Abstract
Introduction
Previous studies found that neuron specific enolase promoter (Nse-BMP4) transgenic mice have increased expression of the nociceptive mediator, substance P and exaggerated local injury responses associated with heterotopic ossification (HO). It is of interest great to know the pain responses in these mice and how the opioid signaling is involved in the downstream events such as mast cell (MC) activation.
Materials and methods
This study utilized a transgenic mouse model of HO in which BMP4 is expressed under the control of the Nse-BMP4. The tactile sensitivity and the cold sensitivity of the mice were measured in a classic inflammatory pain model (carrageenan solution injected into the plantar surface of the left hind paw). The MC activation and the expression profiles of different components in the opioid signaling were demonstrated through routine histology and immunohistochemistry and Western blotting, in the superficial and deep muscle injury models.
Results
We found that the pain responses in these mice were paradoxically attenuated or unchanged, and we also found increased expression of both Methionine Enkephalin (Met-Enk), and the µ-opioid receptor (MOR). Met-Enk and MOR both co-localized within activated MCs in limb tissues. Further, Nse-BMP4;MOR−/− double mutant mice showed attenuated MC activation and had a significant reduction in HO formation in response to injuries.
Conclusions
These observations suggest that opioid signaling may play a key role in MC activation and the downstream inflammatory responses associated with HO. In addition to providing insight into the role of MC activation and associated injury responses in HO, these findings suggest opioid signaling as a potential therapeutic target in HO and possibly others disorders involving MC activation.
Keywords: Mast cell (MC), Bone morphogenetic protein (BMP), Heterotopic ossification (HO), Substance P (SP), µ-Opioid receptor (MOR), Methionine enkephalin (Met-Enk)
Introduction
Heterotopic ossification (HO) is bona fide bone formation outside of the normal skeletal system. HO can either be an acquired complication of tissue damage or a rare inherited condition, such as fibrodysplasia ossificans progressiva (FOP), which is caused by gain-of-function mutations of a bone morphogenetic protein (BMP) type I receptor (ACVR1). There are currently no effective treatments for either acquired HO or for FOP. The precise mechanisms underlying HO are unknown, although numerous signaling pathways and cellular components, both local and systemic, have been implicated.
Mast cells (MCs) are immune cells that were originally known for their roles in allergy and anaphylaxis. However, MCs play multiple roles in diverse diseases, including atherosclerosis [1], ischemia–reperfusion injury [2], autoimmune disorders [3], bladder pain syndrome [4], neuropathic pain [5], autism [6], Alzheimer’s disease [7], and obesity and diabetes mellitus [8]. Massive MC infiltration is found in all stages of lesions in FOP and in HO induced by BMP signaling, especially at early stages of lesions. Furthermore, MC deficiency significantly inhibits BMP-dependent HO and the associated inflammatory responses [9, 10]. Accumulating data suggest that MC-mediated dysregulation of inflammatory responses may be a common underlying mechanism for MC involvement in many of the above-mentioned disorders [11].
Pain is a complex physiological process that is a key early warning signal to the body [12]. Numerous neurotransmitters and neuromodulators serve as nociceptive mediators within pain pathways and modulatory circuits to relay and amplify pain transmission [13, 14]. Conversely, endogenous antinociceptive factors, including opioids [15], directly target pain modulation pathways to inhibit pain signaling and increase pain thresholds [16]. In addition, numerous other factors, including abnormal neuroimmune interactions [17] or dysregulated transforming growth factor beta (TGF-β) signaling [18], disrupt the homeostasis of pain perception. The observation that neurological pain and sensory abnormalities are prevalent in patients with FOP [19] is consistent with a role for TGF-β/BMP signaling in modulating pain pathways. We therefore examined pain perception in mice that overexpress BMP4 under control of the neuron-specific enolase (Nse) promoter (Nse-BMP4), an animal model of HO [9]. Surprisingly we found that these mice have attenuated pain responses despite the substantial inflammatory response associated with formation of HO and increased levels of the nociceptive transmitter, substance P (SP) in the lesions [9]. This observation prompted examination of antinociceptive pathways in these animals. Our findings suggest that opioid signaling through the µ-opioid receptor (MOR) is involved in MC activation and the abnormal injury responses associated with HO.
Materials and methods
Animals and injury models
The Nse-BMP4 transgenic mice used in this study have been described previously [9]. MOR−/− mice were obtained from the Jackson Laboratory. All animal experiments were approved by the Animal Care and Use Committee at Northwestern University.
Superficial and deep muscle injury models
The superficial and deep muscle injury models were preformed according to previous descriptions [9]. Briefly, a deep skin incision caused disruption of skin and subcutaneous tissue as well as injury of the superficial panniculous carnosus muscle. Deep intramuscular injection of 100 µl of 10 mM cardiotoxin (Calbiochem) caused deep muscle injury.
Inflammatory pain model
100 µl carrageenan solution (10 mg/ml in physiological saline) was injected into the plantar surface of the left hind paw, and 100 µl physiological saline was injected into the right hind paw.
Tactile sensitivity
Paw withdrawal thresholds to von Frey filament stimulation were used to assess mechanical sensitivity of the hind paws. Animals were placed in a Plexiglas box with a wire grid floor and allowed to habituate for 15 min. At this point, filaments of various forces (Stoelting, USA) were applied to the plantar surface of each hind paw. Filaments were applied in a descending or ascending pattern, determined by the response of the animal. Each filament was applied for a maximum of 2 s, and paw withdrawal in response to the filament was considered a positive response. 50 % thresholds were calculated according to Chaplan et al. [20].
