Abstract
Objective(s):
In the current study antioxidant capacities of five different extracts of Artemisia ciniformis aerial parts were evaluated by cell-free methods. Then seven fractions of the potent extract were selected and their antioxidant capacity was assayed by cell free and cell based methods.
Materials and Methods:
Antioxidant ability was measured using the: 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging test, β-carotene bleaching (BCB) method and ferrous ion chelating (FIC) assay. Total phenolic contents (TPC) of all the samples also were determined. The cytoprotective effect of fractions was evaluated by measuring the viability of cells after exposure to doxorubicin (DOX). The mechanism of action was studied by investigating caspase-3, mitochondrial membrane potential (MMP), the level of super-oxide dismutase (SOD) and intracellular reactive oxygen species (ROS).
Results:
Hydroethanolic extract exhibited a notably higher antioxidant activity and phenolic content. Among the fractions (A to G) of hydroethanolic extract, the highest antioxidant capacity was observed in the Fraction E. Moreover, 24 hr pretreatment of PC12 cells with fractions B, C and D decreased DOX-induced cytotoxicity. In addition, pre-treatment of cells with fraction B resulted in significant decrease in generation of the reactive oxygen species (ROS) and increase in the activity of SOD. We were able to demonstrate remarkable reduction in the activity of caspase-3 and increase in MMP in PC12 cells following pretreatment with fraction B.
Conclusion:
Our observations indicated that the fraction B of A. ciniformis hydroetanolic extract possessed protective effect on oxidative stress and apoptosis induced by DOX in PC12 cells.
Keywords: Apoptosis, Artemisia ciniformis, Oxidative stress, PC12 cell line
Introduction
Reactive oxygen species (ROS) are considered as harmful products of normal aerobic metabolism of living organisms. The perpetual existence of these chemically reactive oxygen-containing molecules is believed to be in balance with natural antioxidant defense mechanisms. Whenever antioxidants and pro-oxidants can no longer maintain the defense stability, the situation is shifted toward the ROS generation and creation of oxidative stress with various implications on human health (1-4).
Recently, evidences to support plant polyphenols contribution in several important anti mutagenic (5), neuroprotective (6), anti-inflammatory and anticancer (7, 8) activities and hence the interest in plant-derived polyphenols as natural antioxidants have increased noticeably (9). Phenolic compounds commonly found in plants have shown profound antioxidant capacities (9-12). Other types of natural antioxidants such as nitrogen containing compounds (13, 14), terpenoids (15, 16) and polysaccharide fractions (17-19) exert their antioxidant activitywith rather different mechanisms including prevention of lipid peroxidation, inhibition of protease and RNase activity, scavenging of free radicals, reducing power, and metal chelating ability.
The genus Artemisia, Compositae (Asteraceae) is described as small herbs and shrubs belonging to the Anthemideae tribe (20). One of the species that growing widely in Iran is Artemisia ciniformis Krasch. & Popov ex Poljakov with the Persian names of Dermaneye talaaie” and “Dermaneye sakhreroo” (21, 22). Investigations on volatiles from aerial parts of A. ciniformis have resulted in the identification of some mono- and sesquiterpenoids (23, 24). Research on A. ciniformis extracts has revealed outstanding cytotoxicity of petroleum ether and dichloromethane extracts against a wide range of cancer cell lines (25-27). Moreover, in vitro leishmanicidal activity of ethanolic extract (28) and antimalarial activity of dichloromethane extract of the species have been demonstrated (29).
Doxorubicin (DOX) is one of the most effective anti-cancer drugs used for the treatment of various malignancies (30). Like any other anticancer agent, DOX is associated with numerous undesirable side effects on some organs such as the brain manifesting as decrease in hippocampal neurogenesis and volume (31). The mechanism by which DOX induces neuronal injury remains a matter of controversy. However some reports suggest that ROS may be involved in the neurotoxicity induced by DOX. Park et al showed that DOX generates free radicals in cultured astrocytes and induces cytotoxicity dose-dependently (32). Similarly, the findings from our previous study indicated that ROS plays an important role in DOX-induced neurotoxicity (33).
Recently, we reported the protective effect of different extracts of A. ciniformis on H2O2-induced cytotoxicity in cardiomyoblast H9c2 cells (34). The present study investigated the antioxidant potential and the total phenolic contents of different extracts and fractions of A. ciniformis. Furthermore, the protective effect of the resulting fractions from selected extract on doxorubicin-induced oxidative stress and apoptosis in PC12 cells was also determined. PC12 cells, derived from an adrenal tumor, possess neuronal cell functions. This cell line is a good in vitro model to study the neurotoxic effects of chemotherapeutic agents (35).
Materials and Methods
Reagents and chemicals
All chemicals, reagents and kits that were used in this study were purchased according to the following description: Fluorescent probe 2,7-dichlorofluorescein diacetate (DCF-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT), Triton X-100, FBS and rhodamine-123, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), β-carotene, doxorubicin and caspase-3 detection kit from Sigma, Aldrich (St Louis, MO, USA); DMEM-F12 from Gibco (Gibco, Grand Island, NY, USA); super oxide dismutase assay kit from Cayman; gallic acid, linoleic acid, sodium carbonate, ferrous chloride, dimethyl sulfoxide (DMSO), chloroform, EDTA, Tween® 40, Folin-Ciocalteu’s phenol reagent, butylatedhydroxyltoluene (BHT), LiChroprep® RP-18 (15-25 µm) from Merck; ascorbic acid from VWR; ferrozine iron reagent from Acros; organics and all the solvents used for extraction and fractionation from Scharlau (Sentmenate, Spain).
