Abstract
Purpose of review
Most individuals who develop pain following an inciting event will return to a healthy state as the injury heals. However, a small percentage continue to suffer, that is, transition to chronic pain. Chronic pain may persist for years and is accompanied by cognitive abnormalities, as well as diminished quality of life. In animals, persistent pain is characterized by peripheral and spinal cord reorganization, and recent evidence in humans also indicates cortical reorganization. Yet, despite more than 30 years of research, there is little agreement on the neural mechanisms that mediate the transition from acute to chronic pain.
Recent findings
In a longitudinal brain-imaging study, individuals who developed an intense back pain episode were followed over a 1-year period, during which pain and brain parameters were collected repeatedly. A smaller number of healthy individuals and chronic back pain patients were also studied concomitantly, as positive and negative controls. At the time of entry into the study, strength of synchrony between the medial prefrontal cortex and nucleus accumbens (i.e. functional connectivity) was predictive (>80% accuracy) of individuals who subsequently transition to chronicity 1 year later.
Summary
Properties of the brain’s emotional learning circuitry predict the transition to chronic pain. The involvement of this circuitry in pain remains mostly unexplored. Future human and animal model studies are necessary to unravel underlying mechanisms driving pain chronicity, with the potential of advancing novel therapeutics for preventing pain chronification.
Keywords: addiction, brain reorganization, functional MRI, sensitization
INTRODUCTION
Pain remains the primary reason why people seek healthcare. Every day, 50 million Americans are either partially or totally disabled by pain. If left untreated, this pain can culminate in depression, insomnia, depressed immune function, changes in eating patterns, impaired cognitive function, and other long-term deleterious effects.
When pain is the consequence of an acute injury and/or inflammatory process, it can often be alleviated through attenuation of the noxious stimulus or disease processes driving the inflammation. Most acute pain conditions are managed with analgesic medications: largely cyclo-oxygenase inhibitors, opioids or their combination. However, in modern societies chronic pain is common and, by definition, incompletely responsive to currently available therapies. The US Institute of Medicine of the National Academies recently released a comprehensive report and recommendations on chronic pain (www.iom.edu, released on 29 June 2011). They summarize: ‘chronic pain affects at least 116 million American adults – more than the total affected by heart disease, cancer, and diabetes combined. Pain also costs the nation up to $635 billion each year in medical treatment and lost productivity in the USA.’ The second major priority proposed to improve health in the nation was ‘the prevention of pain.’
There is little scientific information regarding mechanisms for the transition from acute to chronic pain. Determination of mechanisms underlying this transition provides new opportunities for the battle against chronic pain. It enables the identification of individuals who are vulnerable to developing chronic pain, thereby providing an opportunity to arrest the transition to chronicity and relieving individuals from the potential of a lifetime of suffering, as well as dramatically reducing the resulting healthcare burden. Recent human brain-imaging studies in back pain populations suggest the possibility of identifying individuals at risk for pain chronification, pioneering a new field: ‘the science of chronic pain prevention.’
CHRONIC BACK PAIN IS A MAJOR HEALTH PROBLEM
Low back pain is a public health problem affecting between 70 and 85% of adults at some point in their lives [1]. The annual prevalence of chronic low back pain ranges from 15 to 45%, with point prevalence averaging 30% [2]. In the United States, chronic and acute back pain are the most common causes of activity limitations in people under 45 years of age, and the second most frequent reason for visits to physicians [3,4]. Data from other Western countries are similar. An article by Deyo [5] states that: ‘. ․ ․ ․․ Clearly, back pain is one of society’s most significant nonlethal medical conditions. And yet the prevalence of back pain is perhaps matched in degree only by the lingering mystery accompanying it.’
CURRENT MANAGEMENT OF LOW BACK PAIN REMAINS INADEQUATE
The majority (>90%) of individuals with acute low back pain (0–7 days of back pain) recover full function within days or weeks, with little or no lingering pain. Yet a small number of individuals with acute low back pain (approximately 5% or less) go on to develop subacute back pain (SBP, 4–12 weeks of back pain), then chronic low back pain (CBP, >3–6 months of back pain) [1]. A high percentage of these patients fail to respond to treatment, continue to have debilitating degrees of pain, are significantly limited in their functional capacity, and become emotionally altered by the suffering conferred by the chronic pain state.
