Abstract
Objectives:
The purpose of this study was to investigate the antifungal effect of bee venom (BV) and sweet bee venom (SBV) against Candida albicans (C. albicans) clinical isolates.
Methods:
In this study, BV and SBV were examined for antifungal activities against the Korean Collection for Type Cultures (KCTC) strain and 10 clinical isolates of C. albicans. The disk diffusion method was used to measure the antifungal activity and minimum inhibitory concentration (MIC) assays were performed by using a broth microdilution method. Also, a killing curve assay was conducted to investigate the kinetics of the anti- fungal action.
Results:
BV and SBV showed antifungal activity against 10 clinical isolates of C. albicans that were cultured from blood and the vagina by using disk diffusion method. The MIC values obtained for clinical isolates by using the broth microdilution method varied from 62.5 μg/ mL to 125 μg/mL for BV and from 15.63 μg/mL to 62.5 μg/mL for SBV. In the killing-curve assay, SBV behaved as amphotericin B, which was used as positive control, did. The antifungal efficacy of SBV was much higher than that of BV.
Conclusion:
BV and SBV showed antifungal activity against C. albicans clinical strains that were isolated from blood and the vagina. Especially, SBV might be a candidate for a new antifungal agent against C. albicans clinical isolates.
Keywords: anti-fungal activity, bee venom, clinical Candida albicans, sweet bee venom
1. Introduction
Yeasts are microorganisms commonly found in nature. They are present in the normal flora (in moist places like the intestinal system, mouth etc.) in the human body. The dimorphic yeast Candida albicans (C. albicans) is the most prevalent opportunistic pathogen in humans. Candidiasis ranges from superficial and mucosal infections to life-threatening septicaemic mycosis in debilitated patients [1-3], and this fungus is known to be the fourth leading cause of nosocomial infections [4, 5]. Over the last few decades, the incidence of candidal infections due to C. albicans has been increasing, paralleling the growing numbers of immune-compromised patients [4-7]. To the patients, disseminated candidiasis is sometimes a serious disease that often results in death [8]. In addition, the fungus causes local infections such as vaginitis and thrush. For medical treatment of fungal infections, amphotericin B has been considered as the drug of choice [9, 10], and the azoles are mainly used in common clinical situations. However, the toxicity of and the resistance to these antifungal drugs are major problems [4, 5]. In the case of amphotericin B, an increased amount of amphotericin B must be administered to patients due to its poor permeability across the membrane [11], which can result in severe side effects, for example, renal damage [12, 13]. To overcome these problems of side-effects, natural products have been considered to be promising antifungal agents with less profound adverse effects. The antifungal activities of several phytochemicals including polyphenols, phenolics, terpenoids, and alkaloids have been reported [14].
Bee venom (BV) from honey bees (Apis mellifera L.) has been utilized for centuries as a pain reliever, anti-coagulant and anti-inflammatory agent for the treatment of chronic diseases, such as arthritis, rheumatism, tendonitis, bursitis, fibrosis and multiple sclerosis [15-18]. In Korea, apitherapy, which uses the stings of live honey bees, has shown therapeutic value for treating piglets, calves and dairy cows with several respiratory diseases [19]. BV, called apitoxin, is a mixture of proteins: melittin (main component 52%), apamin, adolapin, phospholipase A2, hyaluronidase, histamine, dopamine and protease inhibitor [20]. Two major components of BV, melittin and phospholipase A2, are generally thought to play an important role in the induction of the irritation and the allergic reactions associated with bee stings [16]. Also, sweet bee venom (SBV), in which hyper-molecular materials, such as enzymes and histamines that act as allergens in BV pharmacopuncture, have been removed, has been developed [21]. Melittin, a 26 amino acid polypeptide, is known to have anti-bacterial effects [22-24]. However, information on the antifungal efficacies of BV and SBV is not available.
This study was performed to investigate the antifungal activities of BV and SBV against C. albicans clinical isolates cultured from blood and the vagina.