Preparation of extracts and fractions
Aerial parts of A. ciniformis Krasch. & Popov ex Poljakov were freshly collected from Tandoureh National Park, Razavi Khorasan province, Iran, during September 2010 and authenticated by Dr V Mozaffarian at Research Institute of Forest and Rangelands, Tehran, Iran. A voucher specimen (No. 12569) is kept in the herbarium, Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Air-dried aerial parts of A. ciniformis were grounded and used (160 g) to make the extracts with petroleum ether (40-60), dichloromethane, ethyl acetate, ethanol, and ethanol-water (1:1 v/v) respectively (Sequential maceration with ca. 3×1.6 lit of each solvent). The extracted solvents were then filtrated and dried using rotary evaporator at a temperature below 45 °C and under reduced pressure in order to obtain 8.52, 18.42, 0.64, 5.24 and 33.23 g of each extract. The amount of 18 g from the most promising sample in antioxidant assays (hydroethanolic extract) was subjected to a vacuum liquid chromatography (VLC) system (reversed-phase RP-18 (25-40 µm), 90 g) with H2O containing increasing amounts of MeOH (5%, 10%, 20%, 40%, 60%, 80% and 100%) to yield seven fractions (A, B, C, D, E, F and G respectively) (Table 1).
Table 1.
Antioxidant performance and total phenolic contents of the extracts/fractions from A. ciniformis
| Sample | Extraction/fractionation yield (g) | EC50(µg/ml) | TPC (mg GAE /g) | ||
|---|---|---|---|---|---|
| DPPH assay | FIC assay | BCB assay | |||
| PE | 8.52 | 7230.74 ±984.22 | 458.29 ± 40.56 | 214.88 ± 24.51 | 1.98 ± 0.22 |
| DCM | 18.42 | 361.69 ± 37.74 | 198.69 ± 41.29 | 198.55 ±17.33 | 15.80 ± 0.48 |
| EA | 0.64 | 76.63 ± 3.59 | 100.57 ± 15.05 | 45.14 ± 6.51 | 55.94 ± 0.59 |
| EtOH | 5.24 | 44.29± 1.22 | 309.70 ± 58.10 | 42.37 ± 11.75 | 76.89 ± 0.92 |
| EtOH/Wt | 33.23 | 36.91 ± 1.58 | 970.19 ± 172.22 | 9.04 ± 0.27 | 134.67 ± 0.49 |
| Fr. A | 3.81 | 734.63 ± 223.93 | 389.58 ± 26.47 | 262.40 ± 38.29 | 0.78 ± 0.14 |
| Fr. B | 3.23 | 106.47 ± 2.45 | 640.97 ± 57.38 | 89.40 ± 10.84 | 12.37 ± 0.46 |
| Fr.C | 1.11 | 54.23 ± 1.69 | 163.91 ± 8.06 | 19.75 ± 0.28 | 106.86 ± 0.98 |
| Fr. D | 1.08 | 44.44 ± 1.81 | 227.71 ± 27.54 | 9.35 ± 0.37 | 221.68 ± 3.19 |
| Fr. E | 3.23 | 23.48 ± 1.04 | 330.79 ± 52.32 | 5.60 ±0.38 | 242.13 ± 1.32 |
| Fr. F | 3.78 | 33.16 ± 0.30 | 272.80 ± 21.65 | 5.72± 0.47 | 175.47 ± 1.44 |
| Fr. G | 0.75 | 46.98 ± 0.45 | 731.01± 87.15 | 7.05 ± 0.26 | 70.14 ± 0.67 |
| BHT | --- | 4.88±0.57 | --- | 0.458±0.07 | --- |
| VitC | --- | 4.51±0.07 | --- | --- | --- |
| EDTA | --- | --- | 18.00 ± 3.02 | --- | --- |
| Quercetin | --- | --- | 87.24 ± 3.94 | --- | --- |
DPPH: 2,2-diphenyl-1-picrylhydrazyl; FIC: ferrous ion chelating; BCB: β-carotene bleaching; TPC: Total phenolic contents; GAE: gallic acid equivalent
Cell-free assays
Measurement of total phenolic contents
The total phenolic content (TPC) of extracts was determined by applying the Folin–Ciocalteu method (32) with some modification (33). To 500 µl of each sample, 2.5 ml of Folin-Ciocalteu reagent (0.2 N) and 2 ml of Na2CO3 solution (75 g/lit) were added. The optical density was measured after 2 hr standing in the dark at 760 nm against a blank. The final results were calculated based on calibration curve of gallic acid and expressed as milligrams of gallic acid equivalent (GAE) per gram of dried samples.
Measurement of DPPH radical scavenging activity
In order to measure the radical scavenging activity, Hatano et al method with slight modifications was used (36). Briefly, 1.5 ml of DPPH solution in methanol (0.2 mM) was added to the equal volume of all test samples. Mixtures were shaken vigorously and maintained in dark for 30 min and absorbance was then measured at 517 nm against a blank. We used BHT and ascorbic acid as standard references. The scavenging activity was calculated using this formula (1) in which Ac= absorbance of control and A= absorbance of a tested sample in 30 min:
I%=(A_c-A)/A_c × 100 (1)
Determination of metal chelating activity
The chelating activity of samples with ferrous ions Fe2+ was determined following the ferrous iron– ferrozine complex method (37) with some modifications. Briefly, 25 µl of FeCl2 solution (2 mM) was added to the mixtures of 1.5 ml of H2O and 2 ml of the test samples in methanol at different concentrations. The reaction initiation was by the addition of ferrozine solution (50 µl, 5 mM) to each test tube after 30 sec followed by shaking and incubation (10 min, room temperature). Absorbance of the solution was then measured at 562 nm. EDTA and quercetin were used as positive controls. The ability of the extracts and fractions to chelate ferrous ion were figured through adopting the formula (1).
