Abstract
Objective(s):
Diabetic neuropathy (DN) is a common complication of diabetes that leads to allodynia, impaired nerve conduction, and progressive sensory loss. The aim of this study was to observe the effect of a high-affinity cannabinoid receptors agonist, WIN 55,212-2, on thermal hyperalgesia, nerve conduction velocity and sciatic nerve histopathology in diabetic rats.
Materials and Methods:
Diabetes was induced in rats using a single dose of streptozotocin (45 mg/kg IP).
Results:
Intrathecal (IT) administration of WIN55, 212-2 (1, 10, 100 µg/10 µl, IT), produced antinociceptive effects in the hot plate test and also improved nerve conduction velocity (100 µg/10 µl, IT) and sciatic nerve histology.
Conclusion:
These data show that cannabinoids have potent antinociceptive effects through direct actions in the spinal dorsal horn of nociceptive pathway. This suggests that intrathecally administered cannabinoids may offer hopeful strategies for the treatment of diabetic neuropathic pain.
Keywords: Diabetes; Intrathecal; Neuropathy; Pain; WIN 55,212-2
Introduction
Cannabinoids are a known class of analgesics, which exert their effect through spinothalamic tract (1). Type one cannabinoid receptors (CB1) are mainly found in periaqueductal gray matter, dorsal horn of the spinal cord and dorsal root ganglion (DRG) neurons (2-5). It has been shown that the naturally occurring D9-tetrahydrocannabinol (D9-THC) and synthetic cannabinoids such as WIN55,212-2 and CP-55,940 can suppress responses to noxious thermal and mechanical stimuli in acute pain models such as the hot plate, tail flick or paw pressure tests (2). There is now considerable evidence supporting the role of cannabinoids in chronic pain conditions and spontaneous pain-related behavior, as well as hyperalgesia and allodynia conditions induced by peripheral inflammation (6), capsaicin administration (7) or sciatic nerve constriction (8, 9). In persistent nociceptive conditions, particularly associated with neuropathic pain, up-regulation of CB1 receptor expression (10), has been proposed to mediate the cannabinoid-induced antinociceptive effects (11-15). In addition, CB2 receptors demonstrated antinociceptive effect in inflammatory hyperalgesia (16-21), and neuropathic pain (15, 17).
Similar to peripheral nerve injury models, diabetes mellitus also results in neuropathic pain in up to 50% of patients (22). This neuropathy is associated with hyperalgesia (increased sensitivity to noxious stimuli), hypoalgesia (decreased sensitivity to painful stimuli), and allodynia (nociceptive responses to normally innocuous stimuli) (23).
In addition, elevated blood glucose levels lead to several metabolic abnormalities such as enhanced polyol production via aldose reductase and reduced Na+/K+-ATPase activity which leads to impairment of the nerves function (24, 25). Peripheral nerves dysfunction results in slower nerve conduction velocity, which is induced after the onset of diabetes (26). A relationship has been established between reduced nerve conduction velocity and structural lesions, such as axo-glial disjunction and axonal atrophy, in chronically diabetic animals (27, 28).
Despite the widespread occurrence and serious complications of diabetes, there are still no treatments available for diabetic peripheral neuropathy other than glycemic control and diligent foot care. Therefore, it is important to study the pathological mechanisms and look for more specific therapeutic approaches (29, 30).
Recently, a cannabinoid receptor agonist, WIN 55,212-2 that was topically applied has shown to be effective against noxious heat-evoked activity in spinal wide dynamic range (WDR) neurons. Moreover, it is reported that WIN 55,212-2 is effective against hyperalgesia and allodynia in painful unilateral mononeuropathy (22, 31).
Hence, the aim of this study was to observe the local effect of intrathecal administration of a high-affinity cannabinoid agonist, WIN 55,212-2, on thermal hyperalgesia, nerve conduction velocity and histopathology in diabetic rats. This attempt may provide a better understanding of the use of local cannabinoids as a new treatment approach for diabetic neuropathic pain.