Treatments for persistent back pain are unreliable and typically inadequate. Placebo-controlled trials conducted to date support the use of NSAIDs and antidepressants in treating back pain, but the effectiveness is usually not sufficient to be clinically significant (>25% decrease in pain from placebo). For example, a meta-analysis of NSAID studies found no evidence that these drugs were effective in treating low back pain once it had become chronic [6] (see also [7,8]). A systematic review of antidepressant treatment for chronic back pain also concluded that these compounds produce only moderate symptom reduction [9], and a review concluded that: ‘Many drugs used for back pain are no more, or only slightly more, effective than placebos… no drug regimen can be legitimately recommended for back pain’ [10]. The WHO Advisory Panel likewise concluded that ‘there is no single treatment superior to others for relieving chronic back pain’ [11].
EXISTING BIOMARKERS OF BACK PAIN HAVE LOW PREDICTIVE POWER
To date, previously explored psychological, physiological, and genetic risk factors have failed to provide clinically meaningful predictive value in determining who will transition to pain chronicity. However, recent evidence from our laboratory has revealed that brain biomarkers may critically drive the transition to the chronic pain state.
Anatomical and genetic risk factors
The probability that the cause of back pain can be identified by radiography is less than 1% [12]. Nevertheless, the histological composition of herniated disc material seems to correlate with clinical symptoms, such as reported pain [13]. Another cause of pain and radicular symptoms seems due to pressure on the nerve from ligamentum flavum and facet joints [14]. However, the low incidence of these biomarkers suggests that they are unlikely targets for prevention-focused interventions.
Findings from two adult female twin studies indicate that 50–70% of the variation in disc degenerative processes is due to genetic factors [15,16]. A similar Danish study also showed a genetic influence, albeit with more modest results [17]. Other experiments have identified a number of candidate genes that underlie disc degeneration and pain [18]. However, these genetic factors remain uninformative in providing novel targets for therapy.
Psychosocial factors
Given that peripheral physical factors have failed to show a strong relationship with back pain, a long list of psychosocial and demographic factors have been studied. Cumulatively, however, these factors provide weak predictions regarding chronic pain. Depression is ranked as one of the strongest predictors of low back pain, and this association has been observed by multiple studies. One of the largest studies involved a national survey (n = 91 347) and 2-year follow-up survey (n = 55 690), and the findings indicated that depression and low back pain are interrelated (correlation of 0.4), with associational odds ratios (ORs) increasing with the intensity of back pain and the severity of depression [19] (see also [20]). Regarding psychosocial factors, the best predictors have been intensity and duration of pain, expectations of recovery and perception of health change, obesity, workplace risk factors [21,22], and smoking [23]. Yet attempts to create models of chronic back pain based upon psychosocial parameters have been unproductive [24–27]. In summary, there are no dominant physical or psychosocial parameters that can substantially explain chronic pain.
