Abstract
Objective(s):
With regard to pharmacological effects of carvacrol on the respiratory system, its effect on cytokines genes expression in splenocytes of asthmatic mice was examined in this study.
Materials and Methods:
Splenocytes were isolated from non-sensitized (control group), sensitized mice to ovalbumin (OVA) (group S), and S animals treated with dexamethasone, and three concentrations of carvacrol. IL-4, IFN-γ, TGF-β, FOXP3, and IL-17 genes expression were carried out in cultured splenocytes using the real-time PCR method.
Results:
Compared to the control group, IFN-γ and FOXP3 genes expression were significantly decreased (P<0.001 for both cases), but IL-4 and IL-17 genes expression were significantly increased in the S group (P<0.001 and P<0.05, respectively). IL-4 gene expression due to treatment of all concentrations of carvacrol, TGF-β gene expression due to its two higher concentrations, and IL-17 gene expression due to its high concentration were significantly decreased compared to group S (P<0.01 to P<0.001). IFN-γ gene expression was significantly increased due to last carvacrol concentration (300 µg/ml, P<0.01), and FOXP3 due to its two last concentrations (150 and 300 µg/ml, P<0.05 and P<0.001, respectively) in treated S splenocytes. Dexamethasone treatment of sensitized splenocytes only showed significant inhibitory effect on IL-4 and TGF-β genes expression (P<0.001 for both cases).
Conclusion:
These results showed the immunomodulatory effect of carvacrol indicating increased IFN-γ and FOXP3 but decreased IL-4, TGF-β, and IL-17 genes expression, which was more selective than the effect of dexamethasone in sensitized mice splenocytes, which indicates its possible therapeutic value in allergy, autoimmunity, and infectious diseases.
Keywords: Carvacrol, Cytokines, Gene expression, Real-time PCR, Splenocyte
Introduction
The pathologic manifestations of asthma include airway inflammation, airway remodeling and mucus hypersecretion (1). In asthmatics, CD4+ T cells productions such as IL-4, IL-5, and IL-13 have been identified in bronchoalveolar lavage (BAL) and airway biopsies. These cytokines are secreted in the airways of patients with mild or asymptomatic asthma (2, 3). Th2 cells have been identified in the airways of asthmatics, and because Th2 cytokines are required for the development of airway eosinophilia and IgE, it has been proposed that Th2 cells stimulate an inflammatory response that results in asthma (1). Th1 has proved to inhibit Th2 responses, and thus one goal of asthma therapy should be focusing on increasing the activity of Th1 (4). The forkhead/winged helix transcription factor FOXP3 serves as a master regulator for Treg (regulatory T cell) development and is currently found to be the most specific Treg marker (5-7). It has been shown, in both animals and humans that the absence of FOXP3 is associated with the development of immune abnormalities such as severe allergic inflammation and high immunoglobulin E (IgE) levels (8). IL-17 has been proposed to have an important role in the development of autoimmune disorders and in the induction and maintenance of chronic inflammation (9). IL-17 also appears to play an upstream role in T cell-triggered inflammation and hematopoiesis by stimulating stromal cells to secrete other cytokines and growth factors (10). Carvacrol or cymophenol, C6H3CH3(OH)(C3H7) is one of the main constituents of several medicinal plants including Zataria multiflora (11) that have various therapeutic effects on respiratory diseases (11, 12).
All plants containing carvacrol have shown relaxant effects on different smooth muscles including trachea (13-19) and have also shown anti-inflammatory effects (20-27). The relaxant effect of essential oil from Carum copticum on on tracheal chains has been shown to be mainly due to its fraction 2, which is suggested to be carvacrol (28). The relaxant effect of carvacrol on tracheal smooth muscle has also been observed in another study (29). Various effects of carvacrol on sensitized guinea pigs including effect on tracheal responsiveness, pathological changes in the lung, inflammatory cells, and inflammatory mediators were also demonstrated in previous studies (29-34). These studies indicate that carvacrol or plant containing this chemical may affect asthma therapy.
Therefore, in the present study, the effect of carvacrol on genes expression of some cytokines in asthmatic mice splenocytes was studied.
Materials and Methods
Experimental animals and their sensitization
Pathogen-free, 6 to 8 week-old, male BALB/c mice, weighing 18 to 20 g, were purchased from Razi Institute (Mashhad, Iran) and maintained in a pathogen-free animal room in School of Medicine. They were kept in hygienic cages and in air-conditioned rooms 12 hr light/12 hr dark cycle with food and water available ad libitum.
The mice were sensitized by two intraperitoneal injections, on days 0 and 14 of the experiment, with 10 μg/0.1 ml chicken egg albumin (Ovalbumin, grade V, 98% pure; Sigma, St. Louis, MO, USA) together with Al (OH)3 as an adjuvant. The animals were then exposed to an aerosolized 2.5% ovalbumin (OVA) concentration for 30 min/day, three days/week for eight weeks beginning from the 21st day of the study. The mice in the control group received IP and inhaled normal saline instead of OVA using the same procedure as OVA administration in sensitized animals (35, 36). Exposures were carried out in a whole body inhalation exposure chamber. A solution of 2.5% OVA in normal saline was aerosolized by delivery of compressed air to a jet nebulizer (37).