Materials and Methods
Animals and surgery
Male Wistar albino rats, weighing 230-250 g, were fed with a standard rodent diet and had free access to food and water. Animals were accommodated in groups of six to eight in a temperature-controlled room (24± 1°C) with a 12 hr/12 hr light/dark cycle (lights were turned on at 7:00 hr). Diabetes was induced by a single intraperitoneal injection of 45 mg/kg of streptozotocin (Sigma-Aldrich, St. Louis, MO) dissolved in citrate buffer (10 mM, pH 4.5), after an overnight fasting (23, 27). Rat tail vein blood glucose was measured 72 hr after STZ injection and rats with a blood glucose level of >300 mg/dl were considered diabetic and used for further experiments. Control animals received buffer solution. All studies were performed in accordance with institutional ethical guidelines for the care and use of laboratory animals, and the protocols were approved by Tehran University of Medical Sciences (TUMS), Tehran, Iran.
Intrathecal catheterization and drug delivery
Intrathecal catheters were implanted four days before behavioral tests as previously described by Yang et al (32). Briefly, animals were divided into two groups, including diabetic and control animals that were first anesthetized with ketamine–xylazine (75 and 15 mg/kg, respectively, IP). Once anesthetized, a 1–2 cm midline longitudinal incision was made at the level of the iliac crests. Then, a polyethylene catheter (PE-10, BD, USA) that implanted to a depth of about 1.0 cm from the incision between the lumbar vertebrae L5 and L6 to the dorsal spinal surface and the surgical wound was sutured. The intrathecal catheter implementation was confirmed with the paralysis of the bilateral hind limbs after 2% lidocaine (10 µl) injection through the catheter (33, 34).
Behavioral tests
Two weeks after the induction of diabetes, the analgesic effect of intratechal WIN 55,212-2 (1, 10 and 100 µg/10 µl) (2) (Sigma Chemical Co.) was evaluated with the tail flick test which is one of the standard tests for measuring analgesic effects of chemicals. Control rats received 50% DMSO in saline via a similar route. The rats were held gently, and a radiant heat source was focused 3 cm from the tip of the tail and the latency to tail flick was recorded automatically. A 15-sec cut-off was employed to prevent tissue damage. Withdrawal latencies were measured immediately prior to (predose) and then up to 6 hr following drug or vehicle administration.
In order to generate a dose–response curve against antinociception, data were converted to percentage of maximal possible effect (MPE) by using the following equation:
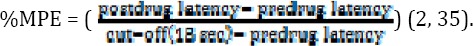
Also, hot plate test was used to measure the sensitivity to pain as this method assesses paw withdrawal latency to a thermal nociceptive stimulus. The plate thermal degree was maintained at 52 ±2 °C and the pain response time was recorded when the animal started hind paw licking or jumping. To prevent tissue damage, the cut-off latency was considered 60 sec (6, 36).
Electrophysiological examination
Electrophysiological parameters of the sciatic and sural nerves were recorded before STZ injection and two weeks after STZ injection. The animals were anesthetized with pentobarbital (45 mg/kg). The nerve conduction velocity (NCV) was calculated as the ratio between the distance from one point of stimulation to the next and the corresponding latency difference (24, 37).
Histological examination
For pathologic studies, two weeks after diabetes induction, sciatic nerve was carefully dissected from the proximal aspect of the thigh to the knee joint proximal to its point of division into common peroneal, tibial, and sural nerves and then fixed in 10% formaldehyde. These samples were compared with sciatic and sural nerve samples taken from rats of the control, diabetic and also treated groups. Nerves were cut into 5µm sections and stained with hematoxylin and eosin (H and E).
Statistical analysis
Data from the behavioral tests for the tail flick and hotplate (latency in sec) were expressed as the mean±SEM and analyzed by using repeated ANOVA measurements to detect the overall differences among treatment groups. When significant main effects were observed, post hoc tests were performed to determine the sources of differences. Statistical analysis was performed using GraphPad prism 5.0 software (GraphPad software Inc, California, USA). Differences were considered to be statistically significant at the level of P<0.05.