Direct examination of the brain in chronic pain
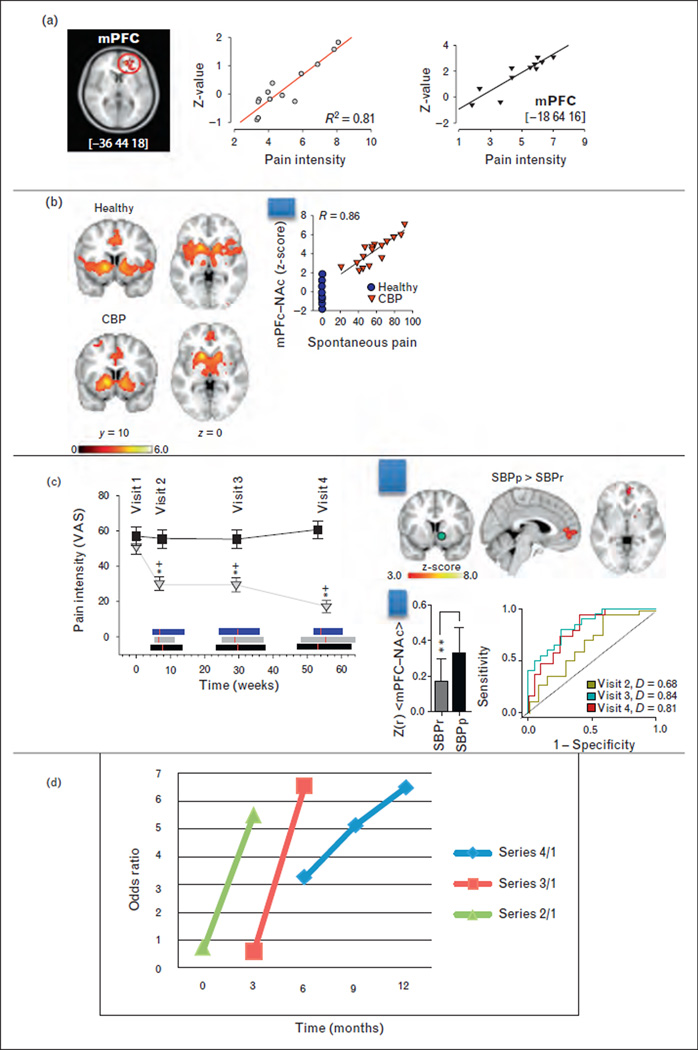
Over the last 10 years, our research group has pioneered the development of brain-imaging methods that can be specifically used to study brain function in humans with chronic pain. A large portion of this work targets the brain of patients with CBP. This group produced the first study demonstrating that cortical grey matter density decreases regionally in CBP [28]; since this work was published, over 50 studies have described similar brain morphological changes across various chronic pain conditions. It was argued that this pattern of changes in brain morphometry may be related to the shift in CBP pain perception from sensory (nociceptive) to emotional (hedonic) areas of the brain. This hypothesis was corroborated by evidence that CBP patients exhibit impaired emotional decision-making in direct proportion to the magnitude of their back pain [29], implying that the emotionally salient nature of the back pain interferes with other emotional tasks. This hypothesis was further supported by functional imaging, wherein we sought to characterize the actual pain experienced by back pain sufferers by identifying brain regions related to fluctuations of spontaneous (unprovoked) back pain. This approach yielded the novel finding that the spontaneous pain of CBP engages the medial prefrontal cortex (mPFC), a brain region that modulates emotional evaluation relative to the self (Fig. 1a) [30]. Furthermore, this work revealed a double dissociation between acute thermal pain applied to the back and spontaneous back pain representations in the brain, with the former encoded primarily in the insula and the latter in the mPFC. More recently it was shown that brain activity elicited by thermal pain is equivalent between healthy controls and CBP patients in the brain areas that are thought to encode painful stimulus information or the perception of pain; the only brain activity that differentiated these groups was localized in the bilateral nucleus accumbens (NAc). NAc activity encoded a salience signal at the onset of painful thermal stimuli, as well as an analgesia-related reward signal at stimulus offset. This analgesia-related reward signal was reversed in direction in CBP, indicating the abnormal valuation of acute pain relief. Furthermore, the strength of functional connectivity between the mPFC and NAc was proportional to the magnitude of back pain in the CPB group (Fig. 1b) [31].
FIGURE 1.