Measurement of inhibition of β-carotene bleaching antioxidant potential of the samples was specified according to a slightly modified version of the β-carotene bleaching method (36). A solution of β-carotene in chloroform (5 mg/10 ml) was prepared and to 750 µl of this solution, 33 µl of linoliec acid and 225 mg of Tween 40 were added. The solvent was completely removed using a rotary evaporator. In order to obtain emulsion A, 75 ml of oxygenated distilled water was added and the mixture was then emulsified for 15 min in a sonicator. Aliquots of 3.5 ml of this emulsion were moved into a series of stopper test tubes containing 1 ml of samples dissolved in water or DMSO in different concentrations. Optical density (OD) readings were recorded at 470 nm for all samples immediately (t=0) and at the end of the assay time (t=120). A second emulsion consisting of 50 ml of oxygenated water, 22 µl of linoleic acid and 150 mg of Tween 40 was also supplied and used as the blank to zero the spectrophotometer. The percentage inhibition was calculated according to the formula (2) where AA(120) is the absorbance of the sample at 120 min, AC(120) is the absorbance of the control at 120 min, and AC(0) is the absorbance of the control at 0 min:
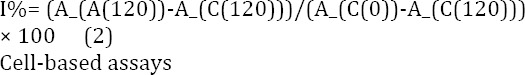
Cell culture and treatments
Rat pheochromocytoma-derived cell line, PC-12, was obtained from Pasteur Institute of Iran (Tehran, Iran). The PC-12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM-F12) with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin at 37 °C, 5% CO2 in a humidified incubator. Stock solutions of DOX and fractions of A. ciniformis hydroethanolic extract were prepared in DMSO. Non-cytotoxic concentrations of fractions of A. ciniformis hydroethanolic extract and IC50 concen-tration of DOX were established based on preliminary tests for cell viability using the methyl thiazol tetrazolium bromide (MTT) assay. A phase contrast inverted microscope (Motic, China) at 40 x magnifications was applied for observation of morphological changes of the cells.
Measurement of intracellular ROS
Intracellular ROS was monitored by using 2, 7-dichlorofluorescein diacetate (DCF-DA) as a non-fluorescent lipophilic ester which once crosses cell membrane, is oxidized to 2,7-dichlorofluorescein (DCF) fluorescent probeby unspecific esterases (38). Pretreated cells with selected fraction were treated with DOX for an additional 24 hr then washed with PBS and incubated with DCF-DA (20 μl) at 37 °C for 30 min. The fluorescence was measured with standard Argon laser for 480-nm excitation and 530-nm band pass (FL1) filter by flow cytometery (PartecTM cytometer, Germany).
Determination of superoxide dismutase (SOD) activity
Superoxide dismutases are metallo enzymes that catalyze partitioning of superoxide anion to molecular oxygen and hydrogen peroxide and hence form an essential part of the cellular antioxidant defense mechanism (39). The SOD activity was measured by commercial SOD assay kit (Cayman, USA), following the manufacturer protocol. Cayman superoxide dismutase assay kit utilizes a tetrazolium salt to detect superoxide radicals made by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme required to exhibit 50% dismutation of superoxide radicals. Values were expressed as U/mg protein.
Measurement of mitochondrial membrane potential (MMP)
Mitochondrial dysfunction has been shown to participate in the induction of apoptosis (30, 40). Accumulation of rhodamine 123 fluorescent cationic dye in mitochondria as a direct function of the membrane potential was used to measure variations of MMP. Depolarization of mitochondrial membrane during cell apoptosis results in the loss of Rh123 from mitochondria and a decrease in intracellular fluorescence intensity (41). Cells were incubated with rhodamine 123 for 30 min at 37 °C. The fluorescence was measured with standard Argon laser for 480-nm excitation and 530 nm band pass (FL1) filter by flow cytometry (PartecTM cytometer, Germany).
Determination of caspase-3 activity
The activation of caspase-3 was determined using commercial caspase-3 assay kit (Sigma, USA) following manufacture recommendations. Briefly, cells were collected and lysed (1×106 /50 μl lysis buffer) and incubated on ice for 10 min followed by 5 min centrifugation at maximum speed. The mixture of cell lysate (10 μl) and an equal amount of substrate reaction buffer, containing a caspase-3 colorimetric substrate, was then incubated for 2 hr at 37 °C. The pNA light emission was quantified using a microplate reader at 400 or 405 nm (BioTek, H1M). Caspase-3 activity was presented as percentage of control and protein content was determined by Bradford method with bovine serum albumin being used as standard.
Statistical analyses
All experiment were performed in triplicate and the results were presented as mean±SEM. One-way analysis of variance (ANOVA) followed by the Tukey test was used to compare the differences between means with the value of p<0.05 considered to be statistically significant. In the cell free assessment, the Pearson’s correlation coefficients (r) between total phenolic contents of the samples and calculated EC50 values were determined in each antioxidant assay.
Results
Cell-free assays
Total phenolic contents
The total phenolic contents (TPC) of A. ciniformis extracts and fractions calculated from the regression equation of the calibration curve (r2=0.997, y= 0.011x+0.057) are shown in Table 1. The total phenolic contents that are expressed in GAE as milligrams per gram of the extracts or fractions (mg GAE/g extract or fraction) showed large variations, between 0.78 ± 0.14 and 242.13 ± 1.32 mg GAE/g sample. The extracts contained a combination of phenolic compounds at different levels in the following order: hydroethanol> ethanol > ethyl acetate> dichloromethane> petroleum ether.
Calculated TPCs for two fractions (D and E) of the hydroethanolic extract were higher than 200 mg GAE/g fraction.