Results
STZ-induced diabetic rats,72 hr after STZ injection, revealed significantly increased blood glucoselevels (407.25±74.78mg/dl) compared to the vehicle-treated control (F(2,102)=456.5, P <0.001). The animals showed hyperglycemia and a marked decline in body weights following induction of diabetes by STZ (F(2,78)=28.55, P<0.001) (Table 1). Four fold increases in blood sugar levels were produced in the diabetic animals and this increase in blood sugar level (BSL) was stable during the study period. Treatment with WIN 55, 212-2had no effect on the body weight and blood sugar levels of the animals (Table 1).
Table 1.
Body weight and blood glucose of the rats before and after the induction of diabetes.Values are expressed as mean ± SEM
| Control | 3 days after diabetes induction | 2 weeks after diabetes induction | |
|---|---|---|---|
| Weight (g) | 254/19±5/44 | 212/82±5*** | 197±6/27*** |
| Blood glucose (mg/dl) | 99/21±1/11 | 407/25±11/27*** | 478/66±18/97*** |
P< 0.001 versus control
The hot-plate latency of STZ-induced diabetic rats was higher compared to the normal group. Weekly examinations of the tail flick and hot-plate latency showed that there was a dip in the latency around 14 days after the induction of diabetes which was normalized and augmented further till the fifth week of diabetes.
Baseline tail-flick latencies were found to be 6.70±1.28 and 5.64 ± 0.98 sec in control and diabetic groups, respectively, that were not significantly different. Also, we plotted the dose–response curves of IT administration of WIN 55, 212-2 in control and diabetic rats. WIN 55, 212-2 (1, 10 and 100 µg/10 µl) significantly prolonged the tail-flick latencies ina dose-dependent manner in diabetic rats 360 min after administration (F(12,125)=2.62)(Figure 1).
Figure 1.
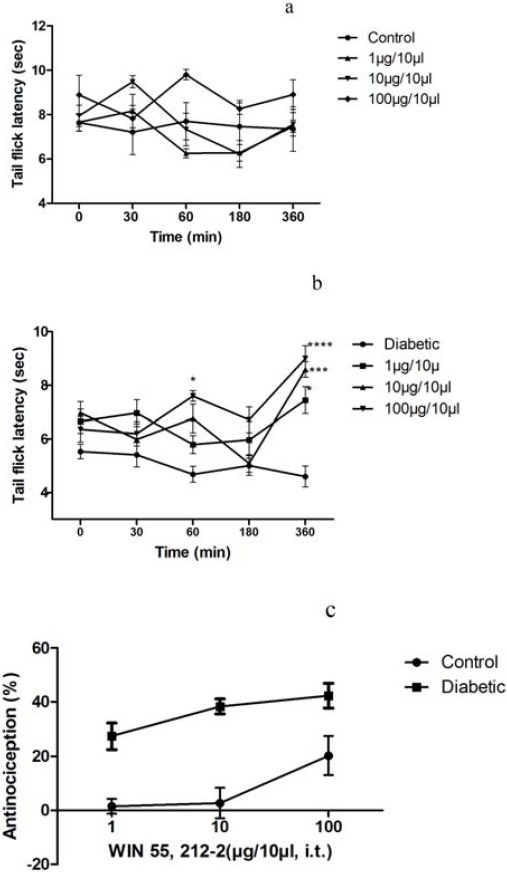
Animals received WIN 55, 212-2 (1, 10 and 100 µg/10 µl, IT) and tested the tail-flick latencies in control (a) and diabetic (b) rats at the indicated time.(c) dose-response curves for suppression of hyperalgesia behavior by WIN 55, 212-2 in tail flick test. Y-axis: percent of maximum possible effect (% MPE); X-axis:dose (μg). n= 6–8 ratsper group
Thermal sensitivity of animals in the hot-plate test was found to be 18.75 ± 2.98 and 11.75 ± 0.5 sec in control and diabetic rats, respectively. In the present study, we showed that WIN 55, 212-2 produced dose-dependent antinociceptive effects in diabetic rats. The antinociceptive potential of WIN 55, 212-2 was found in diabetic rats as it restored the decreased withdrawal thresholds to baseline control levels (F(21,200)=3.70, P<0.0001) (Figure 2).
Figure 2.