Brain circuitry predicting pain chronification. (a) Medial prefrontal cortex (mPFC) activity reflects intensity of back pain in two separate groups of patients. Adapted with permission from [30]. (b) Functional connectivity of nucleus accumbens (NAc) in healthy individuals and in chronic low back pain (CBP) patients, during a thermal pain task. In healthy individuals connectivity is mainly to insula, whereas in CBP it preferentially connects to mPFC. Scatter plot shows that mPFC–NAc connectivity strength in CBP reflects magnitude of back pain. Adapted with permission from [31]. (c) Left panel: Change in back pain over 1 year in subacute back pain (SBP) patients. SBP was subdivided to recovering (SBPr, grey triangles) and persisting (SBPp, black squares). Ordinate is mean ± SD pain intensity assessed by visual analog scale (VAS; 0–100). Horizontal bars represent the range of time from visit 1 for SBPp (black), SBPr (grey), and healthy controls (blue). Red lines are the mean. Right panel: Top: whole-brain functional connectivity contrast for NAc (green) between recovering and persistent SBP (SBPp > SBPr) identifies mPFC. Bar graph: mean and SD of mPFC–NAc connectivity strength. Receiver operator curves and probability of discrimination D are shown for predicting SBPp and SBPr groups, at three time points from initial brain scans. Note visit 2 is 3 months, and visits 3 and 4 are 6 and 12 months from initial scan. Thus, predicting future pain chronicity seems to improve at longer times from scan. (d) Odds ratio (OR) for predicting SBPp and SBPr based on functional connectivity at different time intervals. Series 2/1 is OR for prediction based on visits 1 and 2, series 3/1 is based on visits 1,2, and 3, whereas series 4/1 is based on visits 1, 2, 3, 4. In all cases, the longer the time interval the better is the prediction. Adapted with permission from [32▪▪].
PREDICTING TRANSITION TO CHRONIC PAIN
Given that brain anatomy and functional properties are distinct in chronic pain patients, it was necessary to determine the temporal causal relationship between brain reorganization and the transition to chronic pain. Therefore, a longitudinal observational brain-imaging study was undertaken [32▪▪], wherein SBP patients were tracked over a year as they transitioned to either persistent pain (SBPp; persisting to chronicity) or into recovery (SBPr) (Fig. 1c), thereby enabling comparisons of brain parameters during this time window, and in contrast to healthy and CBP patients.
Longitudinal tracking of these participants’ pain demonstrated that 50% of SBP patients continue to suffer from the same magnitude of back pain 1 year later (Fig. 1c). Thus, this is the back pain population most vulnerable to pain chronicity, and, importantly, preventing transition to chronic pain within this group would represent a large impact on the future prevalence of chronic pain in society.
We observed that grey matter density decreases only in SBPp patients, but that this is a slow process and is preceded by functional connectivity differences that are detectable by the first brain scan session. That is, as soon as the patients are scanned, the functional connectivity strength between the medial prefrontal cortex and nucleus accumbens (mPFC–NAc) at this time distinguishes between SBPp and SBPr (P < 10−3) and predicts the groupings at high accuracy (81% at 1 year from the first scans) (Fig. 1c). This is the first time that a specific brain circuitry in humans pinpoints the transition from acute to chronic back pain. The identified circuit is fully consistent with earlier human brain-imaging studies, earlier rodent studies [33–35,36▪,37], as well as recent studies in rodents in other laboratories [38–41]. This result identifies the more critical elements that lead to pain chronicity.
The receiver operator curves in Fig. 1c show that the prediction of pain chronification seems to vary with time elapsed from the measurement of subacute brain activity and the determinations of persistent and recovery groups. As the brain activity and pain measures were available at all four visits, we calculated the time dependence of prediction accuracy (Fig. 1d). The result illustrates that the longer the interval between brain activity measurement and the pain outcomes, the better the prediction. This observation is counterintuitive to the general principles of prediction from a pure information theoretical viewpoint, given that one expects prediction to be more accurate the shorter the interval between a causal measurement and related outcome. As the Nobel Prize physicist Niels Bohr stated, ‘Making a prediction is hard, especially about the future.’ This same statement is also occasionally credited to baseball legend Yogi Berra. Practically this fact was recently best illustrated by Nate Silver’s FiveThirtyEight (www.fivethirtyeight.com) forecasting the 2012 presidential elections, in which the most salient change in time regarding predictions was the continuous shrinkage of related confidence intervals with the approach of day of election. In contrast to quantum physics, batting averages, and presidential elections, the prediction for pain chronification shows an opposite pattern. Yet this is exactly expected if it is in fact predicting chronification, that is a brain reorganization that only evolves slowly in time, but is causally dependent on the initial brain response to the inciting event.
