Abstract
The aim of the present study was to investigate the associations between the expression of forkhead box protein A1 (FOXA1) and differential clinicopathological characteristics in breast cancer using a meta-analysis method. Eligible studies that investigated the correlation between FOXA1 expression and the clinical characteristics of breast cancer were collected through searching numerous databases, including PubMed, EMBASE, the Chinese National Knowledge Infrastructure and the VIP database. In total, eight studies were included in the meta-analysis. Following a systematic analysis, the expression of FOXA1 was found to be significantly associated with the estrogen receptor α status, the progesterone receptor status, lymph node metastasis and the histological grade in breast cancer. However, no statistically significant association was observed between FOXA1 expression and the human epidermal growth factor receptor-2 status in breast cancer patients.
Keywords: forkhead box protein A1, breast cancer, meta-analysis
Introduction
Breast cancer is one of the most common cancer types in females. The incidence of breast cancer has increased in previous years, with the annual rate of increase reaching 3.1% (1). Therefore, an in-depth understanding of the molecular mechanisms underlying breast cancer, and the identification of novel anticancer drug targets are of great significance.
At present, several clinical features, including the estrogen receptor α (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor-2 (HER-2) status, lymph node metastases and histological grade, have major prognostic value in breast cancer (2). These parameters reflect the biological features of the tumor; however, they are not useful as outcome predictors for the individual patient. Thus, the identification of novel prognostic markers, which are associated with the clinical features, is required to provide a more accurate prediction of clinical outcome, in addition to the identification of new therapeutic targets.
Forkhead box protein A1 (FOXA1) plays an important role in the occurrence and development of various human tumors, and FOXA1 expression has been shown to be closely associated with breast cancer (3,4). Therefore, FOXA1 may be a new target for breast cancer prevention and control. Increasingly, studies have investigated the associations between FOXA1 expression and the clinical pathological features of breast cancer; however, the conclusion of each study has not always been consistent (5–12). Therefore, a comprehensive analysis was required to clarify the inconsistencies. In the present study, a meta-analysis was performed based on the collected literature, and the association between FOXA1 expression and the clinical pathological features of breast cancer was investigated comprehensively. Subsequently, the use of FOXA1 as a biomarker for the diagnosis, treatment and prognosis of breast cancer was assessed.
Materials and methods
Search strategy
A systematic search for eligible studies that reported on the expression of FOXA1 and the associations in patients with breast cancer was conducted in PubMed, EMBASE, the Chinese National Knowledge Infrastructure and the VIP databases. Publications were first identified by using combinations of the following words: FOXA1 and breast cancer. Subsequently, the references in the identified publications were screened and examined for any other potentially relevant studies. Retrieval time range from the time when the databases were established to May 2014. If certain studies lacked information, the authors were contacted to obtain as much available information as possible.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) Texts were published and available on line; ii) studies investigated the association between FOXA1 and clinical features of breast cancer; iii) sufficient information was provided to estimate the odds ratio (OR) and 95% confidence interval (CI); iv) measurement methods and experimental groups were similar among the studies. Exclusion criteria were as follows: i) Review articles or letters; ii) included investigation of non-human samples; iii) articles with duplicated data.
Data extraction
Eligible articles were reviewed independently by two investigators and disagreements were resolved by consensus. The following data were retrieved from the studies: Name of the first author, year of publication, country of origin, methods of testing and definition of positivity (cut-off value).
Statistical analysis
The strength of an association was expressed as pooled OR values along with the corresponding 95% CI. Heterogeneity between studies was assessed by χ2-based Q-tests and I2 tests, where I2 >50% or P<0.10 was considered to indicate significant heterogeneity (13). A random-effects model (DerSimonian-Laird) was used to assess the pooled ORs when significant heterogeneity was observed (14). In cases without significant heterogeneity, a fixed-effects model (Mantel-Haenszel) was used. Rev-Man 4.2 software (The Cochrane Collaboration, Oxford, UK) was used to conduct the meta-analysis.