2. Materials and Methods
The BV and the SBV used in this study were produced at the Korean Pharmacopuncture Institute (Seoul, Korea). Lyophilized whole BV and SBV were dissolved in distilled water at different concentrations, and were then used in this experiment. To examine the antifungal activities of BV and SBV, C. albicans (KCTC 7965) as a standard strain from the Korean Collection for Type Cultures (KCTC) at the Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea; the 10 C. albicans clinical isolates used as test organisms were furnished from the Korean Collection of Medical Fungi (KCMF) at the Konyang Institute of Medical Mycology (KIMM), Daejeon, Korea. Fungal cells were cultured on sabouraud dextrose agar (SDA; BD, Difco, Sparks, MD, USA) plates, and then incubated for 48 hours at 35°C. Amphotericin B (Sigma, St. Louis, MO, USA) and fluconazole (Sigma, St. Louis, MO, USA) were dissolved in 2% dimethyl sulfoxide (DMSO) as a stock solution, and then subsequently diluted.
Disk diffusion was carried out with Mueller-Hinton agar glucose methylene blue (MH-GMB; BD, Difco, Detroit, MI, USA). The inoculum was prepared by using 24-hour plate cultures of C. albicans on SDA. The 0.5 McFarland inoculum was swabbed in three directions on the MH-GMB agar plate. A disk (Advantec filter paper No. 26, size 6 mm) was placed on the surface agar, and a 20 μL sample (10 mg/mL each of BV and SBV) was loaded. Amphotericin B (100 μg) and fluconazole (100 μg) were used as a positive control. The plates were incubated for 24 hours at 35°C. The diameters (cm) of the zone of inhibition were measured by using a metric ruler.
A minimun inhibitory concentration (MIC) assay using a broth microdilution method was performed as described in M27-A2 (CLSI) with modifications [25]. The medium used was Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma Chemical Co., Missouri, USA) with L-glutamine buffered to pH 7.0 by using 0.165 mol/L of morpholino- propanesulfonic acid (MOPS), supplemented with 2% glucose. The strain suspension was prepared in 0.85% saline, with an optical density equivalent to a 0.5 McFarland standard, and was diluted to 1 : 100 in RPMI 1640 medium to obtain a final concentration of 1 − 5 × 104 colony-forming units per milliliter (CFU/mL). This suspension was inoculated into each well of a microtiter plate previously prepared with BV and SBV at concentrations from 500 μg/mL down to 0.9 μg/mL by using RPMI medium. The 96-well microtiter plate was incubated with agitation for 24 hours at 35°C. The control drug for each strain was amphotericin B and fluconazole diluted in DMSO; the concentrations tested ranged from 50 μg/mL down to 0.09 μg/mL in RPMI 1640 medium. The MIC was defined as the lowest concentration at which the optical density (OD) was reduced to 50% of the OD in the growth control well as measured by using an enzyme-linked immunosorbent assay (ELISA) microplate reader (Biotek).
The kinetics of the antifungal actions of BV and SBV against C. albicans cells were evaluated by using killing curve assays. Log-phased fungal cells (2 × 104 CFU/mL) in RPMI 1640 medium were incubated with 125 μg/mL of BV and 62.5 μg/mL of SBV or with 0.1 μg/mL of amphotericin B (at the MIC), which was used as a positive control. The culture was obtained and spread on a SDA agar plate, then, the CFUs were counted after incubation for 24 hours at 35°C [26]. The values represented the averages of measurements conducted in triplicate of three independent assays.
All data were taken in triplicate, and statistical analyses were performed by using the student’s t-test, with (P < 0.05) being considered significant.