The single brain state parameter, mPFC–NAc connectivity, predicted pain chronification with an OR of around 6. However, when this parameter was combined with early versus late analgesic use (regardless of type of analgesic), the overall OR increased to about 12, and, importantly, the model revealed a protective role of medication use against pain chronification only when the early treatment variable was included in the model with the brain parameter. The treatment parameter by itself conferred no significant predictive value for estimating future pain chronification. The observation suggests that treatment outcome is contingent on the brain state. However, as the multiple regression model was a post-hoc exploratory analysis, it remains to be more rigorously tested. To generalize from this first and only longitudinal brain-imaging study to other chronic pain conditions, we presume that the properties of the brain corticolimbic circuitry play a critical role for pain chronification, in which the specifics of the underlying functional networks remain to be elucidated.
A MODEL FOR PAIN CHRONIFICATION
The above results, together with functional MRI studies in other chronic pain conditions [42–46] and additional brain morphometry studies [44,47], have prompted the proposal of a new mechanistic model for the transition to chronicity. This model was developed in three review articles [48–50]. It proposes that learning mechanisms within the limbic circuitry give rise to the transition from acute to chronic pain and render the pain more emotional (Fig. 2). The model suggests that one might characterize chronic pain as a pathologically emotional state.
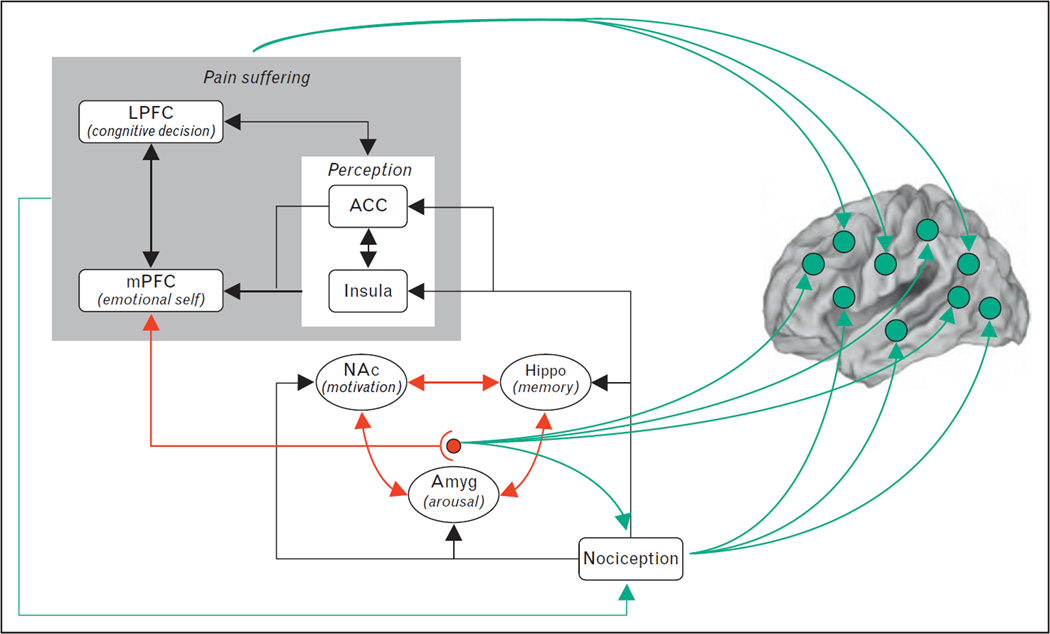
FIGURE 2.