Preparation of splenocytes culture and experimental groups
Splenocytes were removed and suspended in complete RPMI 1640 with 15% FBS at a density of 5×106/ml.
The study was carried out in the following groups (n=5 for groups 1 to 3 and n=6 for treated groups with carvacrol):
(1) Non-sensitized splenocytes (control group).
(2) Sensitized splenocytes (group S).
(3) Sensitized splenocytes treated with dexamethasone (0.1 mM/L, Sigma Chemicals, LTD.), (group D).
(4) Sensitized splenocytes treated with carvacrol at 3 concentrations of 75, 150, and 300 µg/ml (groups C1, C2, and C3).
Splenocytes in all groups were cultured as follows; 5×106 cells were suspended in culture environment (15%) and dexamethasone (0.1 mM/L), carvacrol (75, 150, and 300 µg/ml) were added and were incubated for 18h (T:37°, PCO2:5%). Then, cells were separated and 1 ml Tripure per every microtube was added.
RNA extraction and real-time PCR analysis
Total RNA was isolated from splenocytes with Tripure Isolation Reagents (Roche Applied Science, Germany) according to the manufacturer’s instructions.
Samples were incubated for 10 min at room temperature. Then 200 μl chloroform solution was added to the microtubes, and the vortexing intensity was15 sec. It was incubated for 15 min at 4°C in dim light. Samples were centrifuged at 4°C with 12000 rpm for 15 min. Clear supernatant was removed carefully and transferred to the other microtubes. Cold isopropanol 500 μl was added and incubated for 10 min at 4°C in dim light, then centrifuged for 10 min at 12000 rpm and 4°C. At the end of this stage, sediment RNA was visible as a tiny white pellet. The supernatant was removed and washed, the RNA precipitated by alcohol 75%, and then centrifuged at 4°C and 7500 rpm for 5 min. Supernatant was carefully and completely emptied and the remaining ethanol in the microtubes was removed by air flow. DEPS water was added to microtubes containing RNA, and for the duration of 10 min at 56°C was placed on a dry-block device; eventually moved to -20°C and held until cDNA synthesis.
Synthesis of cDNA
Due to the low half-life of extracted mRNA, it must be transcribed to cDNA, which was performed by the enzyme reverse transcriptase. This DNA sequence was then used as a template in PCR and second strand DNA synthesis by gene-specific primers.
A fixed volume of input RNA (1 µl) was used for each cDNA reaction. Reverse transcription reaction was carried out with Revert Aid™H Minus M-MulV First Strand (Fermentase, Germany) according to manufacturer’s instructions.
Quantitative real-time PCR (qPCR)
The real-time PCR primers and quantitation probes were designed with mRNA sequences for β2M (β2-Microglobin), IFN-γ (Interferon gamma), IL-4, IL-17, and TGF-β (Transforming growth factor beta), and FOXP3 was obtained from the National Center for Biological Information website (http://www.ncbi.nlm.nih.gov), using the Beacon Designer software version 7.9. The criteria for primers and probes design included a melting temperature (Tm) for the primers of 58–60°C, a Tm for the probe of 10°C higher than that of the primers and a maximum amplicon length of less than 150 base pairs (bp). The primers and probes set crossed the intron-exon boundaries, ensuring amplification of mRNA only. The primers and probe were as follows:
1. β2-Microglobin;5-GCCGAACATACTGAACTGCTAC-3(forward)
5-CTTGCTGAAGGACATATCTGACATC-3(reverse)
5-AACACAGTTCCACCCGCCTCACATTGA-3 (probe).
2. IFN-γ;5-GTATTGCCAAGTTTGAGGTCAAC-3(forward)
5-GCTTCCTGAGGCTGGATTC-3(reverse)
5-CCACAGGTCCAGCGCCAAGCATTCAA-3 (probe)
3. IL-4;5-TCCTCACAGCAACGAAGAAC-3 (forward)
5-CAAGCATGGAGTTTTCCCATG-3 (reverse)
5-AGCACCTTGGAAGCCCTACAGACGAGC-3(probe)
4. IL-17; 5-CAGACTACCTCAACCGTTCC-3 (forward)
5-TTCCCTCCGCATTGACAC-3 (reverse)
5-ACTCTCCACCGCAATGAAGACCCTGA-3 (probe).