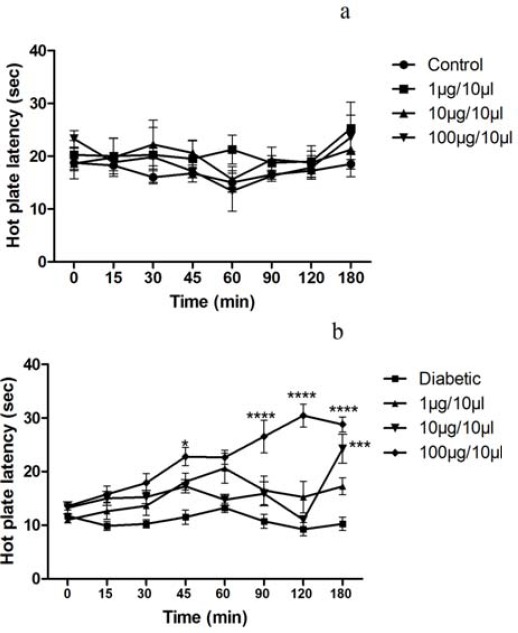
The effects of intrathecal administration of WIN 55, 212-2 in control and diabetic rats (a and b) as assessed by hot plate test; n= 6–8 rats per group
The motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV) values of normal and diabetic group showed that diabetes attenuated the nerve conduction velocity of the experimental animals. A significant decrease in NCV was observed two weeks after diabetes induction (P<0.05).
SNCV in diabetic rats was 38.37 ±1.67 m/sec as compared to control group (50.62± 1.06 m/sec, P<0.05). Treatment with WIN 55, 212-2 (100 µg) produced a significant reversal of the sensory nerve conduction velocity when compared to diabetic group (F(2,21)=11.89, P<0.001) (Figure 3).
Figure 3.
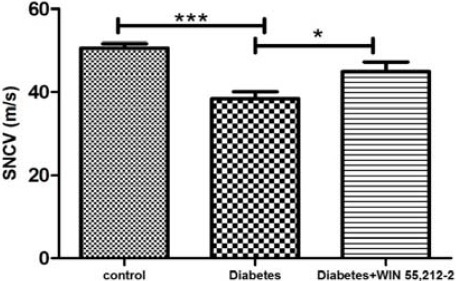
Effect of treatment with WIN 55, 212-2 (100 µg/10 µl, IT) on sensory nerve conduction velocity.Values are expressed as mean ± SEM. *P<0.05 compared to diabetes group and ***P<0.001 compared to control group
MNCV in WIN 55, 212-2-treated animals did not reach the levels observed in the control group (P<0.05). In diabetic rats, a significant deficit in nerve conduction velocity was observed (38.12±1.35 m/sec) as compared to control group (50.50±1.64 m/sec, F(2,21)=9.28, P<01). There was no significant improvement by intervention treatment on MNCV in diabetic rats observed (Figure 4).
Figure 4.
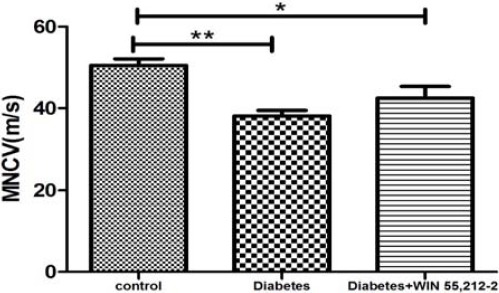
Effect of treatment with WIN 55, 212-2 (100 µg/10 µl, IT) on motor nerve conduction velocity. Values are expressed as mean ± SEM. *P<0.05 and **P<01 compared to control group
The microscopic preparations of sciatic and sural nerves indicated normal histology in the normoglycemic group. In diabetic rats, an increase in cellular infiltrate or locally extensive areas of moderate to marked edema was observed. The WIN 55, 212-2-treated group showed small focal areas of mild edema, reduction in cellular infiltration, lack of destruction and damage to the nerve cells, lack of necrosis and apoptosis and also slightly-increased micro vessels, a sign of healingand recovery, compared to diabetic group (Figure 5).
Figure 5.