Cold sensitivity
To measure sensitivity to cold, a blunt needle connected to a syringe was used to drop 50 µl of acetone on the lateral hind paw. Mice were observed for 5 min and their withdrawal behavior and the duration of their withdrawal reaction (0–4 scale), was recorded according to Choi et al. [21]. Minimal and maximal cut-offs were assigned at 0.5 and 20 s, respectively. Paw withdrawals due to locomotion or weight shifting were not counted and such trials were repeated.
Histology and immunohistochemistry
Immunostaining for different markers was performed as previously described [14]. Briefly, sections were pre-fixed with 4 % paraformaldehyde in PBS. Nonspecific binding was blocked with 10 % normal serum diluted in 1 % bovine serum albumin (BSA; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and 0.25 % Triton X-100 (Sigma) for 1 h at room temperature. The sections were then incubated with primary antibodies diluted with 1 % BSA + 0.25 % Triton X-100 at 4 °C overnight. The sections were then incubated with appropriate conjugated secondary antibodies Cy3 or Cy2 (Jackson ImmunoResearch Laboratories) diluted with 1 % BSA + 0.25 % Triton X-100 or Alexa Fluor 488, Alexa Fluor 594, and Alexa 647 (1:1,000, Invitrogen) in the dark at room temperature for 2 h. Counterstaining was then performed with 4,6-diamidino-2-phenylindole (1:5,000). Primary antibodies against rabbit anti-mast cell tryptase alpha/beta-1 (TPSAB1; Abcam ab134932), rabbit anti-LAMP2 (Life-Span Biosciences), guinea pig anti-mu opioid receptor (neuromics, GP10106), rabbit anti-delta opioid receptor (neuromics, RA19072), mouse anti-kappa opioid receptor (neuromics, MO15098), rabbit anti-methionine enkephalin (neuromics, RA21006), rabbit anti-endomorphin 1 (neuromics, RA21001), and rabbit anti-endomorphin 2 (neuromics, RA10111) were used in this study.
Results
Nse-BMP4 mice have attenuated or normal pain responses
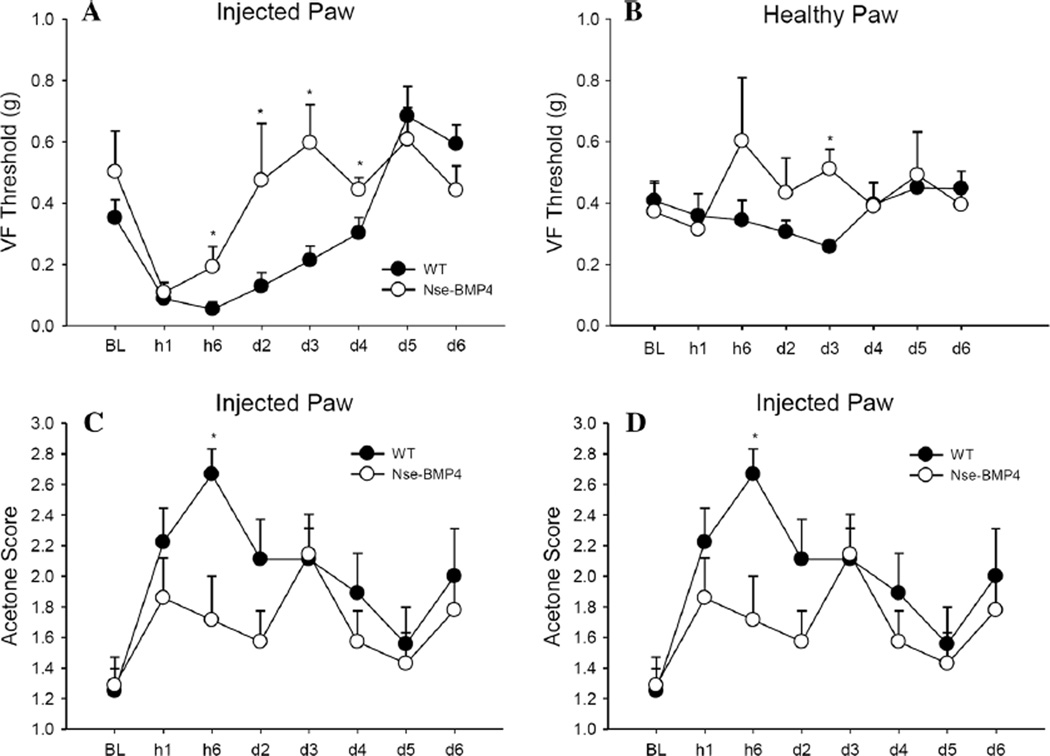
Expression of SP, a nociceptive mediator, is markedly increased in early lesional tissue in patients who have either acquired HO or FOP, and in three independent mouse models [9]. We reasoned that since SP is a potent nociceptive mediator, this up-regulation might cause exaggerated pain responses. We therefore directly assessed pain thresholds in mice in response to temperature and mechanical stimulation following carrageenan injection, a well-established acute pain model [22]. The pain thresholds of adult Nse-BMP4 were compared with wild-type (WT) control mice at different points (1 h, 6 h, and 2, 3, 4, 5, and 6 days) after the carrageenan injection. Surprisingly we found that the pain responses of Nse-BMP4 mice were attenuated or unchanged (Fig. 1a, b).