5. TGF-β; 5-CCTGGATACCAACTATTGCTTCAG-3 (forward)
5-CAGACAGAAGTTGGCATGGTAG-3 (reverse)
5-TCCACTTCCAACCCAGGTCCTTCCT-3 (probe)
6. FOXP3; 5-GGTACACCCAGGAAAGACAG-3 (forward)
5-GCTTGGCAGTGCTTGAGA-3 (reverse)
5-TGGCTCCTCGAAGACCTTCTCACAACC-3 (probe)
Beta 2 microglobulin (β2M) was used as reference gene to normalize the mRNA expression levels and control the errors between samples.
The real-time PCR reactions were performed in glass capillaries (Qiagen, Germany) in a final volume of 10 µl containing 2 µl cDNA template, 0.4 µl of primer pairs (200 nM, β2M, IFN-γ, IL-4, IL-17, TGF-β, and FOXP3), and 5 µl of the probe Master Mix. Two standard curve methods were used for target and reference genes quantification by a Rotor-Gene Q Real-Time PCR machine (Corbett Research, Australia). The Rotor-Gene 6000 software (Corbett Research, Australia) was used to analyze the standards and the unknown RNA copy numbers. The relative quantity of each mRNA was normalized to the relative quantity of β2M mRNA. Then the relative gene of interest expression level for each sample was calculated by an equation:
Normalized index¼ copy number of gene of interest (IFNγ, IL-4, …)/copy number of reference gene (β2M). Then the fold change in expression of gene of interest for each group was calculated by the following equation:
Mean of gene of interest Normalized Index of test group/mean of gene of interest Normalized Index of healthy controls.
Statistical analysis
All data were expressed as mean ± SEM. SPSS 16 software was used to analyze the data. The statistical analyses were carried out using one-way ANOVA with Tukey-Kramer post hoc tests. The results were considered statistically significant if the P value was less than 0.05.
Results
Comparison of gene expression between sensitized and control splenocytes
In sensitized splenocytes, IFN-γ and FOXP3 genes expression significantly decreased (P<0.001), but IL-4 (P<0.001) and IL-17 genes expression increased (P<0.05), compared to the control group. In addition, IFN-γ/IL-4 ratio was significantly decreased in group S compared to the control group (P<0.001). However, there was no significant difference in TGF-β gene expression between the two groups (Figures 1–6).
Figure 1.
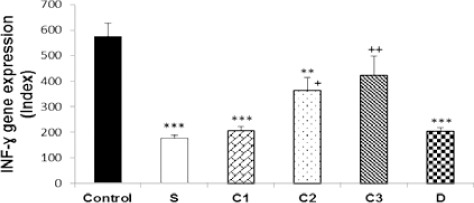
Values (mean±SEM) of IFN-γ gene expression in splenocytes from control mice (control) and sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150, and 300 µg/ml (C1, C2, and C3, respectively), (n=5 for groups C, S, and D, and n=6 for groups treated with carvacrol). The levels of gene expression were gene fold/mL but presented as Normalized Index, as explained in the methods section. Significant difference between control and other groups: **P<0.01, ***P<0.001. Significant difference between dexamethasone and carvacrol vs sensitized group: +P<0.05, ++P<0.01. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Figure 2.
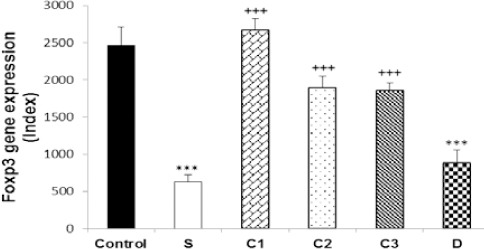
Values (mean±SEM) of FOXP3 gene expression in splenocytes from control mice (control) and sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150, and 300 µg/mL (C1, C2, and C3, respectively), (n=5 for groups C, S, and D, and n=6 for groups treated with carvacrol). The levels of gene expression were gene fold/ml but presented as Normalized Index as explained in the methods section. Significant difference between control and other groups: ***P<0.001. Significant difference between dexamethasone and carvacrol vs sensitized group: +++P<0.001. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Figure 3.
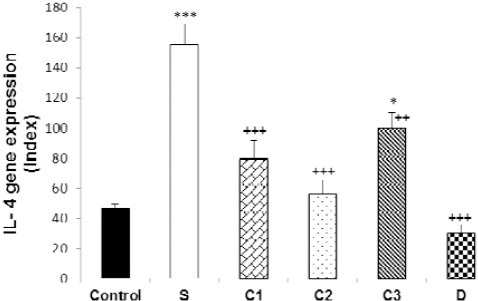
Values (mean±SEM) of IL-4 gene expression in splenocytes from control mice (control) and sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150, and 300 µg/ml (C1, C2, and C3, respectively), (n=5 for groups C, S, and D, and n=6 for groups treated with carvacrol). The levels of gene expression were gene fold/ml but presented as Normalized Index as explained in the methods section. Significant difference between control and other groups: *P<0.05, ***P<0.001. Significant difference between dexamethasone and carvacrol vs sensitized group: ++P<0.01, +++P<0.001. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Figure 4.