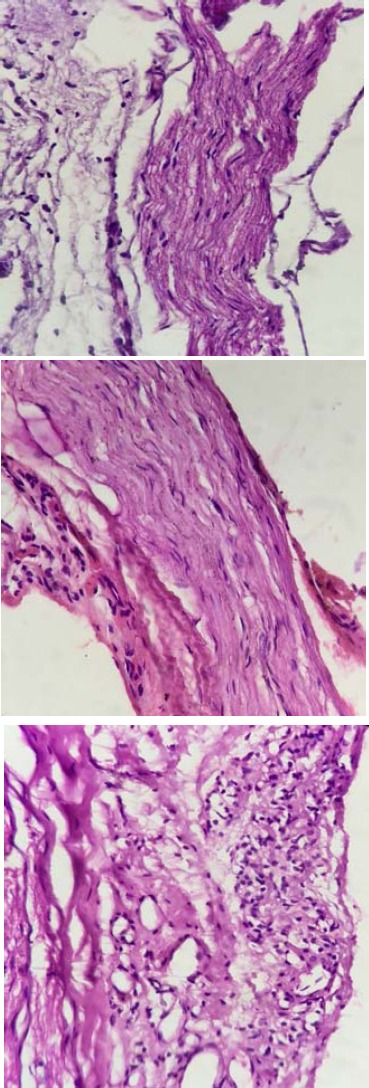
Cross sections of sciatic nerve from control (a), diabetic (b) and WIN 55, 212-2-treated animals (c and d). An increase in cellular infiltration and edema are seen in the diabetic rats. Treated animals show reduction in cellular infiltration, lack of destruction and damaged nerve cells, lack of necrosis and apoptosis and increasing microvessels
Discussion
The present study showed that intrathecally administered WIN 55,212-2 produced a dose-dependentaugmentation in the latency in both hot plate and tail flick tests in diabetic rats. Current evidence indicates that STZ-induced diabetic mice are significantly less responsive to the antinociceptive effects of opioids in tail-flick test (35, 38, 39). It has been accepted that both opioids and cannabinoids are effective antinociceptive agents in rats without pathological pain (40). However, there are some intractable pathological pain syndromes including painful peripheral neuropathy where cannabinoid analgesia system is considered superior to opioids in alleviating pain (40) as in painful peripheral neuropathy, opioid antinociception was reduced but cannabinoid antinociception was not altered (35, 40). Therefore, cannabinoid system can be a therapy of choice for management ofneuropathic pain.
Several studies have focused on cannabinoids action in neuropathic pain conditions, and cannabinoids are proposed to be more effective against chronic rather than acute pain (41-43). In this regard, WIN 55,212-2 was used as an antihyperalgesic and antiallodynic agent in nerve injury-induced neuropathic pain (35, 44). In contrast, little information about the effects of cannabinoids on diabetes-induced neuropathic pain exists (6, 22, 35).
Hence, we studied the effect of spinally administered WIN 55,212-2 on thermal hyperalgesia, electrophysiological and histopathological changes in diabetic rats. Regarding diabetic hyperalgesia (evident in the 2nd week of our experiment), we also focused on pathological changes in the sciatic and sural nerve that result in increased cellular infiltration and edema of peripheral nerves, as previously reported (23, 45, 46).
Thermal hyperalgesia, assessed by the hot-plate and tail flick tests, developed within 14 days in diabetic rats after STZ injection is consistent with previous studies(22, 47). However, although thermal hyperalgesia is fairly consistent with reduction in thermal threshold as demonstrated in STZ diabetic models, reports on changes in thermal nociceptive thresholds have been highly variable, from thermal hyperalgesia observed in some studies to no changes or decreased thermal sensitivity reported by others (35, 48). This discrepancy might be described by the fact that there are so many variables in these tests including species of animals, methodology, standards of response, duration of being diabetic, etc. (23, 45).
The findings of the present study demonstrate that spinally administered WIN 55, 212-2 blocks thermal hyperalgesia in diabetic rats. However, the mechanism by which WIN 55, 212-2 blocked thermal hyperalgesia in diabetic rats, is not clear. It has been demonstrated that cannabinoids act at spinal and supraspinal sites and also in the periphery to alleviate pain (49, 50). The potential importance of supraspinal modulation of pain processing by cannabinoid has been emphasized by the peripheral noxious stimulation that induces the release of anandamide in the PAG, one of the most important supraspinal sites for modulation of nociceptive information (51).