Fig. 1.
Nse-BMP4 mice have attenuated pain responses. Nse-BMP4 animals have significantly higher mechanical pain thresholds than WT mice in carrageenan-injected paws. Von Frey 50 % withdrawal thresholds (g) of Nse-BMP4 and WT littermates in the carrageenan-injected (a) and saline-injected paws (b) showed a lack of mechanical allodynia in Nse-BMP4 mice, starting at 6 h until day 4. Significant increases in Nse-BMP4 thresholds are also seen in the saline-injected paw on day 3, indicating some general hyperalgesia. Nse-BMP4 have lower acetone scores at 6 h (c), and lower reaction times at 6 h (d), compared to WT animals, indicating less cold allodynia than WT animals. Error bars are SEM, *P<0.05
Increased opioid signaling is observed in peripheral tissues but not in the central nervous system (CNS) of Nse-BMP4 mice
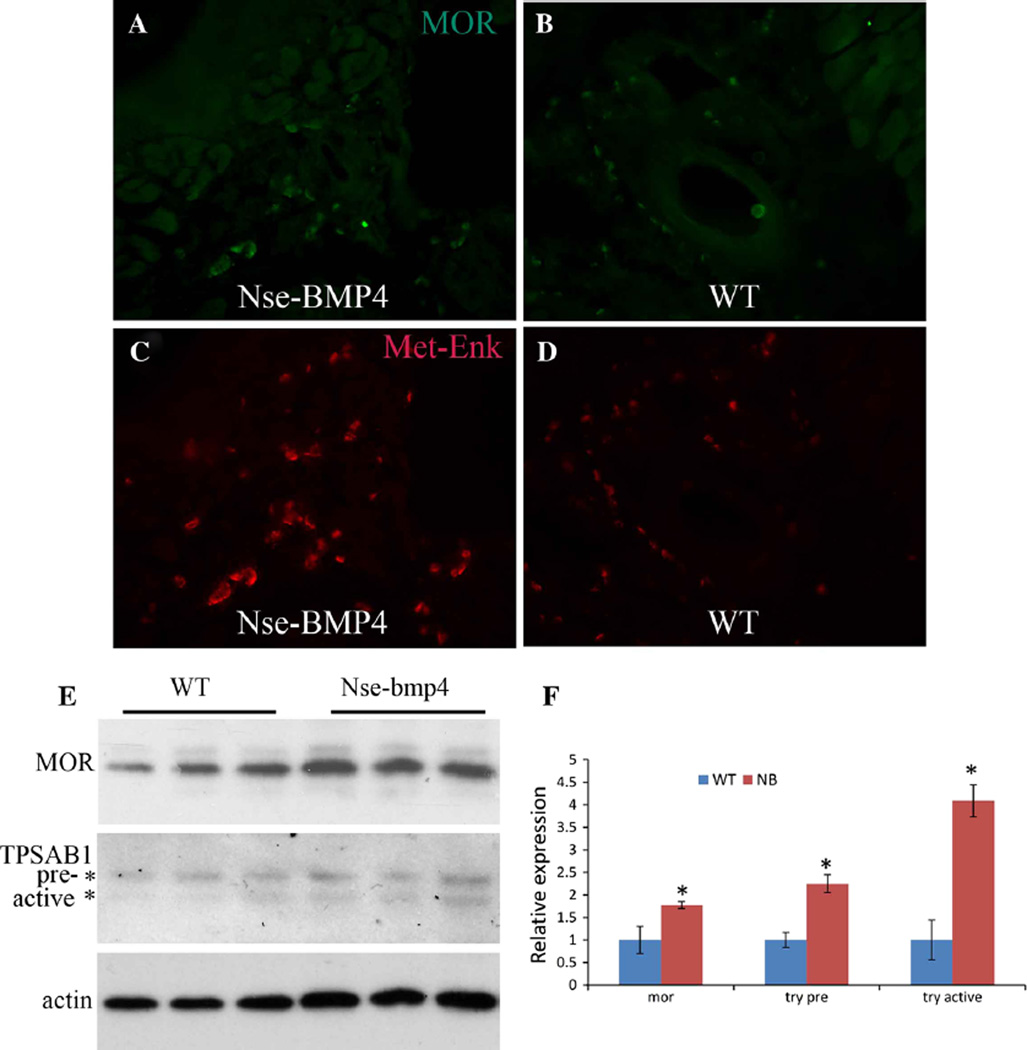
To explain why Nse-BMP4 mice might have attenuated or normal pain responses despite the increase in SP expression, we hypothesized that there might be concurrent expression of antinociceptive factors, such as endogenous opioids and/or opioid receptors. After confirming the specificity and the sensitivity of antibodies for methionine β-endophin (β-endo), methionine enkephalin (Met-Enk), endomorphin 1 (End1), endomorphin 2 (End2), MOR, δ-opioid receptor (DOR) and κ-opioid receptor (KOR) (Supplementary Figs. 1–3), we immunostained sections of brain from Nse-BMP4 and control mice with these antibodies. None of the tested factors were appreciably changed in the CNS either by immunohistochemistry or by Western blotting (Supplementary Figs. 4–7). By contrast, expression of Met-Enk and MOR were significantly increased in hind limb tissues in a transgene-dependent manner (Fig. 2). Western blotting with homogenized tissue from the hind limbs of WT and Nse-BMP4 mice further confirmed the transgene dependent increase in MOR expression. In addition, Western blotting also indicated that expression of TPSAB1), a specific MC marker, was increased. Importantly, levels of the mature active form of TPSAB1 were increased more than the precursor form, suggesting that MCs might be abnormally activated in Nse-BMP4 mice (Fig. 2e, f).