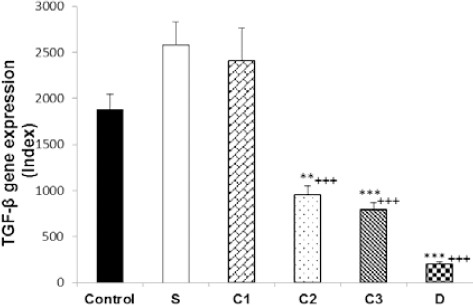
Values (mean±SEM) of TGF-β gene expression in splenocytes from control mice (control) and sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150, and 300 µg/ml (C1, C2, and C3, respectively), (n=5 for groups C, S, and D, and n=6 for groups treated with carvacrol). The levels of gene expression were gene fold/ml but presented as Normalized Index as explained in the methods section. Significant difference between control and other groups: **P<0.01, ***P<0.001. Significant difference between dexamethasone and carvacrol vs sensitized group: +++P<0.001. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Figure 5.

Values (mean±SEM) of IL-17 gene expression in splenocytes from control mice (control) and sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150, and 300 µg/mL (C1, C2, and C3, respectively), (n=5 for groups C, S, and D, and n=6 for treated groups with carvacrol). The levels of gene expression were gene fold/ml but presented as Normalized Index as explained in the methods section. Significant difference between control and sensitized group: *P<0.05. Significant difference between dexamethasone, and carvacrol vs sensitized group: +P<0.05. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Figure 6.

Values (mean±SEM) of IFN-γ/IL-4 gene expression ratio in splenocyte control mice (control), sensitized animals (S), and S groups treated with dexamethasone (D) and carvacrol at 3 concentrations of 75, 150 and 300 µg/ml (C1, C2, and C3 respectively), (n=5 for groups C, S and D and n=6 for treated groups with carvacrol). Significant difference between control and other groups: ***P<0.001. Significant difference between dexamethasone, and carvacrol vs sensitized group: +P<0.05, +++P<0.001. The statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test
Cytokine gene expression in splenocytes of sensitized mice treated with carvacrol
IFN-γ gene expression was significantly increased due to two higher carvacrol concentrations (150 and 300 µg/ml), (P<0.05 and P<0.01, respectively), but FOXP3 gene expression was increased by all concentrations (P<0.001 for all cases) in treated compared to non-treated sensitized splenocytes. IL-4 gene expression was significantly decreased due to all carvacrol concentrations (P<0.001 for 75 µg/ml and 150 µg/ml, P<0.01 for 300 µg/ml); TGF-β gene due to its two higher concentrations (150 and 300 µg/ml, P<0.001 for both cases), and IL-17 gene expression due to its last concentration (300 µg/ml, P<0.001) in treated compared to non-treated sensitized splenocytes. IFN-γ/IL-4 ratio was significantly increased in the treated groups with two higher carvacrol concentrations (P<0.001 for 150 and P<0.05 for 300 µg/ml) compared to group S (Figures 1–6). However, the IFN-γ genes expression in groups treated with C1 and C2, IL-4 gene expression in group treated with C3, TGF-β in groups treated with C2 and C3 and IFN-γ/IL-4 ratio in groups treated with all carvacrol concentrations were significantly different from those of the control group (Figures 1, 3, 4 and 6).
Cytokine gene expression in splenocytes of sensitized mice treated with dexamethasone
Dexamethasone treatment of sensitized splenocytes showed a significant inhibitory effect on IL-4 and TGF-β genes expression (P<0.001 for both cases). However, dexamethasone treatment of sensitized splenocytes did not significantly affect IFN-γ, FOXP3, and IL-17genes expression. In addition, IFN-γ/IL-4 ratio was significantly increased in the group treated with dexamethasone (P<0.001) compared to group S (Figures 1–6). IFN-γ, FOXP3, and TGF-β genes expression and IFN-γ/IL-4 ratio were significantly different in animals treated with dexamethasone than those of the control group (Figures 1, 2, 4, and 6).
Comparison of the effects of carvacrol and dexamethasone treatments on cytokine gene expression in splenocytes of sensitized mice
The effects of high carvacrol concentration treatment on IFN-γ gene expression in sensitized splenocytes were significantly higher than the effect of dexamethasone treatment (P<0.01, Table 1). The effects of all carvacrol concentrations treatments on FOXP3 gene expression in sensitized splenocytes were significantly higher than the effect of dexamethasone treatment (P<0.01 to P<0.001, Table 1). The effects of higher and lower carvacrol concentrations treatments on IL-4 gene expression in sensitized splenocytes were significantly lower than the effect of dexamethasone treatment (P<0.001 and P<0.05 for high and low carvacrol concentrations, respectively, Table 1). The effect of low carvacrol concentration treatment on TGF-β gene expression in sensitized splenocytes was significantly lower than the effect of dexamethasone treatment (P<0.001, Table 1). However, the effects of low and high carvacrol concentrations on IFN-γ/IL-4 ratio were significantly lower than the effect of dexamethasone (P<0.001 and P<0.05 for low and high concentrations, respectively, Table 1).