DPPH radical scavenging activity
Except for petroleum ether and dichloromethane extracts, as well as the fractions A and B, a good to moderate inhibitory activity (calculated EC50 values less than 100 µg/ml) with respect to the DPPH radical was observed. The highest activity was obtained by the fraction E followed by the fraction F and hydroethanolic extract.
Ferrous ion chelating (FIC) effect
The highest ferrous ion chelating effect among the samples was observed in ethyl acetate extract followed by the fraction C and dichloromethane extract. Regardless of the records of decreases in absorbance readings, there were not any remarkable color changes in other samples.
Inhibition of β-carotene bleaching (BCB)
Table 1 displays the inhibitory activity of A. ciniformis extracts and derived fractions on β-carotene bleaching. Fraction E showed the best inhibitory performance whereas fraction A exhibited the lowest.
Pearson’s correlation coefficients between TPC and calculated EC50s for DPPH, FIC and BCB assays showed the values of -0.392, -0.106, and -0.745, respectively. The lowest correlation was observed between TPC of samples and their capacity to chelate ferrous ions and there was no significant correlation between TPC of the samples and the relative DPPH radical scavenging activities. However, a significant correlation between the ability of the samples to inhibit the bleaching of β-carotene and their total phenolic contents was observed.
Cell based assay
Fraction B protection against cytotoxicity and oxidative stress induced by DOX
Our results showed that fractions A-D and F were all non-cytotoxic at concentration below 50 µg/ml, while the maximum safe concentrations for fractions E and G were 5 and 25 µg /ml, respectively (relative MTT activity > 80%) (Figure 3). Moreover, DOX significantly reduced PC12 cells viability as compared with control (IC50= 2.500±0.09 µM). When we used the nontoxic concentrations of fractions to evaluate their effects on induced cytotoxicity by DOX, fractions B, C and D proved to have outstandingly protective effects against DOX-induced toxicity in PC12 cells (Table 2). Next, we were interested in examining the ability of the selected fractions to protect PC12 cells from oxidative damage caused by DOX. As anticipated, adding DOX to PC12 cells induced a significant increase in the ROS level. Interestingly, only fraction B was able to reduce the ROS levels (Figure 4). Therefore, fraction B was used for subsequent experiments. Figure 5 shows that DOX significantly decreased SOD activity (61% of control level) but the presence of fraction B significantly increased SOD activity in PC12 cells.
Figure 1.
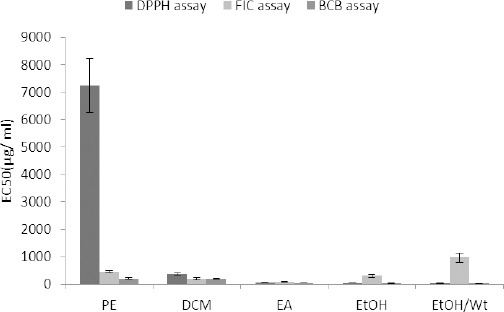
Antioxidant activity of A. ciniformis extracts from petroleum ether (PE), dichloromethane (DCM), ethyl acetate (EA), ethanol (EtOH) and ethanol/water (EtOH/Wt) DPPH: 2,2-diphenyl-1-picrylhydrazyl, FIC: ferrous ion chelating, BCB: β-carotene bleaching
Figure 2.
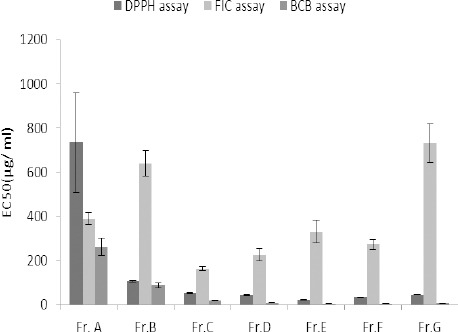
Antioxidant activity of different fractions fromhydroethanolic extract of A. ciniformis. DPPH: 2,2-diphenyl-1-picrylhydrazyl, FIC: ferrous ion chelating, BCB: β-carotene bleaching, Fr: fraction
Figure 3.
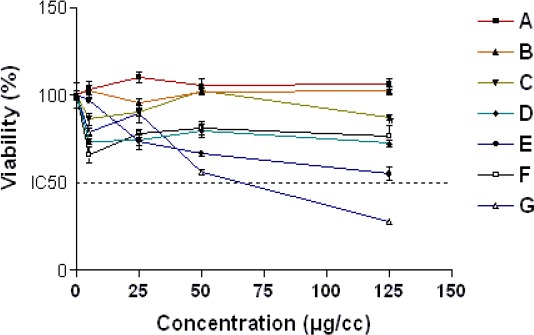
The dose–response curve of A-G fractionsof hydroethanolic extract of A. ciniformis for 24 hr exposure time. Cell viability was determined by MTT assay as described in material sand methods. Data are expressed as the mean±SEM of three separate experiments
Table 2.
Effect of fractions B, C and D of hydroethanolic extract of A. ciniformis pretreatment on DOX induced cytotoxicity
| Group | IC50±SEM (µM)* |
|---|---|
| DOX | 2.500 ± 0.09 |
| DOX+ Fraction B | >> 20 |
| DOX+ Fraction C | 9.29±1.03 |
| DOX+ Fraction D | 18.31±1.51 |
Data are expressed as the mean±SEM of three separate experiments
Figure 4.