3. Results
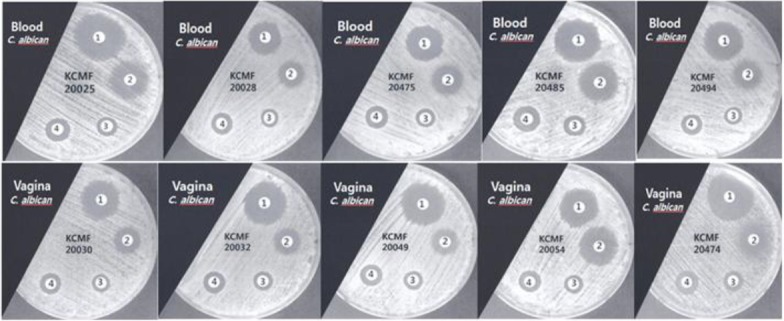
The antifungal efficacies of BV and SBV against C. albicans (KCTC 7965) as a standard strain and 10 C. albicans clinical isolates were evaluated by the disk diffusion method with MH-GMB medium, and the results are shown in Table 1 and Fig. 1. The results showed that the diameters of the inhibition zones for C. albicans blood isolates and C. albicans vaginal isolates were 9 − 12 mm and 10 − 12 mm, respectively, for BV and 12 − 18 mm and 12 − 19 mm, respectively, for SBV. The diameters of the inhibition zones for BV and SBV for the C. albicans standard strain were measured as 11 mm and 15 mm, respectively. In the blood isolates and the vaginal isolates, the diameters of the inhibition zones for BV and SBV were not significantly different (P > 0.05). However, the inhibition zone diameters of SBV compared with that of BV in clinical isolates were significantly different (P < 0.05).
Table. 1. Anti-microbial activities of BV and SBV against Candida albicans clinical isolates as measured by using disk diffusion assay.
| Sources | Candida albicans | Diameter of Zone Inhibition (mm) | |||
|---|---|---|---|---|---|
| Amphotercin B | Fluconazole | BV | SBV | ||
| Blood | KCMF 20025 | 27 | 20 | 11 | 13 |
| KCMF 20028 | 20 | 14 | 10 | 12 | |
| KCMF 20475 | 20 | 17 | 10 | 18 | |
| KCMF 20485 | 25 | 22 | 12 | 15 | |
| KCMF 20494 | 23 | 15 | 9 | 12 | |
| Geometric mean | 23 | 17.6 | 10.4 | 14 | |
| Vagina | KCMF 20030 | 22 | 15 | 10 | 12 |
| KCMF 20032 | 25 | 15 | 11 | 12 | |
| KCMF 20049 | 25 | 15 | 11 | 13 | |
| KCMF 20054 | 28 | 23 | 11 | 12 | |
| KCMF 20474 | 30 | 20 | 12 | 19 | |
| Geometric mean | 26 | 17.6 | 11 | 13.6 | |
| KCTC 7965* | 21 | 13 | 11 | 15 | |
*Standard strain. BV, bee venom; SBV, sweet bee venom; KCMF, Korean Collection of Medical Fungi; KCTC, Korean Collection for Type Cultures.
Fig. 1. Antifungal activities against 10 Candida albicans clinical isolates that were cultured from blood and the vagina as measured by using the disk diffusion method: 1, amphotercin B; 2, fluconazole; 3, bee venom; 4, sweet bee venom.
For the clinical isolates, the MIC values, which were obtained by using the broth microdilution method, varied from 62.5 μg/mL to 125 μg/mL for BV and from 15.63 μg/ mL to 62.5 μg/mL for SBV. The MIC values for BV and SBV for the C. albicans standard strain were 125 μg/mL and 62.5 μg/mL, respectively. The MIC values for BV and SBV for all clinical isolates were similar to those for the standard strain (Table 2).
Table. 2. MIC of BV and SBV against 10 Candida albicans clinical isolates as measured by using the broth microdilution method.
| Sources | Candida albicans | Minimal Inhibitory Concentrations (μg/mL) | |||
|---|---|---|---|---|---|
| Amphotercin B | Fluconazole | BV | SBV | ||
| Blood | KCMF 20025 | 0.195 | 0.39 | 125 | 62.5 |
| KCMF 20028 | 0.39 | 0.39 | 125 | 31.25 | |
| KCMF 20475 | 0.39 | 0.39 | 125 | 15.63 | |
| KCMF 20485 | 0.09 | 0.39 | 125 | 62.5 | |
| KCMF 20494 | 0.195 | 0.39 | 125 | 31.5 | |
| Geometric mean | 0.252 | 0.39 | 125 | 40.63 | |
| Vagina | KCMF 20030 | 0.195 | 0.39 | 125 | 62.5 |
| KCMF 20032 | 0.195 | 0.39 | 125 | 31.25 | |
| KCMF 20049 | 0.195 | 0.39 | 62.5 | 31.25 | |
| KCMF 20054 | 0.39 | 0.39 | 125 | 31.25 | |
| KCMF 20474 | 0.39 | 0.78 | 125 | 62.5 | |
| Geometric mean | 0.273 | 0.47 | 112.5 | 43.75 | |
| KCTC 7965* | 0.09 | 0.39 | 125 | 62.5 | |
*Standard strain. MIC, minimal inhibitory concentrations; BV, bee venom; SBV, sweet bee venom; KCMF, Korean Collection of Medical Fungi; KCTC, Korean Collection for Type Cultures.