Table 1.
The values of IFN-γ, IL-4, TGF-β, FOXP3 and IL-17 genes expression as well as IFN-γ/IL-4 ratio in control (C), non-treated sensitized (S), sensitized treated with dexamethasone (0.1 mM/L) and carvacrol at concentrations of 75, 150, and 300 µg/ml (C1, C2, and C3, respectively) mice
| Variable | IFN-γ | FOXP3 | IL-4 | TGF-β | IL-17 | IFN-γ/IL-4 ratio |
|---|---|---|---|---|---|---|
| S+C1 | 205.33 ±17.80 | 2671.82±148.10 *** | 79.33±12.29 * | 2412.16±351.47 *** | 3176.42±377.06 | 2.88 ± 0.52 *** |
| S+C2 | 363.6 ±49.52 | 1898.93±150.80 ***# | 56.00± 9.06 | 950.16±100.30 *### | 2968.46±309.14 | 7.33 ± 1.52 ### |
| S+C3 | 422.16 ±74.88 **## | 1857.75±100.90 **# | 99.66± 10.61 *** | 793.16±74.25 ### | 2728.16±432.77 | 4.30 ± 0.63 * |
| S+D | 203.00 ±16.86 | 915.20± 172.92 | 29.80± 5.76 | 200.40±21.99 | 3789.71±381.44 | 7.46 ± 0.90 |
Values are presented as mean±SEM. The level of each gene expression (IFN-γ, IL-4, IL-17, TGF-β, and FOXP3) was gene fold/ml but presented as Normalized Index which was explained in the methods section.
Significant difference between treated groups with carvacrol vs dexamethasone (D):
P<0.05,
P<0.01,
P<0.001.
Significant difference between S+C2 and S+C3 vs S+C1 treatment groups:
P<0.05,
P<0.01,
P<0.001.
Statistical comparisons were made using ANOVA with Tukey–Kramer multiple post hoc test.
Comparison of the effects of three concentrations of carvacrol on cytokine gene expression in splenocytes of sensitized mice
There was no significant difference in IL-4 and IL-17 genes expression of sensitized splenocytes among three carvacrol concentrations (Table 1). However, the effect of only high carvacrol concentration (300 µg/ml) treatment on IFN-γ gene expression in sensitized splenocytes was significantly higher than the effect of its low concentration (75 µg/ml), (P<0.01, Table 1). The effect of lower carvacrol concentration (75 µg/ml) on FOXP3 expression potentiation was also significantly higher compared to its other two concentrations (P<0.05 for both cases, Table 1). In addition, the effects of the last two carvacrol concentrations (150 and 300 µg/ml) on reduction of TGF-β gene expression in sensitized splenocytes were significantly higher compared to its lower concentration (P<0.001 for both cases, Table 1). The effect of medium carvacrol concentration (150 µg/ml) treatment on IFN-γ/IL-4 ratio was significantly higher than its low concentration (P<0.001, Table 1).
Discussion
The results of the present study showed decreased IFN-γ and FOXP3 genes expression but increased IL-4 and IL-17 genes expression in sensitized animals. The IFN-γ/IL-4 ratio was also decreased in sensitized animals compared to the controls.
The immunologic features of asthma is a balance shift from Th1 to Th2 (31). Th2 productions such as IL-4, IL-5, and IL-13 have been identified in BAL and airway biopsies of patients with mild or asymptomatic asthma (2, 3). Th2 cytokines are required for the development of airway eosinophilia and IgE, stimulating an inflammatory response that results in asthma (1). IL-17 has also been demonstrated to have an important role in the development of autoimmune disorders and chronic inflammation (9). This cytokine also plays an upstream role in T cell-triggered inflammation and hematopoiesis by secretion of other cytokines and growth factors from stromal cells (10). IL-17 might be a potential player in the cytokine network involved in asthma, and a possible indirect effect of IL-17 on airway remodeling is suggested (38). IL-17 is up-regulated in asthma, which in turn increases synthesis of IL-6 and IL-11 by bronchial fibroblasts derived from bronchial biopsies of asthmatic subjects (38). Molet et al reported that Th2-type cytokines, including IL-4 and IL-13, enhance IL-17 induced release of IL-6 from fibroblasts, suggesting that IL-17 might be involved in asthma, which is a Th2-mediated disease (38). IL-17 has been reported to be involved in inflammatory cell recruitment into airways, particularly neutrophils (38). Th1 has proved to inhibit Th2 response by secretion of IFN-γ, IL-2, and TNFβ and thus one goal of asthma therapy should be increasing the balance of Th1/Th2 (39). In addition, it was shown that the absence of FOXP3 is associated with the development of immune abnormalities, such as severe allergic inflammation and high immunoglobulin E (IgE) levels (8). The reduction of FOXP3 protein expression is reported in patients with stable asthma (40). Therefore, reduction of IFN-γ and FOXP3 genes expression and IFN-γ/IL-4 ratio but increased IL-4 and IL-17 genes expression in sensitized animals confirmed their sensitization and indicates the induction of an animal model of asthma in mice.