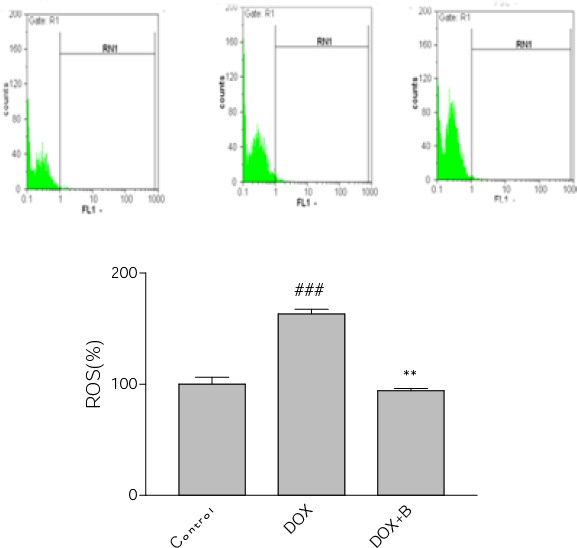
Effect of fraction B of hydroethanolic extract of A. ciniformis on DOX–induced ROS generation. Flow cytometry detection of ROS with DCF-DA. a) Representative of flow cytometry with DCF-DA plots of PC12 cells in different groups. b) Column bar graph of mean cell florescent for DCF-DA. Data are expressed as the mean±SEM of three separate experiments. ### P-value<0.0_1 vs. Control, ** P-value <0.01 vs. DOX treated cells
Figure 5.
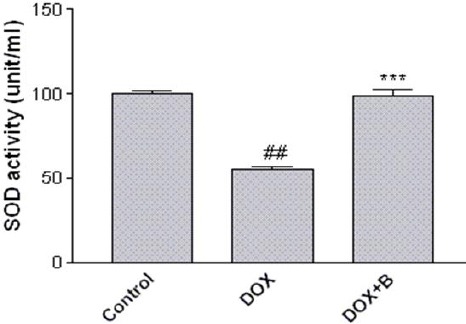
Effect of fraction B of hydroethanolic extract of A. ciniformis on the activity of SOD. Cells were pretreated with fraction B for 24 hr before exposure to 2.5 μM of DOX. The activity was measured using a colorimetric assay kit, and activity was represented as the percent inhibition of the superoxide anions. ## P-value <0.01 vs. control, *** P-value <0.001 vs. DOX treated cells
Fraction B protection against DOX-induced apoptosis
The importance of activation of caspase cascade in initiation of apoptosis in various biological systems is well known and caspase-3 has been shown to be a significant regulator of apoptosis (42). Caspase-3 activation can therefore be employed as a sensitive marker for apoptosis and for evaluating the ability of fraction B to inhibit DOX-induced apoptosis in PC12 cells. The obtained results showed that DOX was able to increase significantly caspase-3 activation in PC12 cells. Pretreatment with fraction B also decreased caspase-3 activation dramatically in comparison with DOX-treated cells (Figure 6). In addition, we examined MMP in the PC12 cell line. Flow cytometry analysis, showed a decrease of fluorescence indicating the rapid collapse of MMP when PC12 cells were exposed to DOX (2.5±0.098). Pretreatment of cells with the active fraction also promoted the inhibition of MMP reduction induced by DOX (Figure 7).
Figure 6.
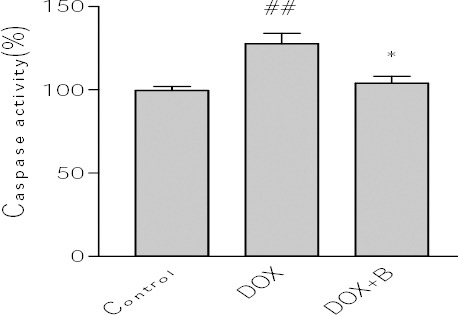
Effect of fraction B of hydroethanolic extract of A. ciniformis on caspase-3 activity. Cell pretreated with fraction 24 before exposure to 2.5 μM of DOX. Caspase-3 activity was measured by colorometric detection of p-nitroanilide and expressed as percent of control Data are expressed as the mean±SEM of three separate experiments. ## P-value <0.01 vs. Control, *** P-value <0.001 vs. DOX treated cells
Figure 7.
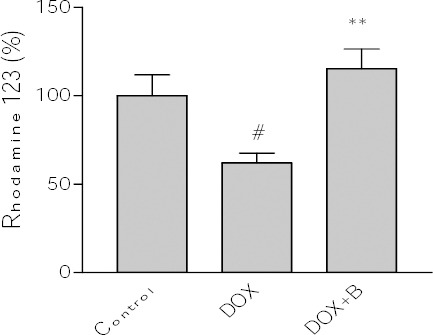
Effect of fraction B of hydroethanolic extract of A. ciniformis on DOX -induced MMP collapse. Data are expressed as the mean±SEM of three separate experiments. # P-value <0.05 vs. Control, * P-value <0.05 vs. DOX treated cells
Discussion
Various types of secondary metabolites of plants are known to have neuroprotective activity. Tomatine and tomatidine are examples of steroidal alkaloids with reported protective activity on glutamate-induced toxicity in SH-SY5Y neuroblastoma cells (43). Neuroprotective effect with different degrees against H2O2-, CoCl2- and Aβ25-35-induced cytotoxicity in SH-SY5Y cells have also been demonstrated in isolated alkaloids from the genus Lycoris (44). A terpenoid rich fraction from Hygrophila auriculata has been reported to possess neuroprotective potential against transient global cerebral ischemia induced byoxidative stress (45). Another study on Asterias rollestoni showed neuroprotective activities in a neurotoxicity model of Parkinson’s disease for a glucan and a mannoglucan sulfate with a higher antioxidant activity for the second compound (46). Moreover, there are other compounds such as organosulfur that are considered as natural antioxidants (47) or phenolic compounds which are considered as potential neuroprotective agents (48) and include a vast variety of constituents with aromatic ring(s) bearing one or more hydroxyl substituents (49). Some of them such as the novel isolated phenolic compounds from rhizomes of Gastrodiaelata (50), coumarin glycosides from the stems of Hydrangea paniculata (51) and a well-known phenyl propanoid glucoside, verbascoside, isolated from Buddleja officinalis (52) as well as an isolated flavone from Dracocephalum kotschyi (53) have shown significant protective activities in PC12 cells. However, some of the results are not in agreement with the presence of a direct and perpetual relationship between the results of cell-free antioxidant and cell-based neuroprotective assays. Jin et al (2014) showed that the DPPH-radical scavenging effect did not contribute to the neuroprotective effect of heteropolysaccharides from Sargassum naozhouense although there was a correlation between neuroprotective activity of heteropolysaccharides from