Fig. 2.
Expression of Met-Enk, MOR and TPSAB1 is increased in the limbs of Nse-BMP4 mice. Typical images of MOR staining of the sections of hind limbs from Nse-BMP4 (a) and WT mice (b). Typical images of Met-Enk staining of sections of hind limbs from Nse-BMP4 (c) and WT (d) mice. e Western blotting demonstrates increased expression of MOR (upper panel) and TPSAB1 (middle panel) in Nse-BMP4 mice. Note that the increase in the active, mature form of TPSAB1 is greater than that of the precursor. β-actin (bottom panel) was used as loading control. f Quantification of (e). Error bars are SD, *P < 0.05 vs. control
Increased Met-Enk and MOR expression occurs within activated MCs
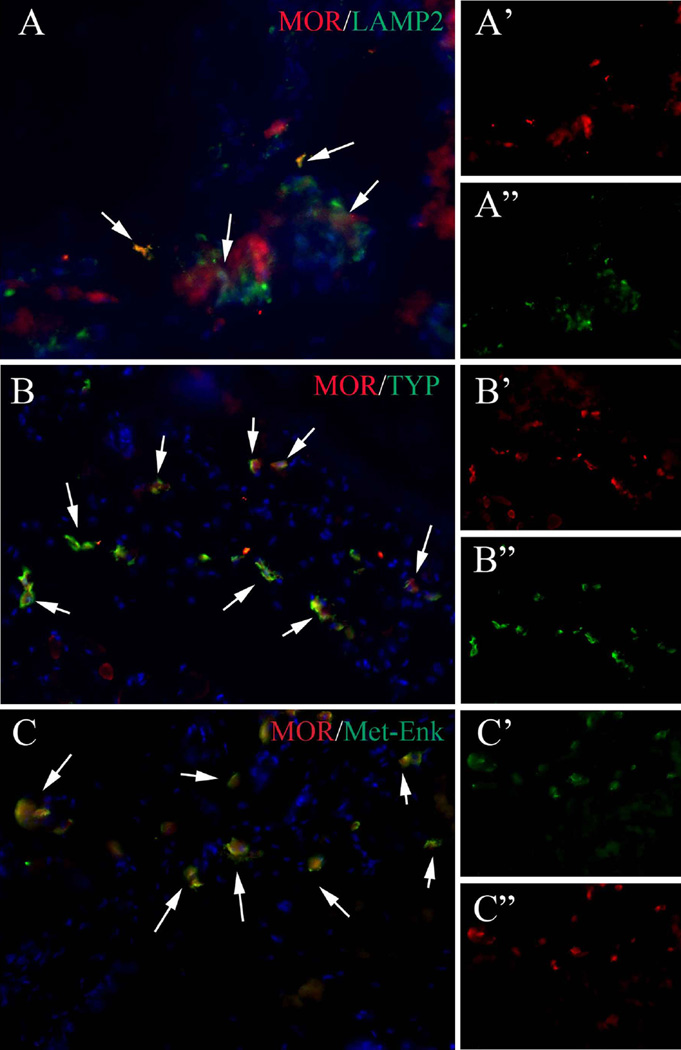
Met-Enk and MOR are expressed by cells in both the nervous and immune systems in different contexts [23, 24]. To understand the cell type(s) that expresses Met-Enk and MOR in our model, we first double stained for MOR and lysosomal-associated membrane protein 2 (Lamp2), a commonly used MC marker. MOR and Lamp2 expression overlapped extensively (Fig. 3). However, Lamp2 also labels cell populations other than MC [25], and some Lamp2+ cells were not MOR+ (Fig. 3). We therefore double stained for MOR and TPSAB1, a more specific marker for activated MC, and we found that MOR was expressed exclusively by cells that co-stained for TPSAB1 (Fig. 3). Furthermore, we found that MOR and Met-Enk co-localized to the same cells in peripheral tissues (Fig. 3) as well as in the CNS (Supplementary Fig. 8). These findings indicate that both Met-Enk and MOR were expressed by activated MCs. This suggested an intimate relationship between MC activation and opioid signaling in the limbs of our mouse model of HO.
Fig. 3.