Systemic cannabinoid administration has been indicated to be effective in decreasing mechanical allodynia in different nerve injury models of neuropathic pain in animals (2, 52) and CB1 receptors activation was shown to be involved in the antiallodynic effects of cannabinoids in those models (2, 37, 40).
Since the adverse effects limit the systemic usage of cannabinoid agonists as effective analgesics, topical administration has some advantages (22). It has been reported that A-fibers have a key function in the allodynia related to peripheral nerve injury (53, 54) and abnormal activity in A-delta and A-beta fiber leads to mechanical allodynia in diabetes.It is notable that CB1 receptors are mostly found in myelinated A-fibers on DRG neurons (54).
In support of the efficacy of cannabinoids in noxious heat-evoked activity, spinally administered WIN 55,212-2 to intact rats, produced antinociceptive effects in the tail-flick test with a time-course and efficacy that paralleled the suppression of noxious heat-evoked activity (1).
These findings propose that the role of cannabinoid in modulating spinal nociceptive effects is mediated via spinal dorsal horn and is associated with the antinociceptive effects of intrathecally administered cannabinoids. More variability in the onset of suppression was prominent for the topical route of administration as compared to systemic (55) or intraventricular administration (1). In contrast, little information on the effects of intrathecally administered cannabinoids in diabetes-induced neuropathic pain exists.
One of the important goals of the current study was to compare the alterations in NCV between STZ-treated and WIN55, 212-2-treated rats. In diabetic rats, a significant impairments in nerve conduction velocity was observed which is not consistent with previous experiment (14, 27, 33, 37); however, intrathecal administration of WIN55, 212-2 recovered these deficits in sciatic nerve. This is the first time that the beneficial effects of WIN55, 212-2 on altered NCV in diabetic rats have been found.
Light microscopy examinations of sciatic and sural nerve showed alterations in neuronal appearance including mild increase of micro vessels that is a sign of healingand recovery in treated rats compared to STZ-treated rats.
Accordingly, our results would suggest that the anti-allodynic effects of WIN 55, 212-2 in STZ diabetic rats might be mediated through the CB1 receptors. However, diabetes induces nerve injury throughout the nervous system including, peripheral nervous system and brain (20, 51). CB1 receptors are also present throughout the central nervous system(51). Therefore, we can propose that the effect of WIN 55, 212-2 mediated by CB1 receptors, may be due to changes in the expression or sensitivity of CB1 receptors (35). These receptors are present not only on the primary afferent fibers but also throughout the nervous system in diabetes (35). To address this issue, further experiments are required to compare the activity and expression of CB1 receptors in nerve tissues in diabetic and nondiabetic control animals (10, 35).
Conclusion
In conclusion, cannabinoid agonist WIN 55, 212-2 undoubtedly has marked antinociceptive effects in diabetic rats. Painful neuropathy is an important diabetic complication, which severely affects the quality of life, and our results showed that cannabinoids could be considered as hopeful treatments for diabetic neuropathic pain.
Acknowledgment
This research was supported by Tehran University of Medical Sciences, Tehran, Iran.