Sargassum thunbergii, S. fusiforme and S. integerrimum and their ability to scavenge the free radicals (54). In addition, isocampneoside II, a phenylethanoid glycoside isolated from the genus Paulownia, with protective effects against H2O2-induced oxidative stress and apoptosis in PC12 cells, eliminated superoxide radical by 80.75% at a concentration of 0.1 mg/ml (55) even though the superoxide-radical scavenging effect did not correlate with the neuroprotective activity of heteropolysaccharides from Sargassum species (54). The neuroprotective activity had also been reported in crude polysaccharide extracted by water from Saccharina japonica but not in its five fractions with stronger hydroxyl-radical scavenging effects and reducing power (56). In a study by Ji et al (2012) pigmented potatoes in general have been reported to contain higher levels of phenolic components and antioxidant activity, measured as their potency in scavenging DPPH radicals, despite the fact that the bioactive components involved in protection of cortical neurons from cell death caused by oxygen glucose deprivation were not dependent on pigmentation of potato clones (57). Some studies have shown a positive correlation exists between total phenolic content and antioxidant activity of the test samples (58).
In cell-free assays, the most promising samples in the FIC method contained relatively low amounts of total phenolic compounds whereas free radical scavenging activities of the samples displayed better correlation to their total phenolic contents as clarified by Pearson’s correlation coefficients (Table 1). In a study on the aerial parts of A. campestris L., the extract with the highest total phenolic content showed the least amount of chelating activity (59). Due to the complexity of the compounds found in plants, it is difficult to deduce a relationship between antioxidant activity and a specific group of secondary metabolites (60). In general, notable cell-free antioxidant activities of A. ciniformis hydroethanolic extract and some of its derived fractions in comparison with other samples could be ascribed to their higher content of phenolic compounds.
In the present study, the obtained results from the cell-based investigation suggested that DOX could decrease the cell viability in the PC12 cell line. DOX could significantly increase the intracellular ROS levels, and inhibit the SOD activity rate, which may eventually lead to PC12 cell death. Furthermore, our data confirmed that DOX-mediated cytotoxicity is mainly executed by apoptosis in this model which all together are in accordance with our previously described findings (33). When the protective effect of seven fractions was examined, we observed that pretreatment of PC12 cells with sub-toxic concentration of fractions B markedly protected the cells from DOX-induced cytotoxicity (Table 2). Notable increase in SOD activity as well as significant reduction of intracellular ROS indicated that fraction B may protect the PC12 cells from oxidative injury through preventing increased oxidative stress (Figure 4, 5). The results of MMP assay clearly showed that the selected fraction prevented DOX-induced collapse of mitochondrial membrane potential in PC12 cells (Figure 7). It is not surprising that in our experiments, pretreatment with potent fractions was also associated with the inhibition of downstream apoptosis signaling pathway and eventually decreasing the caspase-3 activity.
Conclusion
Taking together, in this study we could demonstrate that the fraction B from hydroethanolic extract of A. ciniformis attenuates the oxidative stress injury and apoptosis induced by DOX in PC12 cells. However, in our study the cytoprotective activity of the test fractions did not correlate with their antioxidant potential measured by cell free method. This might be due to the actual antioxidant activity in physiological conditions such as specific target radicals, localization in different phases, their possible interaction and differences in cellular uptake (29). In addition, it could be concluded that there should be some other types of phytochemicals but phenolics which are in charge of acting as antioxidants in cell based assay. It looks to be necessary to isolate and elucidate the structure of the components as the following step.
Acknowledgment
The authors appreciate the Research Council of Kermanshah University of Medical Sciences for the financial supports. This work was performed in partial fulfillment of the requirements for Pharm D of Sajjad Nasseri (grant number: 90159), Kermanshah University of Medical Sciences, Kermanshah, Iran.