A model regarding brain circuitry involved in the transition from acute to chronic pain. Nociceptive information, perhaps distorted by peripheral and spinal cord sensitization processes, impinges on limbic circuitry. The interaction of limbic circuitry with prefrontal processes determines the level at which a certain pain condition transitions to a more emotional state. The limbic circuitry also provides learning/modulation signals to the rest of the cortex inducing functional and anatomical distortions that reflect the suffering and coping strategies. Adapted from [50]. LPFC, lateral prefrontal cortex; mPFC, medial prefrontal cortex; ACC, anterior cingulate cortex; NAc, nucleus accumbens; Amyg, amygdala; Hippo, hippocampus.
IMPLICATIONS
The brain circuitry identified as being critical for pain chronification can be cast within the rubric of appetitive and aversive motivational learning, wherein aversive motivation involves behaviors and drives to escape hedonically unpleasant conditions, with the primary example being pain. As pain provides a teaching signal that enables individuals to avoid future actions and environments associated with harm [38,48], it is an aversive motivation and its relief gives rise to negative reinforcement. Motivational information provided by nociceptive inputs should contribute to the activity of circuitry involved in predicting the utility and costs of competing goals, as well as to behavioral decisions made in the presence of conflict [51–54]. Neural mechanisms of reward valuation and appetitive motivation engage the NAc, ventral tegmental area, and prefrontal cortex [55]. Furthermore, both dopaminergic projections from ventral tegmentum to the NAc and to the cortex, as well as glutamatergic inputs to the NAc from the amygdala, hippocampus, and prefrontal cortex, a network collectively known as the mesolimbic–prefrontal circuit [56,57], participates in appetitive behaviors instructed by conditioned cues. Accumulating evidence by our group and others now shows that this system is also intimately engaged with noxious stimuli, pain [31,40,41,58–60], and pain relief [61▪,62▪]. Moreover, we show that the responses of this system to painful stimuli are distorted in chronic pain [31] and that its connectivity predicts the development of pain chronification [32▪▪].
Dysfunction of the mesolimbic–prefrontal network is a hallmark of addiction, and the corticostriatal circuit is a subportion of this circuit. Moreover, all substances of abuse self-administered by humans that can result in addiction are believed to exert their reinforcing effects by increasing dopaminergic tone in the NAc [63]. The persistent nature of addiction is associated with activity-induced plasticity of neurons within the ventral tegmentum and NAc, with dysfunction of prefrontal activity, with long-term downregulation of dopamine receptors and dopamine production, as well as enhanced glutamatergic transmission from prefrontal regions to the NAc [64].
CONCLUSION
Given that we have identified the main components of this same circuitry in pain chronification, we assume close parallels between mechanisms leading to addiction and those leading to pain chronification. We therefore hypothesize that the transition to chronic pain is dependent on activity-induced plasticity of the corticostriatal circuit, leading to reorganization of the network such that aversive cues lead to aberrantly elevated and prolonged network activity. Within this framework, chronic pain and addictive behavior are viewed as distinct yet mechanistically related manifestations of reorganization of the brain’s motivational learning circuitry [65,66▪]. Thus, similar to addiction, chronic pain may be viewed as a brain disease state, and we presume that the sequelae of the transition to chronic pain within the corticostriatal system should exhibit strong parallels to the reorganization observed for addiction. A corollary to this hypothesis is the idea that chronic pain may be prevented either by blunting the aversive drive impinging on the corticostriatal circuit or by blocking the reorganization of the latter circuit, or by combining both approaches. As recent evidence shows that the striatum can modulate nociception by direct or indirect descending pathways [41,67], the corticostriatal circuit may also be involved in spinal cord sensitization. Given this conceptualization, clinicians and scientists face the challenge of managing, reversing, and ideally preventing the dysfunction of pain addiction.
KEY POINTS.
End-organ parameters are not adequate to explain the transition to chronic pain.
Brain function and structure reorganizes with chronic pain on local and global scales.
The brain’s emotional response to injury appears to predict who transitions to chronicity.
Acknowledgments
The authors would like to thank Howard Fields, Thomas J. Schnitzer, and Marco Martina for their contributions to the concepts in the article. Our work was funded by the National Institute of Neurological Disorders and Stroke, the National Institute of Dental and Craniofacial Research, the National Institute on Drug Abuse, and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 449).