A killing-curve assay against C. albicans KCTC 7965 cells was conducted to assess the killing potencies of BV and SBV. The results showed that BV and SBV exhibited antifungal activities through fungicidal effects. C. albicans cells treated with 50 μg/mL of SBV rapidly decreased in number in a manner similar to the decrease observed with 1 μg/mL of amphotericin B after incubation for 4 hours, however, for 100 μg/mL of BV, the decrease in the number of C. albicans was slow (Fig. 2). Thus, the fungicidal effect of SBV was higher than that of BV.
Fig. 2. Time-killing plots for BV and SBV against Candida albicans KCTC 7965 strain. Fungal cells were incubated with 100 μg/mL of BV, 50 μg/mL of SBV and 1 μg/mL of amphotericin B which was used as a positive control. Viability was determined every 2 hours by using CFUs and was expressed as a percent survival. The error bars represent the SD values for two independent experiments performed in duplicate.
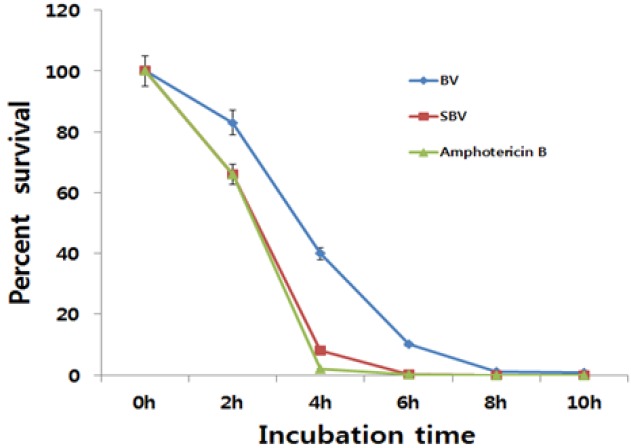
BV, bee venom; SBV, sweet bee venom; KCTC, Korean Collection for Type Cultures; CFUs, colony forming units; SD, standard deviation.
4. Discussion
BV, called apitoxin, is a mixture of proteins: melittin (main component 52%), apamin, adolapin, phospholipase A2, hyaluronidase, histamine, dopamine and protease inhibitor [20]. Two major components of BV, melittin and phospholipase A2, are generally thought to play important roles in the induction of the irritation and the allergic reactions associated with bee stings [16]. Also, SBV which has hyper-molecular materials, such the enzymes and the histamines that act as allergens in BV pharmacopuncture, removed, has been developed [21]. The frequent uses of anti-microbial and antifungal agents such as anti-biotics enable many pathogens to acquire resistances to multiple drugs [27]. The emergence of anti-bacterial-resistant strains of animal pathogens and their potential health risks to humans have captured great attention [28, 29]. Therefore, new anti-bacterial or antifungal agents with fewer adverse effects must be developed for the successful treatment of dermatophytes infections [30].
The anti-bacterial properties of BV as a natural antibacterial agent have been extensively studied, and BV therapy has been suggested for use as an alternative to anti-biotic therapy [31-33]. Strong antibacterial activities of BV against both Gram negative and Gram positive bacteria have been reported [34, 35]. Nakatuji et al [36] reported that BV could control the growth of Staphylococcus aureus, which plays an important role in the pathogenesis of inflamed lesions in the case of acne vulgaris. Moreover, BV exhibited antibacterial activities against skin bacteria such as Propionibacterium acnes, Staphylococcus epidermidis, and Streptococcus pyogenes [37].