Reference
- 1.Halliwell B. Free radicals and antioxidants:a personal view. Nut Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress:oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 3.Clark SF. The biochemistry of antioxidants revisited. Nut Clin Pract. 2002;17:5–17. doi: 10.1177/011542650201700105. [DOI] [PubMed] [Google Scholar]
- 4.Farzaeia MH, Rahimia R, Attarb F, Siavoshic M, Sanieec P, Hajimahmoodid M, et al. Chemical composition, antioxidant and antimicrobial activity of essential oil and extracts of tragopogon graminifolius, a medicinal herb from Iran. Nat Prod Commun. 2014;9:121–124. [PubMed] [Google Scholar]
- 5.Ferguson LR. Role of plant polyphenols in genomic stability. Mutat Res. 2001;475:89–111. doi: 10.1016/s0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- 6.Ghaffari H, Ghassam BJ, Chandra Nayaka S, Ramachandra Kini K, Prakash HS. Antioxidant and neuroprotective activities of Hyptissuaveolens., L. Poit. against oxidative stress-induced neurotoxicity. Cell Mul Neurobiol. 2014;34:323–331. doi: 10.1007/s10571-013-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins AR. Antioxidant intervention as a route to cancer prevention. Eur J Can. 2005;41:1923–1930. doi: 10.1016/j.ejca.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Kundu JK, Chun KS. The Promise of dried fruits in cancer chemoprevention. Asian Pac J Cancer Prev. 2014;15:3343–3352. doi: 10.7314/apjcp.2014.15.8.3343. [DOI] [PubMed] [Google Scholar]
- 9.Ignat I, Volf I, Popa VI. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 11.Velioglu Y, Mazza G, Gao L, Dave OB. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric food Chem. 1998;46:4113–4117. [Google Scholar]
- 12.Kähkönen MP, Hopia AI, Vuorela HJ. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 13.Drolet G, Dumbroff E, Legge R, Thompson JE. Radical scavenging properties of polyamines. Phytochemistry. 1986;25:367–371. [Google Scholar]
- 14.Hussain SS, Ali M, Ahmad M, Siddique GH. Polyamines:natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv. 2011;29:300–311. doi: 10.1016/j.biotechadv.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Topçu G, Ertas A, Kolak U. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. ARKIVOC. 2007;7:195–208. [Google Scholar]
- 16.Xia Q, Zhang H, Sun X, Xia Q, Zhao H, Wu L, et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 2014;19:17478–17535. doi: 10.3390/molecules191117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Chang SC, Hsu BY, Chen BH. Structural characterization of polysaccharides from Zizyphusjujuba and evaluation of antioxidant activity. Int J Biol Macromol. 2010;47:445–453. doi: 10.1016/j.ijbiomac.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Liang Z, Guo YT, Yi YJ. Ganodermalucidum polysaccharides target a Fas/caspase dependent pathway to induce apoptosis in human colon cancer cells. Asian Pac J Cancer Prev. 2014;15:3981–3986. doi: 10.7314/apjcp.2014.15.9.3981. [DOI] [PubMed] [Google Scholar]
- 20.Bora KS, Sharma A. The Genus artemisia:a comprehensive review. Pharm Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian V. A dictionary of Iranian plant names:Latin, English, Persian. Farhang Mo’aser. 1996 [Google Scholar]
- 22.Ghahreman A, Attar F. Biodiversity of plant species in Iran, Central Herbarium of Tehran University. Faculty of Science. 1999 [Google Scholar]
- 23.Rustaiyan A, Masoudi S, Kazemi M. Volatile oils constituents from different parts of Artemisia ciniformis Kraschet M. Pop. exPoljak and Artemisia incana., L. Druce. from Iran. J Essen Oil Res. 2007;19:548–551. [Google Scholar]
- 24.Firouzni A, Vahedi H, Sabbaghi F. Composition of the essential oil of Artemisia ciniformis A kopetdaghensis and A. khorasanica in Iran. Chem Nat Comp. 2008;44:804–806. [Google Scholar]
- 25.Emami A, Zamani Tahgizadeh Rabe SH, Ahi A, Mahmoudi M. Study on toxic effects of Artemisisa spp. fractions from Iran on human cancer cell lines. J Zan Univ Med Sci. 2010;18:58–67. [Google Scholar]
- 26.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm Biol. 2011;49:962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 27.Tayarani-Najaran Z, Hajian Z, Mojarrab M, Emami A. Cytotoxic and apoptotic effects of extracts of Artemisia ciniformis Krasch.&Popov ex Poljakov on K562 and HL-60 cell lines. Asian Pac J Cancer Prev. 2014;15:7055–7059. doi: 10.7314/apjcp.2014.15.17.7055. [DOI] [PubMed] [Google Scholar]
- 28.Emami SA, Zamanai Taghizadeh Rabe S, Ahi A, Mahmoudi M. Inhibitory Activity of Eleven Artemisia Species from Iran against Leishmania Major Parasites. Iran J Bas Med Sci. 2012;15:807–811. [PMC free article] [PubMed] [Google Scholar]
- 29.Mojarrab M, Naderi R Heshmati, Afshar F. Screening of different etracts from artemisia species for their potential antimalarial activity. Iran J Pharm Res. 2015;14:605–608. [PMC free article] [PubMed] [Google Scholar]
- 30.Hosseinzadeh L, Behravan J, Mosaffa F, Bahrami G, Bahrami AR, Karimi GH. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem Toxicol. 2011;49:1102–1109. doi: 10.1016/j.fct.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Lopes AM, Meisel M, Dirnagl D, Carvalho FD, Bastos MDL. “Doxorubicin induces biphasic neurotoxicity to rat cortical neurons. Neuro Toxico. 2008;29:286–293. doi: 10.1016/j.neuro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Park ES, Kim SD, Lee MH, LeeH S, Lee IS, Sung JK, et al. Protective effects of N-acetylcysteine and selenium against doxorubicin toxicity in rats. J Vet Sci. 2003;4:129–136. [PubMed] [Google Scholar]