MOR is co-localized with MC marker, Lamp2, activated MC marker, TPSAB1, and with Met-Enk. a–a″ Typical image of MOR/Lamp2 double staining. a′, a″ Split channel of MOR and Lamp2, respectively. b–b″ Typical image of MOR/TPSAB1 double staining. b′, b″. Split channel of MOR and TPSAB1, respectively. c–c″ Typical image of MOR/Met-Enk double staining. c′, c″. Split channel of MOR and Met-Enk, respectively. Note that the co-localization of MOR and Lamp2 is only partial, whereas the co-localization of MOR/TPSAB1 and MOR/Met-Enk are almost exclusive
Phenotypes of Nse-BMP4;MOR−/− double mutant mice
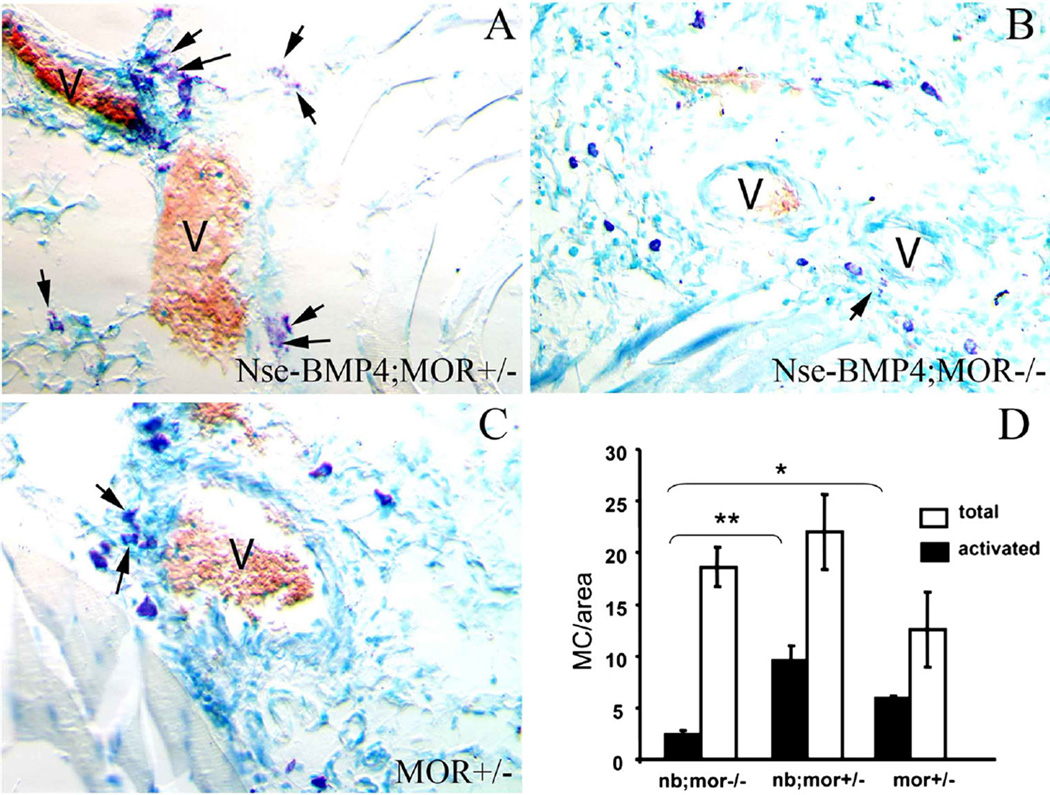
To further understand the roles of MOR and opioid signaling in this model, we crossed MOR−/− mice with Nse-BMP4 mice and then compared the phenotypes of BMP4;MOR−/− double mutant mice with control mice. The goals were to clarify the causal relationship between the abnormal opioid signaling and activation of MC, and to determine the pathological consequences of disruption of this abnormal opioid signaling. Nse-BMP4;MOR−/− double mutant mice showed attenuated MC activation and degranulation, strongly suggesting that MC activation might be MOR dependent (Fig. 4). These data are consistent with previous data about the expression of the activated MC marker, TPSAB1 (Fig. 2), and further support the tenet that opioid signaling is involved in MC activation. More importantly, Nse-BMP4;MOR−/− double mutant mice had a significant reduction in HO formation in response to injuries (Fig. 5), similar to what we observed in MC-deficient Nse-BMP4 mice [9]. This suggests that MC activation, not simply the presence of MCs, is required for the abnormal downstream events that lead to HO. Notably the pain behaviors of Nse-BMP4;MOR−/− double mutant and control mice (Nse-BMP4;MOR−/+) did not differ significantly (Fig. 6), suggesting that the attenuated pain behavior observed in Nse-BMP4 mice is not dependent solely on MOR expression.
Fig. 4.