BV and SBV anti-candidal activities were observed by using the disk diffusion method and the broth microdilution method, confirming that the compounds have a potential for use as anti-candidal agents, although their activities are less efficient as compared with amphotericin B and fluconazole, also, in the disk diffusion assay of C. albicans isolates from blood and the vagina, the antifungal activites of BV and SBV were similar (Tables 1,2, Fig. 1). However, Yu et al [38] reported that the antifungal activities of BV against Trichophyton mentagrophytes and Trichophyton rubrum were much stronger than those of fluconazole, one of the commercial antifungal drugs that is currently being used. In the current study, amphotericin B was used as a positive control: amphotericin B is a fungicidal agent that is widely used to treat serious systemic infections [39]. According to the MIC values, the anti-candidal activities of BV and SBV were less potent than those of amphotericin B and fluconazole as a positive control (Table 2). Most anti- biotics show an anti-microbial effect caused by a cidal or a static action. SBV exhibited an effect similar to that of amphotericin B, which is known a cidal agent for fungi; the anti-candidal activities of SBV are due to its killing action, as described by the time killing curve assay with C. albicans (Fig. 2).
In this study, the MIC values for C. albicans blood and viginal isolates, as obtained by using the broth microdilution method, varied from 62.5 μg/mL to 125 μg/mL for BV and from 15.63 μg/mL to 62.5 μg/mL for SBV (Table 2). Seoung [39] reported that methanol extracts from Galla rhois showed antifungal activity against C. albicans isolated from patients with recurrent vaginal candidiasis and the MIC value was 50 mg/mL for C. albicans. Also, dichloromethane extract from Paeonia japonica showed antifungal activity against C. albicans, and the MIC value was 50 mg/mL for C. albicans [40]. Thus, the anti-candidal activities of BV and SBV are thought to be much stronger than those of extracts from Galla rhois and Paeonia japonica. We demonstrated that SBV had very strong antifungal activity, the antifungal activity of SBV being much stronger than that of BV. Moreover, the anti-candidal activities of BV and SBV were much higher than those of phytochemicals, judging by their effective anti-candidal concentration ranges. This study raises the possibility that BV and SBV can be used as an alternative strategy for treating fungal pathogens, which would reduce anti-biotic use. BV and SBV may be candidates for new antifungal agents against C. albicans clinical isolates. Although this study, demonstrated that BV and SBV had considerable antifungal activites, further experiments for evaluating the in vivo efficacies of BV and SBV for clinical applications are warranted.
5. Conclusion
The antifungal activities of BV and SBV against 10 clinical isolates of C. albicans that were cultured from blood and the vagina were determined by using the disk diffusion assay, the broth microdilution assay and the killing-curve assay. BV and SBV showed antifungal activities against those clinical isolates of C. albicans, and the antifungal activites of BV and SBV showed similar results on the disk diffusion assay. The MIC values obtained for clinical isolates by using the broth microdilution method varied from 62.5 μg/ mL to 125 μg/mL for BV and from 15.63 μg/mL to 62.5 μg/ mL for SBV. In the killing-curve assay, SBV behaved as amphotericin B, which was used as positive control, did. The antifungal efficacy of SBV was much higher than that of BV.
Acknowledgments
The author is thankful to the Korean Collection of Medical Fungi (KCMF) at the Konyang Institute of Medical Mycology (KIMM), Daejeon, Korea. for providing C. albicans clinical to carry out the research work.
Footnotes
Conflict of interest The authors declare that there are no conflict of interest.