Results
Study selection
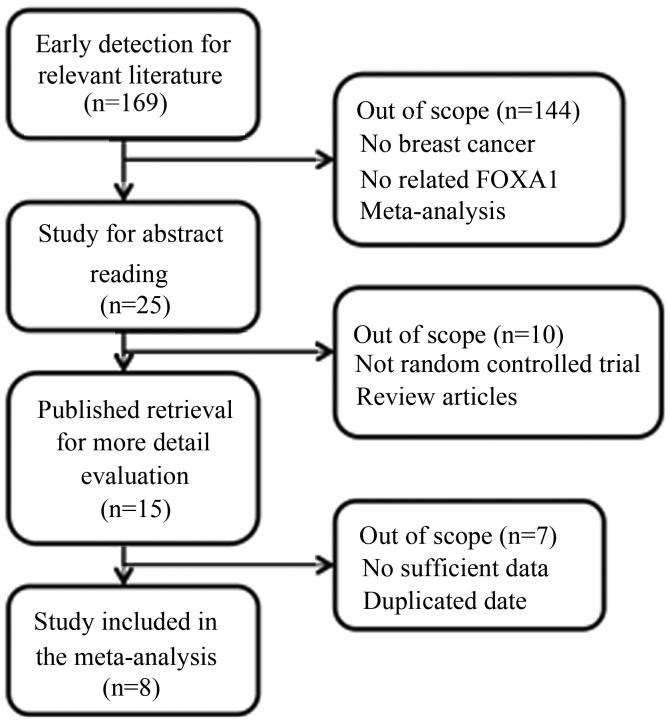
A selection flow chart is shown in Fig. 1. In total, 15 articles were eligible for detailed reading. The studies by Ademuyiwa et al (15), Kawase et al (16), Imamura et al (17) and Badve et al (18) were excluded due to insufficient data. In addition, the studies by McCune et al (19) and Bernardo et al (20) were excluded due to the inclusion of non-human-sample-based trials. There were two studies (5,21) that used the same samples; thus, the study with the smaller sample size was excluded. Finally, there were eight studies included in the meta-analysis (5–12).
Figure 1.
Literature screening and result. FOXA1, forkhead box protein A1.
Study characteristics
Major characteristics of the eight eligible publications are reported in Table I. The studies were conducted in four countries (USA, UK, China and Japan). Six studies were published in English, and the remaining two were published in Chinese. The eight articles used an immunohistochemistry method to determine the FOXA1 status.
Table I.
Main characteristics of the studies included in the meta-analysis.
| First author (ref) | Year | Country | Cases (n) | Method | Cut-off |
|---|---|---|---|---|---|
| Wolf (15) | 2007 | USA | 100 | IHC | Not mentioned |
| Habashy (16) | 2008 | UK | 880 | IHC | Median |
| Thorat (17) | 2008 | UK | 245 | IHC | 3 |
| Albergaria (18) | 2009 | UK | 249 | IHC | 3 |
| Liu (19) | 2010 | China | 213 | IHC | 3 |
| Hisamatsu (14) | 2011 | Japan | 239 | IHC | Median |
| Ijichi (20) | 2012 | Japan | 113 | IHC | 2 |
| Jiang (21) | 2012 | China | 113 | IHC | 3 |
IHC, immunohistochemistry.
Meta-analysis results
No significant heterogeneity was encountered across the studies with regard to the association between FOXA1 and any of the prognostic factors, with the exception of the ER status and HER-2 status. Thus, the random-effects model was selected for the ER status and HER-2 status, while the fixed-effects model was selected for each of the remaining three clinical features.
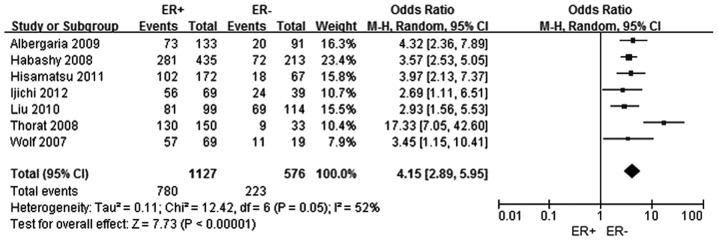
A total of seven studies were selected (5–11) to analyze the association between FOXA1 expression and the ER status in breast cancer. In total, 1,127 patients were included in the ER positive group, while 576 patients comprised the ER negative group. The FOXA1 expression level was shown to be higher in the ER positive breast cancer group when compared with the ER negative breast cancer group, and the difference was statistically significant (OR, 4.15; 95% CI, 2.89–5.95; P<0.0001), as shown in Fig. 2.
Figure 2.
Forest plot assessing the association between forkhead box protein A1 expression and the ER status in breast cancer. The center of the square indicates the study-specific OR and the horizontal lines correspond to the study-specific 95% CI. The area of the square reflects the study-specific variance. The diamond represents the pooled OR and 95% CI. ER, estrogen-receptor α; OR, odds ratio; CI, confidence interval.
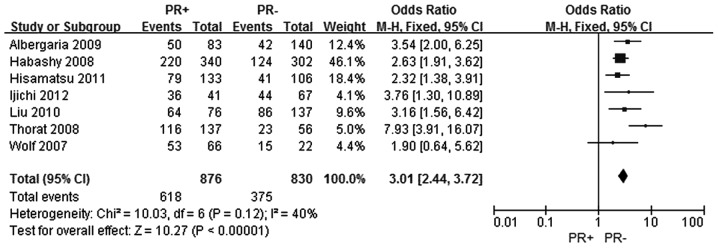
The same seven studies were included to assess the association between FOXA1 expression and the PR status in breast cancer (5–11). In total, 876 patients were included in the PR positive group and 830 patients were included in the PR negative group. The FOXA1 expression level was demonstrated to be increased in the PR positive breast cancer group when compared with the PR negative breast cancer group, and the difference was statistically significant (OR, 3.01; 95% CI, 2.44–3.72; P<0.0001), as shown in Fig. 3.
Figure 3.