MC activation/degranulation is MOR dependent. Typical images of toluidine blue staining of sections from injured hind limbs from Nse-BMP4;MOR+/− (a), Nse-BMP4;MOR−/− (b) and MOR+/− mice (c), show the number, location, size and the characteristic morphology of the metachromatic stained (red–purple) MCs. Light blue is the orthochromatic staining of the background, and red blood cells are brown. Note that the MCs in different groups are clearly different in both size and morphology. MCs in Nse-BMP4;MOR−/− mice are much smaller (immature) than in other groups. Also note that degranulation of MCs in Nse-BMP4;MOR−/− mice is less obvious than in Nse-BMP4;MOR+/− mice, which have the highest number (or percentage) of activated MCs. d Quantification of total numbers and the activated/degranulated MCs in different groups. Error bars are SD, *P < 0.05, **P < 0.01 vs. control
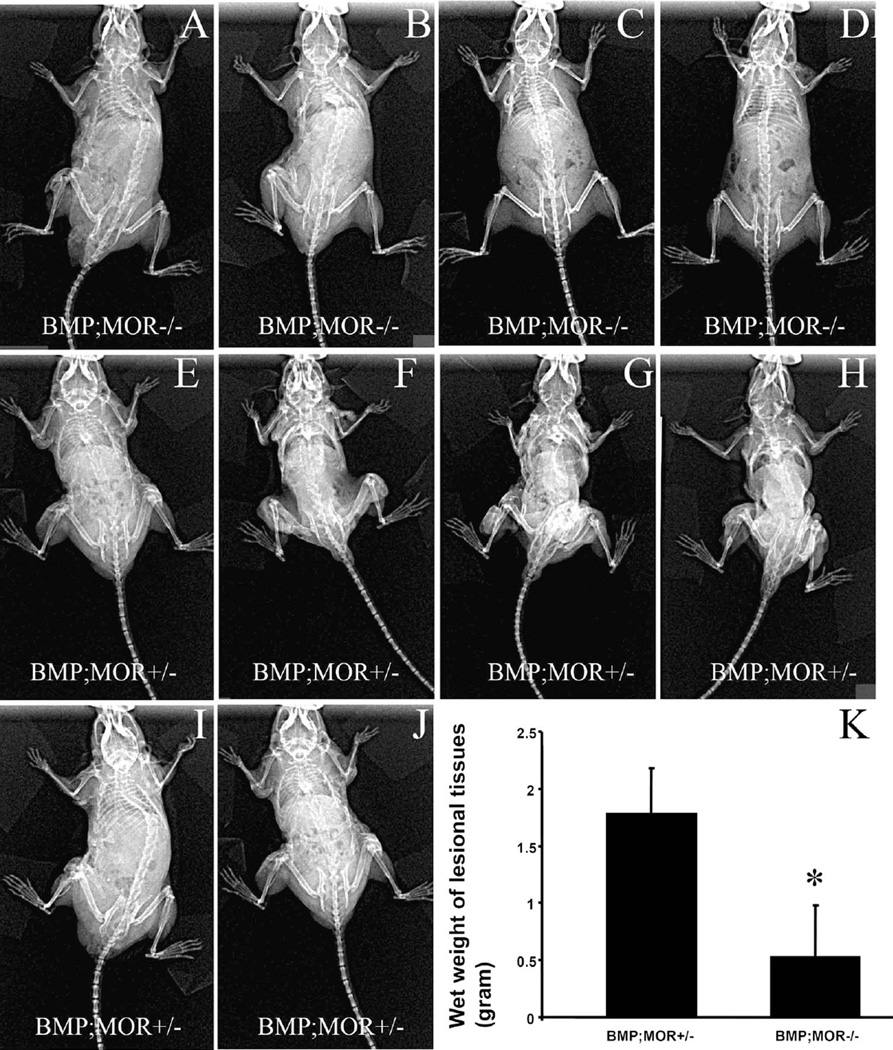
Fig. 5.
Nse-BMP4;MOR−/− double mutant mice form significantly less HO. Whole body X-ray imaging reveals a dramatic difference in the efficacy of HO formation between Nse-BMP4;MOR+/− (a–d) and Nse-BMP4;MOR−/− mice (e–j) after similar injuries. k Quantification of the wet weight of lesional tissues of (a–j). *P < 0.05 vs. control
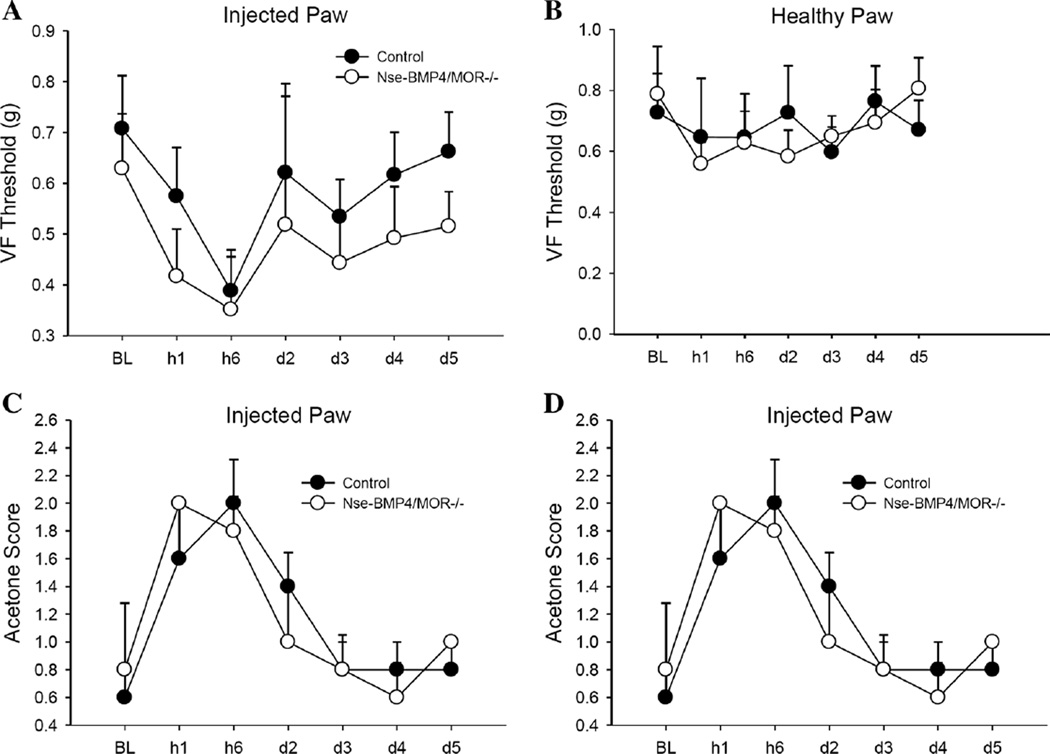
Fig. 6.