- 33.Shokoohinia Y, Hosseinzadeh L, Moieni-Arya M, Mostafaie A, Mohammadi-Motlagh HR. Osthole attenuates doxorubicin-induced apoptosis in PC12 cells through inhibition of mitochondrial dysfunction and ROS production. Biomed Res Int. 2014;2014:156848. doi: 10.1155/2014/156848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mojarrab M, Jamshidi M, Ahmadi F, Hosseinzadeh L. Extracts of Artemisia ciniformis Protect cytotoxicity induced by hydrogen peroxide in H9c2 cardiac muscle Cells through the Inhibition of Reactive Oxygen Species. Adv Pharmacol Sci. 2013;2013:141683. doi: 10.1155/2013/141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendonc LM¸Venancio VP, Bianchi ML, Antunes LM, Machado CS. Co enzyme Q10 protects PC12 cells from cisplatin-induced DNA damage and neurotoxicity. Neurotoxicology. 2013;36:10–16. doi: 10.1016/j.neuro.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Hatano T, Edamatsu R, Hiramatsu M, Moti A, Fujita Y, Yasushara T. Effects of the interaction of tannins with co-existing substances. VI:Effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:2016–2021. [Google Scholar]
- 37.Hatami T, Emami SA, Miraghaee SS, Mojarrab M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iran J Pharm Res. 2014;13:551–558. [PMC free article] [PubMed] [Google Scholar]
- 38.Shokoohinia Y, Rashidi M, Hosseinzadeh L, Jelodarian J. Quercetin-3-O-b-D-glucopyranoside, a dietary flavonoid, protects PC12 cells from H2O2-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015;167:162–167. doi: 10.1016/j.foodchem.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 39.Malstorm B, Andreasson l, Reinhammer B. In: The enzymes. Boyer P XIIB, editor. New York: Academic Press; 1975. p. 533. [Google Scholar]
- 40.Sankari SL, Masthan K, Babu NA, Kasturiv M. Apoptosis in cancer–an update. Asian Pac J Cancer Prev. 2015;13:4873–4878. doi: 10.7314/apjcp.2012.13.10.4873. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Wang J, Lu C, Liu Y. The role of lysosomes in BDE 47-mediated activation of mitochondrial apoptotic pathway in HepG2 cells. Chemosphere. 2015;124:10–21. doi: 10.1016/j.chemosphere.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinzadeh L, Khorand A, Aliabadi A. Discovery of 2-Phenyl –N-5-(trifluoromethyl)–1,3,4-thiadiazol-2-yl) acetamid derivatives as apoptosis inducer via caspase pathway whit potential anticancer activity. Arch Pharm Chem Life Sci. 2013;346:812–818. doi: 10.1002/ardp.201300180. [DOI] [PubMed] [Google Scholar]
- 43.Taveira M, Sousa C, Valentão P, Fereress F, Teixeria JP, Andreid PB. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J Asian Nat Prod Res. 2013;16:192–199. doi: 10.1016/j.jsbmb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Wu WM, Zhu YY, Li HR, Yu HZ, Zhang P, Pi HF. Two new alkaloids from the bulbs of Lycorissprengeri. Steroid Biochem Mol Biol. 2014;140:106–115. [Google Scholar]
- 45.Kanhere R, Anjana A, Anbu J. Neuroprotective and antioxidant potential of terpenoid fraction from Hygrophila auriculata against transient global cerebral ischemia in rats. Pharm Biol. 2013;51:181–189. doi: 10.3109/13880209.2012.716851. [DOI] [PubMed] [Google Scholar]
- 46.Zhang ZC, Su G, Li J, Xcie XD. Two new neuroprotective phenolic compounds from Gastro dia-elata. J Asian Nat Prod Res. 2013;15:619–623. doi: 10.1080/10286020.2013.791286. [DOI] [PubMed] [Google Scholar]
- 47.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YC. Neuroprotective phenolics in medicinal plants. Arch Pharmacol Res. 2010;33:1611–1632. doi: 10.1007/s12272-010-1011-x. [DOI] [PubMed] [Google Scholar]
- 49.Bors W, Heller W, Michel C. Flavonoids and polyphenols:Chem Biol. Antioxidants Health Dis Series. 1996:409–468. [Google Scholar]
- 50.Zhang W, Wang J, Jin W, Zhang Q. The antioxidant activities and neuroprotective effect of polysaccharides from the starfish Asteriasrollestoni. Carbohyd Polym. 2013;5(95):9–15. doi: 10.1016/j.carbpol.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Shi J, Li CJ, Yang JZ, Yuan YH, Chen MH, Zhang DM. Coumarin glycosides and iridoidGlucosides with neuroprotective effects from hydrangea paniculata. Planta Med. 2012;78:1844–1850. doi: 10.1055/s-0032-1315394. [DOI] [PubMed] [Google Scholar]
- 52.Sheng GQ, Zhang JR, Pu XP. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur J Pharmacol. 2002;451:119–124. doi: 10.1016/s0014-2999(02)02240-9. [DOI] [PubMed] [Google Scholar]
- 53.Sarvestani NN, Khodagholi F, Ansari N, Farimini MN. Involvement of p-CREB and phase II detoxifying enzyme system in neuroprotection mediated by the flavonoid calycopterin isolated from Dracocephalumkotschyi. Phytomedicine. 2013;20:939–946. doi: 10.1016/j.phymed.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Jin W, Zhang W, Wang J, Yao J, Xie E, Liu D, et al. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int J Biol Macromol. 2014;67:336–342. doi: 10.1016/j.ijbiomac.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 55.Hu W, Wang G, Li P, Wang Y, Si CL, He J, et al. Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem Toxicol. 2013;59:145–152. doi: 10.1016/j.fct.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 56.Jin W, Zhang W, Wang J, Zhang Q. The neuroprotective activities and antioxidant activities of the polysaccharides from Saccharina japonica. Int J Biol Macromol. 2013;58:240–244. doi: 10.1016/j.ijbiomac.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Ji X, Rivers L, Zielinski MacDugal E, Jancy S, Zhang S. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2011;133:1177–1188. [Google Scholar]
- 58.Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, et al. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003;63:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 59.Djidel S, khennouf S. Radical scavenging, reducing power, lipid peroxidation inhibition and chelating properties of extracts froma artemisia campestris L. Aerial Parts. Ann Res Rev Biol. 2014;4:1691–1702. [Google Scholar]
- 60.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]