- 1.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 2.Andersson GBJ, Frymoyer JW. The adult spine: principles and practice. Vol. 2. Philadelphia, PA: Lippincott-Raven; 1997. The epidemiology of spinal disorders; pp. 93–141. [Google Scholar]
- 3.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Praemer A, Furnes S, Rice DP. Musculoskeletal conditions in the United States. Vol. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1992. pp. 1–99. [Google Scholar]
- 5.Deyo RA. Low-back pain. Sci Am. 1998;279:48–53. doi: 10.1038/scientificamerican0898-48. [DOI] [PubMed] [Google Scholar]
- 6.van Tulder MW, Scholten RJ, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000;25:2501–2513. doi: 10.1097/00007632-200010010-00013. [DOI] [PubMed] [Google Scholar]
- 7.Coats TL, Borenstein DG, Nangia NK, Brown MT. Effects of valdecoxib in the treatment of chronic low back pain: results of a randomized, placebo-controlled trial 1. Clin Ther. 2004;26:1249–1260. doi: 10.1016/s0149-2918(04)80081-x. [DOI] [PubMed] [Google Scholar]
- 8.Pallay RM, Seger W, Adler JL, et al. Etoricoxib reduced pain and disability and improved quality of life in patients with chronic low back pain: a 3 month, randomized, controlled trial. Scand J Rheumatol. 2004;33:257–266. doi: 10.1080/03009740410005728. [DOI] [PubMed] [Google Scholar]
- 9.Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of anti-depressants in the treatment of chronic low back pain. Spine. 2003;28:2540–2545. doi: 10.1097/01.BRS.0000092372.73527.BA. [DOI] [PubMed] [Google Scholar]
- 10.Bogduk N. Pharmacological alternatives for the alleviation of back pain. Expert Opin Pharmacother. 2004;5:2091–2098. doi: 10.1517/14656566.5.10.2091. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81:671–676. [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bosch MA, Hollingworth W, Kinmonth AL, Dixon AK. Evidence against the use of lumbar spine radiography for low back pain. Clin Radiol. 2004;59:69–76. doi: 10.1016/j.crad.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Willburger RE, Ehiosun UK, Kuhnen C, et al. Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine. 2004;29:1655–1661. doi: 10.1097/01.brs.0000133645.94159.64. [DOI] [PubMed] [Google Scholar]
- 14.Okuda T, Baba I, Fujimoto Y, et al. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004;29:1689–1697. doi: 10.1097/01.brs.0000132510.25378.8c. [DOI] [PubMed] [Google Scholar]
- 15.MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51:160–167. doi: 10.1002/art.20236. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Hartvigsen J, Christensen K, Frederiksen H, Petersen HC. Genetic and environmental contributions to back pain in old age: a study of 2,108 Danish twins aged 70 and older. Spine. 2004;29:897–901. doi: 10.1097/00007632-200404150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005;17:134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- 19.Meyer T, Cooper J, Raspe H. Disabling low back pain and depressive symptoms in the community-dwelling elderly: a prospective study. Spine. 2007;32:2380–2386. doi: 10.1097/BRS.0b013e3181557955. [DOI] [PubMed] [Google Scholar]
- 20.Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
- 21.Schultz IZ, Crook J, Meloche GR, et al. Psychosocial factors predictive of occupational low back disability: towards development of a return-to-work model. Pain. 2004;107:77–85. doi: 10.1016/j.pain.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Fransen M, Woodward M, Norton R, et al. Risk factors associated with the transition from acute to chronic occupational back pain. Spine. 2002;27:92–98. doi: 10.1097/00007632-200201010-00022. [DOI] [PubMed] [Google Scholar]
- 23.Feldman DE, Rossignol M, Shrier I, Abenhaim L. Smoking. A risk factor for development of low back pain in adolescents. Spine. 1999;24:2492–2496. doi: 10.1097/00007632-199912010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 25.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2007;136:271–280. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Keeley P, Creed F, Tomenson B, et al. Psychosocial predictors of health-related quality of life and health service utilisation in people with chronic low back pain. Pain. 2007;135:142–150. doi: 10.1016/j.pain.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Clays E, De Bacquer D, Leynen F, et al. The impact of psychosocial factors on low back pain: longitudinal results from the Belstress study. Spine. 2007;32:262–268. doi: 10.1097/01.brs.0000251884.94821.c0. [DOI] [PubMed] [Google Scholar]
- 28.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. First evidence that brain functional properties predict the transition to chronic pain, based on a longitudinal evaluation of patients with subacute back pain.