Pain behaviors of Nse-BMP4;MOR−/− and control mice do not differ. Nse-BMP4;MOR−/− animals do not have significantly different mechanical pain thresholds than littermate controls in carrageenan-injected or control paws. Von Frey 50 % withdrawal thresholds (g) of Nse-BMP4;MOR−/− mice and littermate Nse-BMP4 controls in the carrageenan-injected (a) and saline-injected paws (b) are shown. Cold allodynia score (c) and reaction times (d) are also not significantly different
Discussion
This study focused on MC activation in the context of BMP-dependent HO. Our findings not only support the idea that opioid signaling and inflammation are closely associated processes, but also suggest that MCs may be a key point of convergence for these two processes.
MCs can be activated by immunological and nonimmunological pathways. The traditional, immunological view of MC activation is that antigen-dependent MC activation is initiated by antigen-specific IgE binding to IgE receptors (FcεRI) on MCs, and that the aggregation of FcεRI then leads to an intracellular signaling cascade that culminates in release of mediators [26]. Even though a direct, nonimmunological activation of MCs through pertussis toxin-sensitive heterotrimeric G-proteins has also been proposed [27]. Further studies found that these two pathways are not mutually exclusive. Accumulating evidence indicates that the classic immunological pathway can be significantly modified by many different signaling pathways, including G protein-coupled receptors (GPCRs) [26], which mediate signaling by SP, opioids and many other ligands. The importance of some of the GPCR ligands, such as SP, in modulating MC activation has been well recognized, but for others GPCR ligands, such as opioids, the importance and the underlying mechanisms are still controversial. For example, even though a previous study demonstrated that codeine (a natural isomer of methylated morphine) and meperidine (a synthetic opioid) can specifically induce MC activation [28], the authors argued that MOR may not be directly involved.
Similar to the potential role of MOR in MC activation, the peripheral opioid system has also been implicated in injury and wound healing, although the evidence is controversial. For example, Poonawala et al. [29] observed that topically applied opioids speed up wound closure and angiogenesis. Furthermore, opioid-induced wound healing was inhibited by an opioid receptor antagonist. In contrast, Rook et al. [30] reported that topical opioids (morphine) impair wound closure via inhibition of SP and neurokinin A (NKA) release peripherally into the healing wound. Nevertheless, deletion of a functional NK-1 receptor has little effect on wound healing in response to a simple cut in mouse skin [31].
Our current study found that expression of both Met-Enk and MOR were consistently increased in tissues of Nse-BMP4 mice, and that both co-localized within activated MCs. Since a previous report suggested that there may be nonspecific binding of primary antibodies against regulatory peptides in MC staining [32], we rigorously confirmed the specificity and the sensitivity of our primary antibodies (Supplementary Figs. 1–3) to exclude the possibility of non-specific staining. More importantly, we found that null mutation of the MOR gene attenuated MC activation/degranulation, confirming that MC activation is at least partially MOR dependent. Furthermore, Nse-BMP4;MOR−/− double mutant mice had a significant reduction in formation of HO, consistent with what we found in MC-deficient Nse-BMP4 (Nse-BMP4;c-kitw-sh/w-sh double mutant) mice [9]. It is unclear why the pain behavior of Nse-BMP4;MOR−/− double mutant and Nse-BMP4 mice did not differ. It is possible that another antinociceptive signaling pathway, such as cannabinoid receptor signaling, may be involved in modulating pain behavior in Nse-BMP4 mice [33]. Alternatively the increased expression of SP up-regulation in the mice could be coupled with an unconventional antinociceptive, rather than nociceptive, signaling pathway, analogous to the report by Lin et al. [34] that NK1 receptors on muscle afferents can inhibit activity of the acid-sensing ion channel 3. Although we found no changes in opioid pathways in the CNS of Nse-BMP4 mice, Mutso et al. suggested that abnormal hippocampal neurogenesis in these mice might contribute to the observed pain phenotypes (personal communications). Regardless of the mechanisms regulating the pain responses of these mice, our findings indicate that endogenous opioid signaling can modulate MC activation and the downstream events such as HO. In turn this suggests opioid signaling as a potential therapeutic target in HO and possibly others disorders involving MC activation.
Supplementary Material
Acknowledgments
We appreciate the help from many members of the Kessler Laboratory. LK was supported in part by grants from The Center for Research in FOP and Related Disorders of the University of Pennsylvania School of Medicine, and by NIH grant NS 20013 to JAK, and NS057704 to AVA.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s00011-013-0690-4) contains supplementary material, which is available to authorized users.
Contributor Information
Lixin Kan, Email: l-kan@northwestern.edu, Department of Neurology, Northwestern University, Ward Building 10-233, 303 East Chicago Avenue, Chicago, IL 60611-3008, USA.
Amelia A. Mutso, Departments of Physiology, Northwestern University, Ward Building 10-233, 303 East Chicago Avenue, Chicago, IL 60611-3008, USA
Tammy L. McGuire, Department of Neurology, Northwestern University, Ward Building 10-233, 303 East Chicago Avenue, Chicago, IL 60611-3008, USA
Apkar Vania Apkarian, Departments of Physiology, Northwestern University, Ward Building 10-233, 303 East Chicago Avenue, Chicago, IL 60611-3008, USA.