In splenocytes of sensitized animals treated with carvacrol, IFN-γ gene expression was significantly increased due to its last concentration and FOXP3 due to its last two concentrations. In addition, IL-4 gene expression due to all carvacrol concentrations, TGF-β due to its two higher concentrations and IL-17 due to its high concentration were significantly decreased compared to group S. In addition, IFN-γ/IL-4 ratio was significantly increased in the groups treated with two higher carvacrol concentrations. Therefore, carvacrol treatment showed potentiating of anti-inflammatory and reduction of pro-inflammatory cytokines genes expression in splenocytes of sensitized mice.
These results suggest a preventive therapeutic effect of carvacrol on inflammatory disorders associated with immunological dis-regulation such as asthma. In fact, a previous study showed enhancement effect of carvacrol on serum level of IFN-γ and its reduction effect on IL-4 of sensitized guinea pigs as well as increased IFN-γ/IL4 cytokine (Th1/Th2) balance (31). The results of this study support the findings of the present study. In addition, the effect of carvacrol on tracheal responsiveness (41), pathological changes (30), and inflammatory mediators (31, 41) in sensitized guinea pigs were also shown in previous studies, which also support the possible preventive therapeutic effect of carvacrol on asthma.
The effects of high carvacrol treatment on IL-4, FOXP3 genes expression, and IFN-γ/IL-4 ratio were lower than its other tow concentrations. These results may show that the maximum effect of carvacrol is occurring at its medium concentration.
The excitatory effect of carvacrol on FOXP3 gene expression in sensitized animals was also demonstrated in the present study. Peripheral blood CD4+CD25+ Tregs were found to suppress Th2 cytokine production from both atopic and non-atopic donors (42). Therefore, carvacrol may suppress Th2 cytokine production in this manner, which will help in the treatment of asthma.
Treatment of sensitized animals with carvacrol also caused significant reduction in IL-17 gene expression. The direct role of IL-17 in contributing to the severity of asthma was demonstrated in mouse studies. Mice deficient in IL-17RA or IL-17A have markedly diminished recruitment of neutrophils into the lung in response to allergens or gram-negative bacteria (43, 44). In animal models of asthma, transfer or antigen-specific IL-17 producing Th2 cells triggered influx of heterogeneous leukocytes, including neutrophils, eosinophils, macrophages, and lymphocytes, resulting in distinct pathophysiological features of severe asthma (45). IL-17 producing Th2 cell cytokines may represent the key pathogenic Th2 on promoting exacerbations of allergic asthma (46). The inflammatory response may be propagated in the airway and regulate the maintenance of Th2 and Th17 cell subsets during the chronic phase of the disease (46). Therefore, carvacrol may also have therapeutic effects on asthma by suppression of IL-17 gene expression.
The results of the present study also indicated that carvacrol decreased TGF-β gene expression in splenocytes of sensitized mice. TGF-β has an effective role in origins of airway remodeling in asthma (47) and pro-inflammatory effects on inflammatory cells (47). In addition, TGF-β is synthesized during the late phase of lung inflammation (48). It was shown that TGF-β gene expression increases in asthmatic airways after repeated exposure to low doses of cat allergen, accompanied by asthma symptoms (49). Therefore, these results showed that carvacrol may affect asthma therapy by reducing TGF-β gene expression.
Previous studies have also shown the preventive effect of carvacrol on systemic inflammation, tracheal responsiveness, and lung pathology in guinea pigs model of COPD (22, 50, 51). The anti-inflammatory effects of carvacrol in various inflammatory disorders were also shown previously (20-27). The results of the above studies may also support the findings of the present study, indicating anti-inflammatory effect of carvacrol.
The results also showed that dexamethasone treatment of sensitized splenocytes only inhibited the pro-inflammatory cytokines including IL-4 and TGF-β genes expression in splenocytes of sensitized mice, but dexamethasone had no significant effect on IFN-γ and FOXP3 genes expression. Although dexamethasone significantly increased IFN-γ/IL-4 ratio, this effect was due to suppression of pro-inflammatory rather than the effect on anti-inflammatory cytokines. However, treatment of splenocytes of sensitized mice with carvacrol affects both anti-inflammatory and pro-inflammatory cytokine genes expression. This impact totally differed from dexamethasone’s effect because dexamethasone inhibited the activities of only Th2 cell subpopulations, but carvacrol potentiated Th1 and inhibit Th2 activities. These results showed that carvacrol has more selective therapeutic effects in disorders associated with pro and anti-inflammatory cytokine imbalances such as asthma than dexamethasone. In fact, similar results were observed for carvacrol on serum IFN-γ/IL4 cytokine (Th1/Th2) ratio of sensitized guinea pigs (36), which supports the results of this study.