- 33.Centeno MV, Mutso A, Millecamps M, Apkarian AV. Prefrontal cortex and spinal cord mediated antineuropathy and analgesia induced by sarcosine, a glycine-T1 transpoter inhibitor. Pain. 2009;145:176–183. doi: 10.1016/j.pain.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metz AE, Yau HJ, Centeno MV, et al. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millecamps M, Centeno MV, Berra HH, et al. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2007;132:108–123. doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mutso AA, Radzicki D, Baliki MN, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. Evidence that hippocampal processing is impaired with chronic pain, thereby reinforcing a role of hippocampal-dependent learning in pain chronicity.
- 37.Apkarian AV, Lavarello S, Randolf A, et al. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett. 2006;407:176–181. doi: 10.1016/j.neulet.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 39.Qu C, King T, Okun A, et al. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkis R, Saade N, Atweh S, et al. Chronic dizocilpine or apomorphine and development of neuropathy in two rat models I: behavioral effects and role of nucleus accumbens. Exp Neurol. 2011;228:19–29. doi: 10.1016/j.expneurol.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Exp Neurol. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baliki M, Katz J, Chialvo DR, Apkarian AV. Single subject pharmacological-MRI (phMRI) study: modulation of brain activity of psoriatic arthritis pain by cyclooxygenase-2 inhibitor. Mol Pain. 2005;1:32. doi: 10.1186/1744-8069-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baliki MN, Geha PY, Jabakhanji R, et al. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farmer MA, Chanda ML, Parks EL, et al. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geha PY, Baliki MN, Chialvo DR, et al. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geha PY, Baliki MN, Wang X, et al. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;38:641–656. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geha PY, Baliki MN, Harden RN, et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:s49–s64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fields HL. Proceedings of the 11th World Congress on Pain. Seattle, WA: IASP press; 2006. A motivation-decision model of pain: the role of opioids; pp. 449–459. [Google Scholar]
- 52.Glimcher PW. Decisions, uncertainty, and the brain: the science of neuroeconomics. Cambridge, MA: MIT press; 2003. [Google Scholar]
- 53.Glimcher PW, Camerer CF, Fehr E, Poldrack RA. Neuroeconomics. London, UK: Elsevier; 2009. [Google Scholar]
- 54.Rolls ET. Emotion explained. Oxford: Oxford University Press; 2005. [Google Scholar]
- 55.Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- 56.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becerra L, Breiter HC, Wise R, et al. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 59.Seymour B, O’Doherty JP, Dayan P, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- 60.Seymour B, O’Doherty JP, Koltzenburg M, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 61. Navratilova E, Xie JY, Okun A, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. First animal study to show the specific role of the NAc in pain relief.
- 62. Wanigasekera V, Lee MC, Rogers R, et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A. 2012;109:17705–17710. doi: 10.1073/pnas.1120201109. Interesting insight into the role of NAc activation with pain relief.
- 63.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 64.Volkow ND, Wang GJ, Fowler JS, et al. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hikida T, Kimura K, Wada N, et al. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 66. Hikida T, Yawata S, Yamaguchi T, et al. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci U S A. 2013;110:342–347. doi: 10.1073/pnas.1220358110. Suggests that dopamine receptor subtype 2 pathways may be specific for aversive learning.
- 67.Pertovaara A, Wei H. Dual influence of the striatum on neuropathic hypersensitivity. Pain. 2008;137:50–59. doi: 10.1016/j.pain.2007.08.009. [DOI] [PubMed] [Google Scholar]