John A. Kessler, Department of Neurology, Northwestern University, Ward Building 10-233, 303 East Chicago Avenue, Chicago, IL 60611-3008, USA
References
- 1.Ramalho LS, Oliveira LF, Cavellani CL, Ferraz ML, de Oliveira FA, Miranda Correa RR, et al. Role of mast cell chymase and tryptase in the progression of atherosclerosis: study in 44 autopsied cases. Ann Diagn Pathol. 2013;17(1):28–31. doi: 10.1016/j.anndiagpath.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Huang P, Liu D, Gan X, Zhang R, Gao W, Xia Z, et al. Mast cells activation contribute to small intestinal ischemia reperfusion induced acute lung injury in rats. Injury. 2012;43(8):1250–1256. doi: 10.1016/j.injury.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Costanza M, Colombo MP, Pedotti R. Mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci. 2012;13(11):15107–15125. doi: 10.3390/ijms131115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv J, Huang Y, Zhu S, Yang G, Zhang Y, Leng J, et al. MCP-1-induced histamine release from mast cells is associated with development of interstitial cystitis/bladder pain syndrome in rat models. Mediators Inflamm. 2012;2012:358184. doi: 10.1155/2012/358184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51(2):240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, et al. Mast cell activation and autism. Biochim Biophys Acta. 2012;1822(1):34–41. doi: 10.1016/j.bbadis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Niemi K, Baumann MH, Kovanen PT, Eklund KK. Serum amyloid A (SAA) activates human mast cells which leads into degradation of SAA and generation of an amyloidogenic SAA fragment. Biochim Biophys Acta. 2006;1762(4):424–430. doi: 10.1016/j.bbadis.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Anand P, Singh B, Jaggi AS, Singh N. Mast cells: an expanding pathophysiological role from allergy to other disorders. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(7):657–670. doi: 10.1007/s00210-012-0757-8. [DOI] [PubMed] [Google Scholar]
- 9.Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112(10):2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001;32(8):842–848. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Sharma A. Mast cells: emerging sentinel innate immune cells with diverse role in immunity. Mol Immunol. 2010;48(1–3):14–25. doi: 10.1016/j.molimm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207(1):19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 14.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 15.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):1379–1387. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tramullas M, Lantero A, Diaz A, Morchon N, Merino D, Villar A, et al. BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-beta family in pain modulation. J Neurosci. 2010;30(4):1502–1511. doi: 10.1523/JNEUROSCI.2584-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitterman JA, Strober JB, Kan L, Rocke DM, Cali A, Peeper J, et al. Neurological symptoms in individuals with fibrodysplasia ossificans progressiva. J Neurol. 2012;259(12):2636–2643. doi: 10.1007/s00415-012-6562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 22.Rocha AC, Fernandes ES, Quintao NL, Campos MM, Calixto JB. Relevance of tumour necrosis factor-alpha for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br J Pharmacol. 2006;148(5):688–695. doi: 10.1038/sj.bjp.0706775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langsdorf EF, Mao X, Chang SL. A role for reactive oxygen species in endotoxin-induced elevation of MOR expression in the nervous and immune systems. J Neuroimmunol. 2011;236(1–2):57–64. doi: 10.1016/j.jneuroim.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assis MA, Collino C, Figuerola Mde L, Sotomayor C, Cancela LM. Amphetamine triggers an increase in met-enkephalin simultaneously in brain areas and immune cells. J Neuroimmunol. 2006;178(1–2):62–75. doi: 10.1016/j.jneuroim.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Xu J, Pang S, Bai B, Yan B. Age-related decrease of the LAMP-2 gene expression in human leukocytes. Clin Biochem. 2012;45(15):1229–1232. doi: 10.1016/j.clinbiochem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113(2):59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klinker JF, Seifert R. Morphine and muscle relaxants are receptor-independent G-protein activators and cromolyn is an inhibitor of stimulated G-protein activity. Inflamm Res. 1997;46(2):46–50. doi: 10.1007/s000110050058. [DOI] [PubMed] [Google Scholar]
- 28.Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg. 2004;98(2):364–370. doi: 10.1213/01.ANE.0000097168.32472.0D. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Poonawala T, Levay-Young BK, Hebbel RP, Gupta K. Opioids heal ischemic wounds in the rat. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair. Wound Repair Regen. 2005;13(2):165–174. doi: 10.1111/j.1067-1927.2005.130207.x. [DOI] [PubMed] [Google Scholar]
- 30.Rook JM, McCarson KE. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol. 2007;74(5):752–757. doi: 10.1016/j.bcp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao T, Grant AD, Gerard NP, Brain SD. Lack of a significant effect of deletion of the tachykinin neurokinin-1 receptor on wound healing in mouse skin. Neuroscience. 2001;108(4):695–700. doi: 10.1016/s0306-4522(01)00435-3. [DOI] [PubMed] [Google Scholar]
- 32.Ruck P, Horny HP, Kaiserling E. Immunoreactivity of human tissue mast cells: nonspecific binding of primary antibodies against regulatory peptides by ionic linkage. J Histochem Cytochem. 1990;38(6):859–867. doi: 10.1177/38.6.2335741. [DOI] [PubMed] [Google Scholar]
- 33.Liu YQ, Qiu F, Qiu CY, Cai Q, Zou P, Wu H, et al. Cannabinoids inhibit acid-sensing ion channel currents in rat dorsal root ganglion neurons. PLOS One. 2012;7(9):e45531. doi: 10.1371/journal.pone.0045531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CC, Chen WN, Chen CJ, Lin YW, Zimmer A, Chen CC. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci USA. 2012;109(2):E76–E83. doi: 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.