References
- 1.Odds FC. Candida species and virulence. Am Soc Microbiol News. 1994;60(6):313–318. [Google Scholar]
- 2.Verduyn Lunel, FM, Meis JF, Voss A. Nosocomial fungal infections: candidemia. Diagn Microbiol Infect Dis. 1999;34(3):213–220. doi: 10.1016/S0732-8893(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 3.McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Pilkaytis BD et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis. 2001;33(5):641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 4.Bodey GP. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988;11(Suppl A):411–426. doi: 10.1016/0195-6701(88)90220-4. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JE., Jr Invasive candida infections-evolution of a fungal pathogen. N Engl J Med. 1991;324(15):1060–1062. doi: 10.1056/NEJM199104113241511. [DOI] [PubMed] [Google Scholar]
- 6.Fisher-Hoch SP, Hutwagner L. Opportunistic candidiasis: an epidemic of the 1980s. Clin Infect Dis. 1995;21(4):897–904. doi: 10.1093/clinids/21.4.897. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JE, Jr, Bodey GP, Boeden RA, Buchner T, DePauw BE, Filler SG et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25(1):43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 8.Anttila VJ, Ruutus P, Bondestam S, Jansson SE, Nordling S, Farkkila M et al. Hepatosplenic yeast infection in patients with acute leukemia: a diagnostic problem. Clin Infect Dis. 1994;18(6):979–981. doi: 10.1093/clinids/18.6.979. [DOI] [PubMed] [Google Scholar]
- 9.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12(2):308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Hartsel S, Bolard J. Amphotericin B: new life for an old drug. Trends Pharmacol Sci. 1996;17(12):445–449. doi: 10.1016/S0165-6147(96)01012-7. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka S, Murata M. Cholesterol markedly reduces ion permeability induced by membrane-bound amphotericin B. Biochim Biophys Acta. 2002;1564(2):429–434. doi: 10.1016/S0005-2736(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 12.Fonos V, Cataldi L. Amphotericin B-induced nephrotoxicity: a review. J Chemother. 2000;12(6):463–470. doi: 10.1179/joc.2000.12.6.463. [DOI] [PubMed] [Google Scholar]
- 13.Mayer J, Doubek M, Doubek J, Horky D, Scheer P, Stepanek M. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: animal experiments and clinical study. J Infect Dis. 2002;186(3):379–388. doi: 10.1086/341662. [DOI] [PubMed] [Google Scholar]
- 14.Chee HY, Kim H, Lee MH. In vitro antifungal activity of limonene against trichophyton rubrum. Microbiology. 2009;37(3):243–246. doi: 10.4489/MYCO.2009.37.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YB, Lee HJ, Han HJ, Mar WC, Kang SK, Yoon OB et al. The water-soluble fraction of bee venom produces anti-nociceptive and antiinflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002;71(2):191–204. doi: 10.1016/s0024-3205(02)01617-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HW, Kwon YB, Ham TW, Rho DH, Yoon SY, Lee JH et al. Acupoint stimulation using bee venom attenuates formalin induced pain behavior and spinal cord fos expression in rats. J Vet Med Sci. 2003;65(3):349–355. doi: 10.1292/jvms.65.349. [DOI] [PubMed] [Google Scholar]
- 17.Han SM, Lee KG, Yeo JH, Kweon HY, Kim BS, Kim JM et al. Antibacterial activity of the honey bee venom against bacterial mastitis pathogens infecting dairy cows. Int J Indust Entomol. 2007;14(2):137–142. [Google Scholar]
- 18.Lee SM, Lim J, Lee JD, Choi DY, Lee S. Bee venom treatment for refractory postherpetic neuralgia: a case report. J Altern Complement Med. 2014;20(3):212–214. doi: 10.1089/acm.2013.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SH, Cho SK, Kang SS, Bae CS, Bai YH, Lee SH et al. Effect of apitherapy in piglets with preweaning diarrhea. Am J Chin Med. 2003;31(2):321–326. doi: 10.1142/S0192415X03001004. [DOI] [PubMed] [Google Scholar]
- 20.Park HJ, Lee SH, Son DJ, Oh KW, Kim KH, Song HS et al. Anti-arthritic effect of bee venom: inhibition of inflammation mediator generation by suppression of NF-B through interaction with the p50 subunit. Arthritis Rheum. 2004;50(11):3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- 21.Choi YG, Choi SH, Kwon KR. [Purification of peptide components including melitittin from bee venom using gel filtration chromatography and propionic acid/urea polyacrylamide gel electrophoresis] J Pharmacopuncture. 2006;9(2):105–111. Korean. [Google Scholar]