Conclusion
The results of this study showed that carvacrol potentiated anti-inflammatory cytokines, and IFN-γ and FOXP3 genes expression, but decreased pro-inflammatory, IL-4, TGF-β, and IL-17 genes expression in splenocytes of sensitized mice. However, dexamethasone treatment only reduced pro-inflammatory IL-4 and TGF-β genes expression. Therefore, the results may indicate the more selective therapeutic value in allergy, autoimmunity, and infectious diseases by both potentiating anti-inflammatory and suppressing pro-inflammatory cytokines.
Acknowledgment
This work was financially supported by a grant from Research Council of Mashhad University of Medical Sciences (Code: 901000), Mashhad, Iran. The manuscript is a part of a Ph.D. thesis.
Conflict of interest
The authors declare that they have no conflict of interest.
Reference
- 1.Ray A, Cohn L. Th2 cells and GATA-3 in asthma:new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 4.Boskabady MH, Mehrjardi SS, Rezaee A, Rafatpanah H, Jalali S. The impact of Zataria multiflora Boiss extract on in vitro and in vivo Th1/Th2 cytokine (IFN-gamma/IL4) balance. J Ethnopharmacol. 2013;150(3):1024–1031. doi: 10.1016/j.jep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, NY) 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 8.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–959. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thymi herba“ monographs on the medicinal uses of plant drugs. Exeter UK: European Scientific Cooperative on Phytotherapy; 1997. [Google Scholar]
- 12.Sharafkandi A t. Law in Medicine. Avesina A. 1985:187. [Google Scholar]
- 13.Gharib Naseri MK. Effect of Zataria multiflora Boiss leaf hydroalchoholic extract on rat ileum. Behbood J. 2003;7:18–26. [Google Scholar]
- 14.Gharib Naseri MK, Mazlomi H, Goshaiesh M, Vakilzadeh G, Heidari A. Antispasmodic Effect of Zataria multiflora Boiss. Leaf Extract on the Rat Uterus. IJPR. 2006;5:131–136. [Google Scholar]
- 15.Hajhashemi V, Sadraei H, Ghannadi AR, Mohseni M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J Ethnopharmacol. 2000;71:187–192. doi: 10.1016/s0378-8741(99)00209-3. [DOI] [PubMed] [Google Scholar]
- 16.Boskabady MH, Aslani MR, Kiani S. Relaxant effect of Thymus vulgaris on guinea-pig tracheal chains and its possible mechanism(s) Phytother Res. 2006;20:28–33. doi: 10.1002/ptr.1796. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, AM, Mansuri F, Amery S. Relaxant effect of Satureja hortensis on guinea pig tracheal chains and its possible mechanism(s) DARU J Pharm Sci. 2007;15:199–204. [Google Scholar]
- 18.Boskabady MH, Rakhshandeh H, Moetamed Shariati V. Bronchodilatory and anticholinergic effects of Carum copticum on iso¬lated guinea pig tracheal chains. Med J Islam Rep Iran. 1999;12:329–334. [Google Scholar]
- 19.Reiter M, Brandt W. Relaxant effects on tracheal and ileal smooth muscles of the guinea pig. Arzneimittel-Forschung. 1985;35:408–414. [PubMed] [Google Scholar]
- 20.El-Sayed EM, Mansour AM, Abdul-Hameed MS. Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J Biochem Mol Toxicol. 2015 doi: 10.1002/jbt.21740. [DOI] [PubMed] [Google Scholar]
- 21.Arigesavan K, Sudhandiran G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem Biophys Res Commun. 2015;461:314–320. doi: 10.1016/j.bbrc.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Boskabady MH, Gholami Mahtaj L. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement Altern Med. 2015;15:39. doi: 10.1186/s12906-015-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezhumalai M, Ashokkumar N, Pugalendi KV. Combination of carvacrol and rosiglitazone ameliorates high fat diet induced changes in lipids and inflammatory markers in C57BL/6J mice. Biochimie. 2015;110:129–136. doi: 10.1016/j.biochi.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Kara M, Uslu S, Demirci F, Temel HE, Baydemir C. Supplemental carvacrol can reduce the severity of inflammation by influencing the production of mediators of inflammation. Inflammation. 2015;38:1020–1027. doi: 10.1007/s10753-014-0066-0. [DOI] [PubMed] [Google Scholar]
- 25.El-Sayed EM, Abd-Allah AR, Mansour AM, El-Arabey AA. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J Biochem Mol Toxicol. 2015;29:165–172. doi: 10.1002/jbt.21681. [DOI] [PubMed] [Google Scholar]
- 26.Bonfim RR, Paiva-Souza IO, Moraes JP, Pereira DS, Santos CA, Santana DG, et al. Isopropoxy-carvacrol, a derivative obtained from carvacrol, reduces acute inflammation and nociception in rodents. Basic Clin Pharmacol Toxicol. 2014;115:237–243. doi: 10.1111/bcpt.12220. [DOI] [PubMed] [Google Scholar]