Reference
- 1.Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci Lett. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- 2.Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral cannabinoid 1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 3.Tsou K, Brown S, Sanudo-Pena M, Mackie K, Walker J. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 4.Hohmann A, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia:a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 5.Hohmann A, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- 6.Martin WJ, Loo CM, Basbaum AI. Basbaum, Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Daughters RS, Bullis C, Bengiamin R, Stucky MW, Brennan J, et al. The cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in rats. Pain. 1999;81:25–33. doi: 10.1016/s0304-3959(98)00263-2. [DOI] [PubMed] [Google Scholar]
- 8.Costa B, Siniscalco D, Trovato AE, Comelli F, Sotgiu ML, Colleoni M, et al. AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. Br J Pharmacol. 2006;148:1022–1032. doi: 10.1038/sj.bjp.0706798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang YC, Huang CC, Hsu KS. The synthetic cannabinoids attenuate allodynia and hyperalgesia in a rat model of trigeminal neuropathic pain. Neuropharmacology. 2007;53:169–177. doi: 10.1016/j.neuropharm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Siegling A, Hofmann HA, Denzer D, Mauler F, De Vry J. Cannabinoid CB 1 receptor upregulation in a rat model of chronic neuropathic pain. Eur J Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- 11.Bridges D, Thompson S, Rice A. Mechanisms of neuropathic pain. Br J Anaesth. 2001;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee RG. Cannabis and cannabinoids:pharmacology and rationale for clinical use. Forsch Komplementarmed. 1999;6:12–15. doi: 10.1159/000057150. [DOI] [PubMed] [Google Scholar]
- 13.Rice A, Farquhar-Smith W, Nagy I. Endocannabinoids and pain:spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan CW, Christie MJ. An analgesic role for cannabinoids. Med J Australia. 2000;173:270–272. doi: 10.5694/j.1326-5377.2000.tb125638.x. [DOI] [PubMed] [Google Scholar]
- 15.Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain:pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB 2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 19.Malan TP, Ibrahim MM, Vanderah TW, Makriyannis A, Porreca F. Inhibition of pain responses by activation of CB 2 cannabinoid receptors. Chem Phys Lipids. 2002;121:191–200. doi: 10.1016/s0009-3084(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 20.Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55, 212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanuš L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308:a specific agonist for CB2, a peripheral cannabinoid receptor. Proc Natl Acad Sci. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett. 2004;371:167–170. doi: 10.1016/j.neulet.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi-Farani A, Sahebgharani M, Sepehrizadeh Z, Jaberi E, Ghazi-Khansari M. Diabetic thermal hyperalgesia:role of TRPV1 and CB1 receptors of periaqueductal gray. Brain Res. 2010;1328:49–56. doi: 10.1016/j.brainres.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 24.Becker M, Benromano T, Shahar A, Nevo Z, Pick CG. Changes in the basal membrane of dorsal root ganglia schwann cells explain the biphasic pattern of the peripheral neuropathy in streptozotocin-induced diabetic rats. J Mol Neurosci. 2014;54:704–713. doi: 10.1007/s12031-014-0424-2. [DOI] [PubMed] [Google Scholar]
- 25.Kafri M, Drory VE, Wang N, Rabinowitz R, Korczyn AD, Chapman J. Assessment of experimental autoimmune neuritis in the rat by electrophysiology of the tail nerve. Muscle Nerve. 2002;25:51–57. doi: 10.1002/mus.10011. [DOI] [PubMed] [Google Scholar]
- 26.Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy:from glucose metabolism to neurodegeneration. J Diabetes Res. 2003;4:303–312. doi: 10.1155/EDR.2003.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbi A, Maixent JM, Ansaldi JL, Pierlovisi M, Coste T, Pelissier JF, et al. Fish oil supplementation prevents diabetes-induced nerve conduction velocity and neuroanatomical changes in rats. J Nutr. 1999;129:207–213. doi: 10.1093/jn/129.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Sima A, Prashar A, Nathaniel V, Bril V, Werb M, Greene D. Overt diabetic neuropathy:repair of axo-glial dysjunction and axonal atrophy by aldose reductase inhibition and its correlation to improvement in nerve conduction velocity. Diabet Med. 1993;10:115–121. doi: 10.1111/j.1464-5491.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 29.Siemionow M, Demir Y. Diabetic neuropathy:pathogenesis and treatment. J Reconstr Microsurg. 2004;20:241–252. doi: 10.1055/s-2004-823112. [DOI] [PubMed] [Google Scholar]
