, our results showed that part of the preventive effect of 1,25-dihydroxyvitamin D3 on the onset/progression of SLE could be modulated through down-regulated production of the effector cytokines IFN-γ, IL-17, IL-23 and up-regulating production of IL-4.
Conclusion
Systemic lupus erythematosus is a disease with no cure and current existing therapeutics mostly delay the disease progression. Our results showed that vitamin D3 in combination with other conventional treatments might better control the inflammatory conditions in SLE patients. Vitamin D3 through reducing the expression rate of IL-17, IL-23, IFN-γ, and increasing the expression level of IL-4, could exert some of its beneficial effects in SLE.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Reference
- 1.Tsokos GD. Systemic lupus erythematosus. New Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.D.P D’Cruz, M. A Khamashta, G. R Hughes. “Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JE, Fu SM, Gaskin F. Autoimmunity, end organ damage, and the origin of autoantibodies and autoreactive T cells in systemic lupus erythematosus. Discov Med. 2013;15:85–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotech. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao B, Wu J, Chu YW, Wang Y, Wang DP, Wu HS, et al. Induction of systemic lupus erythematosus-like syndrome in syngeneic mice by immunization with activated lymphocyte-derived DNA. Rheumatology (Oxford) 2005;44:1108–1114. doi: 10.1093/rheumatology/keh656. [DOI] [PubMed] [Google Scholar]
- 6.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, et al. Dysregulated balance of TH17 and TH1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basso AS, Cheroutre H, Mucida D. More stories on TH17 cells. Cell Res. 2009;19:399–411. doi: 10.1038/cr.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balanescu P, Balanescu E, Tanasescu C, Nicolau A, Tanasescu R, Grancea C, et al. TH17 cell population in lupus erythematosus. Rom J Intern Med. 2010;48:255–259. [PubMed] [Google Scholar]
- 9.Apostolidis S A, Crisp´ın JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120–124. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 10.Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J. Imbalance between TH17 and regulatory T-cells in systemic lupus erythematosus. Folia Histochem Cytobiol. 2011;49:646–653. doi: 10.5603/fhc.2011.0088. [DOI] [PubMed] [Google Scholar]
- 11.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet. J Rare Dis. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Menachem E. Review article:systemic lupus erythematosus:A review for anesthesiologists. Anesth Analg. 2010;111:665–676. doi: 10.1213/ANE.0b013e3181e8138e. [DOI] [PubMed] [Google Scholar]
- 13.Crispin JC, Liossis SN, Kis-Toth K, Kis-Toth K, Lieberman LA, Kyttaris VC, et al. Pathogenesis of human systemic lupus erythematosus:Recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutolo M. Further emergent evidence for the vitamin D endocrine system involvement in autoimmune rheumatic disease risk and prognosis. Ann Rheum Dis. 2013;72:473–475. doi: 10.1136/annrheumdis-2012-202538. [DOI] [PubMed] [Google Scholar]
- 15.Szodoray P, Nakken B, Gaal J, Jonsson R, Szegedi A, Zold E, et al. Complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68:261–269. doi: 10.1111/j.1365-3083.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang SH, Chung Y, Dong C. Vitamin D suppresses TH17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–38755. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen ZK, Xu W, Xu L, Cao OH, Wang Y, Chu YW, et al. DNA hypomethylation is crucial for apoptotic DNA to induce systemic lupus erythematosus-like autoimmune disease in SLE-nonsusceptible mice. Rheumatology. 2007;46:1796–1803. doi: 10.1093/rheumatology/kem275. [DOI] [PubMed] [Google Scholar]