- 27.Suntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr. 2015;55:304–318. doi: 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- 28.Boskabady MH, Ramazani M, Tabei T. Relaxant effects of different fractions of essential oil from Carum copticum on guinea pig tracheal chains. Phytother Res. 2003;17(10):1145–1149. doi: 10.1002/ptr.1238. [DOI] [PubMed] [Google Scholar]
- 29.Boskabady MH, Jandaghi P. Relaxant effects of carvacrol on guinea pig tracheal chains and its possible mechanisms. Die Pharmazie. 2003;58:661–663. [PubMed] [Google Scholar]
- 30.Boskabady MH, Tabatabaee A, Jalali S. Potential effect of the extract of Zataria multiflora and its constituent, carvacrol, on lung pathology, total and differential WBC, IgE and eosinophil peroxidase levels in sensitized guinea pigs. J Functional Foods. 2014;11:49–61. [Google Scholar]
- 31.Jalali S, Boskabady MH, Haeri Rohani A, Eidi A. The effect of carvacrol on serum cytokines and endothelin levels of ovalbumin sensitized guinea-pigs. Iran J Basic Med Sci. 2013;16:615–619. [PMC free article] [PubMed] [Google Scholar]
- 32.Boskabady MH, Jafari Z, Pouraboli I. The effect of carvacrol on muscarinic receptors of guinea-pig tracheal chains. Phytother Res. 2011;25:530–535. doi: 10.1002/ptr.3290. [DOI] [PubMed] [Google Scholar]
- 33.Boskabady MH, Tabanfar H, Gholamnezhad Z, Sadeghnia HR. Inhibitory effect of Zataria multiflora Boiss and carvacrol on histamine (H(1)) receptors of guinea-pig tracheal chains. Fundam Clin Pharmacol. 2012;26:609–620. doi: 10.1111/j.1472-8206.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 34.Boskabady MH, Kaveh M, Eftekhar N, Nemati A. Zataria multiflora Boiss and carvacrol affect beta(2)-Adrenoceptors of guinea pig trachea. Evid Based Complement Alternat Med. 2011:857124. doi: 10.1155/2011/857124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma:selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babayigit A, Olmez D, Karaman O, Ozogul C, Yilmaz O, Kivcak B, et al. Effects of Ginkgo biloba on airway histology in a mouse model of chronic asthma. Allergy asthma proc. 2009;30:186–91. doi: 10.2500/aap.2009.30.3187. [DOI] [PubMed] [Google Scholar]
- 37.Hocaoglu AB, Karaman O, Erge DO, Erbil G, Yilmaz O, Kivcak B, et al. Effect of Hedera helix on lung histopathology in chronic asthma. Iran J Allergy Asthma Immunol. 2012;11:316–323. [PubMed] [Google Scholar]
- 38.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 39.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 41.Boskabady MH, Jalali S. Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized guinea pigs. Exp Biol Med. 2013;238:200–208. doi: 10.1177/1535370212474604. [DOI] [PubMed] [Google Scholar]
- 42.Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J Allergy clin Immunol. 2003;111:862–868. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- 43.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 45.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11:388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duvernelle C FV, Frossard N. Transforming growth factor-b and its role in asthma. Pulm Pharmacol Ther. 2003;16:181–196. doi: 10.1016/S1094-5539(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 48.Magnan A, Mege JL, Escallier JC, Brisse J, Capo C, Reynaud M, et al. Balance between alveolar macrophage IL-6 and TGF-beta in lung-transplant recipients. Marseille and Montreal Lung Transplantation Group. Am J Respir Crit Care Med. 1996;153:1431–1436. doi: 10.1164/ajrccm.153.4.8616577. [DOI] [PubMed] [Google Scholar]
- 49.Duvernelle C KO, de Blay F, Krieger P, Spirlet F, Pauli G, Frossard N. Transforming growth factor-b1 (TGF-b1) expression after inhalations of low subclinical doses of cat allergen in asthmatic patients. Am J Respir Crit Care Med. 1998;157 Abstract. [Google Scholar]
- 50.Gholami Mahtaj L, Boskabady MH, Mohamadian Roshan N. The Effect of Zataria multiflora and its constituent, carvacrol, on tracheal responsiveness and lung pathology in guinea pig model of COPD. Phytother Res. 2015;29:730–736. doi: 10.1002/ptr.5309. [DOI] [PubMed] [Google Scholar]
- 51.Mahtaj LG, Feizpour A, Kianmehr M, Soukhtanloo M, Boskabady MH. The effect of carvacrol on systemic inflammation in guinea pigs model of COPD induced by cigarette smoke exposure. Pharmacol Rep. 2015;67:140–145. doi: 10.1016/j.pharep.2014.08.017. [DOI] [PubMed] [Google Scholar]