- 30.Peeraer E, Van Lutsenborg A, Verheyen A, de Jongh R, Nuydens R, Meert TF. Pharmacological evaluation of rat dorsal root ganglion neurons as an in vitro model for diabetic neuropathy. J Pain Res. 2011;4:55. doi: 10.2147/JPR.S15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55, 212-2. Neurosci Lett. 1998;257:119–122. doi: 10.1016/s0304-3940(98)00802-7. [DOI] [PubMed] [Google Scholar]
- 32.Yang P, Wen H, Ou S, Cui J, Fan D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol. 2012;236:19–27. doi: 10.1016/j.expneurol.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Kang S, Kim CH, Lee H, Kim DY, Han JI, Chung RK, et al. Antinociceptive synergy between the cannabinoid receptor agonist WIN 55,212-2 and bupivacaine in the rat formalin test. Anesth Analg. 2007;104:719–725. doi: 10.1213/01.ane.0000255291.38637.26. [DOI] [PubMed] [Google Scholar]
- 34.Cui J, He W, Yi B, Zhao H, Lu K, Ruan H, et al. mTOR pathway is involved in ADP-evoked astrocyte activation and ATP release in the spinal dorsal horn in a rat neuropathic pain model. Neuroscience. 2014;275:395–403. doi: 10.1016/j.neuroscience.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Doğrul A, Gül H, Yıldız O, Bilgin F, Güzeldemir ME. Cannabinoids blocks tactile allodynia in diabetic mice without attenuation of its antinociceptive effect. Neurosci Lett. 2004;368:82–86. doi: 10.1016/j.neulet.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Shabani M, Zangiabadi N, Asadi-Shekaari M. Evidence for positive effects of date extract that attenuates thermal hyperalgesia in a diabetic rat model of neuropathic pain. Neurosci Med. 2013;4:16–22. [Google Scholar]
- 37.Manschot SM, Gispen WH, Kappelle LJ, Biessels GJ. Nerve conduction velocity and evoked potential latencies in streptozotocin-diabetic rats:effects of treatment with an angiotensin converting enzyme inhibitor. Diabetes Metab Res Rev. 2003;19:469–477. doi: 10.1002/dmrr.401. [DOI] [PubMed] [Google Scholar]
- 38.Fox A, Eastwood C, Gentry C, Manning D, Urban L. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain. 1999;81:307–316. doi: 10.1016/S0304-3959(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 39.Ohsawa M, Mizoguchi H, Narita M, Narita M, Kamei J, Nagase H, et al. Effects of a μ-opioid receptor agonist on G-protein activation in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2000;401:55–58. doi: 10.1016/s0014-2999(00)00408-8. [DOI] [PubMed] [Google Scholar]
- 40.Mao J, Price D, Lu J, Keniston L, Mayer D. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- 41.Fuentes J, Ruiz-Gayo M, Manzanares J, Vela G, Reche I, Corchero J. Cannabinoids as potential new analgesics. Life Sci. 1999;65:675–685. doi: 10.1016/s0024-3205(99)00290-8. [DOI] [PubMed] [Google Scholar]
- 42.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 43.Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Therapeut. 2002;95:127–135. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 44.Herzberg U, Eliav E, Bennett G, Kopin IJ. The analgesic effects of R (+)-WIN 55,212–2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- 45.Calcutt NA. Experimental models of painful diabetic neuropathy. J Neurol Sci. 2004;220:137–139. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Kakinoki B, Sekimoto S, Yuki S, Ohgami T, Sejima M, Yamagami K, et al. Orally active neurotrophin-enhancing agent protects against dysfunctions of the peripheral nerves in hyperglycemic animals. Diabetes. 2006;55:616–621. doi: 10.2337/diabetes.55.03.06.db05-1091. [DOI] [PubMed] [Google Scholar]
- 47.Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats:behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- 48.Ulugol A, Karadag HC, Tamer M, Firat Z, Aslantas A, Dokmeci I. Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci Lett. 2002;328:129–132. doi: 10.1016/s0304-3940(02)00491-3. [DOI] [PubMed] [Google Scholar]
- 49.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 50.Hohmann AG, Briley EM, Herkenham M. Pre-and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- 51.Iversen L, Chapman V. Cannabinoids:a real prospect for pain relief. Curr Opin Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 52.Bridges D, Rice A, Egertova M, Elphick M, Winter J, Michael G. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 53.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 55.Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sci. 1995;56:2111–2118. doi: 10.1016/0024-3205(95)00196-d. [DOI] [PubMed] [Google Scholar]