- 22.Eiseman JL, Von Bredow, J, Alvares AP. Effect of honeybee (Apis mellifera) venom on the course of adjuvant- induced arthritis and depression of drug metabolism in the rat. Biochem Pharmacol. 1982;31(6):1139–1146. doi: 10.1016/0006-2952(82)90354-9. [DOI] [PubMed] [Google Scholar]
- 23.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Muller U et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98(7):1676–1683. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon YB, Lee JD, Lee HJ, Han HJ, Mar WC, Kang SK et al. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90(3):271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- 25.Espinel-Ingroff A, Barchiesi F, Cuenca-Estrella M, Pfaller MA, Rinaldi M, Rodriguez-Tudela JL et al. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J Clin Microbiol. 2005;43(8):3884–3889. doi: 10.1128/JCM.43.8.3884-3889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA, Influence of., test Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42(5):1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens WE, Nickerson SC, Boddie RL, Tomita GM, Ray CH. Prevalence of mastitis in dairy heifers and effectiveness of antibiotic therapy. J Dairy Sci. 2001;84(4):814–817. doi: 10.3168/jds.S0022-0302(01)74538-9. [DOI] [PubMed] [Google Scholar]
- 28.Pitkälä A, Haveri M, Pyörälä S, Myllys V, Honkanen-Buzalski T. Bovine mastitis in Finland 2001-prevalence, distribution of bacteria, and antimicrobial resistance. J Dairy Sci. 2004;87(8):2433–2441. doi: 10.3168/jds.S0022-0302(04)73366-4. [DOI] [PubMed] [Google Scholar]
- 29.Nair MK, Joy J, Vasudevan P, Hinckley L, Hoagland TA, Venkitanarayanan KS. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J Dairy Sci. 2005;88(10):3488–3495. doi: 10.3168/jds.S0022-0302(05)73033-2. [DOI] [PubMed] [Google Scholar]
- 30.Komine Y, Komine K, Kai K, Itagaki M, Kuroishi T, Aso H et al. Effect of combination therapy with lactoferrin and antibiotics against staphylococcal mastitis on drying cows. J Vet Med Sci. 2006;68(3):205–211. doi: 10.1292/jvms.68.205. [DOI] [PubMed] [Google Scholar]
- 31.Fennell JF, Shipman WH, Cole LJ. Antibacterial action of melittin, a polypeptide from the venom. Proc Soc Exp Biol Med. 1968;127(3):707–710. doi: 10.3181/00379727-127-32779. [DOI] [PubMed] [Google Scholar]
- 32.Somerfield SD, Stach JL, Mraz C, Gervais F, Skamene E. Bee venom inhibits superoxide production by human neutrophils. Inflammation. 1984;8(4):385–391. doi: 10.1007/BF00918214. [DOI] [PubMed] [Google Scholar]
- 33.Saini SS, Peterson JW, Chopra AK. Melittin binds to secretory phospholipase A2 and inhibits its enzymatic activity. Biochem Biophys Res Commun. 1997;238(2):436–442. doi: 10.1006/bbrc.1997.7295. [DOI] [PubMed] [Google Scholar]
- 34.Stocker JF, Traynor JR. The action of various venoms on Escherichia coli. J Appl Bacteriol. 1986;61(5):383–388. doi: 10.1111/j.1365-2672.1986.tb04300.x. [DOI] [PubMed] [Google Scholar]
- 35.Perumal Samy, R, Gopalakrishnakone P, Thwin MM, Chow TK, Bow H, Yap EH et al. Antibacterial activity of snake, scorpion and bee venoms: a comparison with purified venom phospholipase A2 enzymes. J Appl Microbiol. 2007;102(3):650–659. doi: 10.1111/j.1365-2672.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakatuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL et al. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129(10):2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han SM, Lee KG, Yeo JH, Baek HJ, Park KK. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J Med Plants Res. 2010;4(6):459–464. [Google Scholar]
- 38.Yu AR, Kim JJ, Park GS, Oh SM, Han CS, Lee MY. The antifungal activity of bee venom against dermatophytes. J Appl Biol Chem. 2012;55(1):7–11. doi: 10.3839/jabc.2011.052. [DOI] [Google Scholar]
- 39.Seoung IW. Antifungal activity of the extracts from Galla rhosis against Candida albicans. Korean J Med Mycol. 2007;12(4):175–179. [Google Scholar]
- 40.Seoung IW. Antifungal activity of the extracts from Paeonia japonica against Candida albicans. Korean J Med Mycol. 2006;11(1):19–26. [Google Scholar]