- 18.Apostolidis SA, Lieberman LA, Kis-Toth K, Crispin JC, Tsokos GC. The dysregulation of cytokine networks in systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:769–779. doi: 10.1089/jir.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding-Lei Su ZM, Min-Ning S, Xia L, Ling-Yun S. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotech. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgerton C, Crispín JC, Moratz CM, Betteli E, Oukka M, Simovic M, et al. IL-17 producing CD4+ T-cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting Edge:IL-23 receptor deficiency orevents development of lupus nephritis in C57BL/6–lpr/lprmice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabasi N, Rastin M, Mahmoudi M, Ghoryani M, Mirfeizi Z, Zamani SH, et al. Influence of Vitamin D on cell cycle, apoptosis, and some apoptosis related molecules in systemic lupus erythematosus. Iran J Basic Med Sci. 2015;18:1107–1111. [PMC free article] [PubMed] [Google Scholar]
- 24.Lavi Arab F, Rastin M, Faraji F, Zamani Taghizadeh Rabe S, Tabasi N, Khazaee M, et al. Assessment of 1,25-D3 effects on Treg cells and their related molecules in active chromatin lupus induced Balb/c mice. Immunopharmacol Immunotoxicol. 2015;37:12–18. doi: 10.3109/08923973.2014.968255. [DOI] [PubMed] [Google Scholar]
- 25.Wahono CS, Rusmini H, Soelistyoningsih D, Hakim R, Handono K, Endharti AT, et al. Effects of 1,25(OH)2D3 in immune response regulation of systemic lupus erithematosus (SLE) patient with hypovitamin D. Int J Clin Exp Med. 2014;7:22–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates expression of experimental murine lupus in MRL/1 mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 27.Deluca HF, Cantorna MT. Vitamin D:Its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi T, Nakao Y, Matsui T, Nakagawa T, Matsuda S, Komoriya K, et al. Effects of corticosteroid and 1,24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol. 1985;77:396–404. doi: 10.1159/000233815. [DOI] [PubMed] [Google Scholar]
- 29.Vaisberg MW, Kaneno R, Franco MF, Mendes NF. Influence of cholecalci-ferol (vitamin D3) on course of experimental systemic lupus erythematosus in F1 (NZB x W) mice. J Clin Lab Anal. 2000;14:91–96. doi: 10.1002/(SICI)1098-2825(2000)14:3<91::AID-JCLA2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reihani H, Rastin M, Mahmoudi M, Ghoryani M, Abdlahi N, Tabasi N, et al. Influence of 1 Alpha, 25-Dihydroxyvitamin D3 on T Helper 17 Cells and Related Cytokines in Systemic Lupus Erythematosus, Iran J Immunol. 2015;12:82–93. [PubMed] [Google Scholar]
- 31.Onishi RM, Gaffen SL. Interleukin-17 and its target genes:mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raza A, Yousaf W, Giannella R, Shata M. Th17 cells:interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol. 2012;8:161–168. doi: 10.1586/eci.11.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T-helper (TH) 1/TH17 to a TH2 and regulatory T-cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 35.Ding-Lei Su ZM, Min-Ning S, Xia L, Ling-Yun S. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotech. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25 Dihydroxyvitamin D3 and IL-2 combine to inhibit T Cell production of inflammatory cytokines and promote development of regulatory T Cells expressing CTLA-4 and Foxp3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terrier B, Derian N, Schoindre Y, Charra W, Geri G, Zahr N, et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Therap. 2012;14:R22. doi: 10.1186/ar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh A, Kamen DL. Potential benefits of vitamin D for patients with systemic lupus erythematosus. Dermatoendocrinol. 2012;4:146–51. doi: 10.4161/derm.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghoryani M, Sahebari M, Mahmoudi M, Abdollahi N, Reihani H, Zamani SH, et al. Immunomodulatory Vitamin D effects on regulatory T-cells and cytokines in an in vitro study on patients with systemic lupus erythematosus. Food and Agricultural Immunology. 2016;27:377–387. [Google Scholar]
- 40.Funauchi M, Yu H, Sugiyama M, Ikoma S, Ohno M, Kinoshita K, et al. Increased IL-4 production by NK T-cells in systemic lupus erythematosus. Clin Immunol. 1999;92:197–202. doi: 10.1006/clim.1999.4742. [DOI] [PubMed] [Google Scholar]
- 41.Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4-CD8-(double negative) T-cells in patients with systemic lupus erythematosus:Production of IL-4. Lupus. 2002;11:501–507. doi: 10.1191/0961203302lu234oa. [DOI] [PubMed] [Google Scholar]