Forest plot assessing the association between forkhead box protein A1 expression and the PR status in breast cancer. The center of the square indicates the study-specific OR and the horizontal lines correspond to the study-specific 95% CI. The area of the square reflects the study-specific variance. The diamond represents the pooled OR and 95% CI. PR, progesterone receptor; OR, odds ratio; CI, confidence interval.
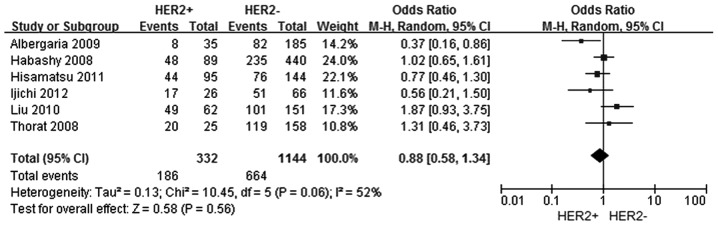
In total, six studies were selected (5–11) to investigate the association between FOXA1 expression and the HER-2 status, among which 332 patients comprised the HER-2 positive group and 1,144 patients were included in the HER-2 negative group. However, no significant association was identified between FOXA1 expression and the HER-2 status in breast cancer patients (OR, 0.88; 95% CI, 0.58–1.3; P=0.56), as shown in Fig. 4.
Figure 4.
Forest plot assessing the association between forkhead box protein A1 expression and the HER-2 status in breast cancer. The center of the square indicates the study-specific OR and the horizontal lines correspond to the study-specific 95% CI. The area of the square reflects the study specific variance. The diamond represents the pooled OR and 95% CI. HER-2, human epidermal growth factor receptor-2; OR, odds ratio; CI, confidence interval.
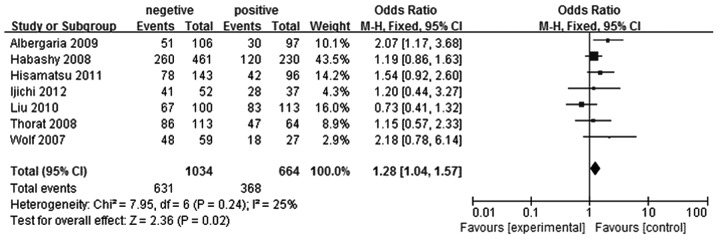
With regard to the analysis of the association between lymph node metastasis and FOXA1 expression in breast cancer, seven studies were selected (5–11). In total, 664 patients were included in the lymph node metastasis positive group and 1,034 patients were included in the lymph node metastasis negative group. FOXA1 expression levels were shown to be higher in the lymph node metastasis negative breast cancer group when compared with the lymph node metastasis positive breast cancer group (OR, 1.28; 95% CI, 1.04–1.57; P=0.02), as shown in Fig. 5.
Figure 5.
Forest plot assessing the association between forkhead box protein A1 expression and lymph node metastasis in breast cancer. The center of the square indicates the study-specific OR and the horizontal lines correspond to the study-specific 95% CI. The area of the square reflects the study-specific variance. The diamond represents the pooled OR and 95% CI. OR, odds ratio; CI, confidence interval.
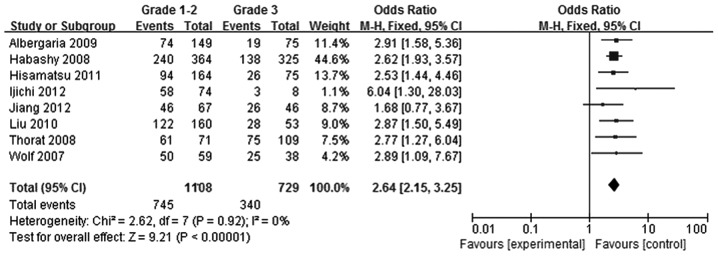
Tumors were divided according to histological grade into a grade 1–2 group and grade 3 group. Using eight studies (5–12), 1,108 patients were included in the grade 1–2 group, while 729 patients were included in the grade 3 group. FOXA1 expression levels in the grade 1–2 breast cancer group were found to be greater compared with those in the grade 3 breast cancer group (OR, 2.64; 95% CI, 2.15–3.25; P<0.00001), as shown in Fig. 6.
Figure 6.
Forest plot assessing the association between forkhead box protein A1 expression and histological grade in breast cancer. The center of the square indicates the study-specific OR and the horizontal lines correspond to the study-specific 95% CI. The area of the square reflects the study-specific variance. The diamond represents the pooled OR and 95% CI. OR, odds ratio; CI, confidence interval.
Discussion
As a member of the FOX family of transcription factors, FOXA1 expression has been observed not only in breast cancer, but also in colon, lung, thyroid, esophageal, prostate and endometrial cancers (22–26). FOXA1 can bind to the promoters of numerous genes associated with regulation of cell signaling and the cell cycle (27). In addition, FOXA1 was identified to not only stimulate growth, but also function as a growth inhibitor (28). When functioning as a trigger, FOXA1 is a ‘pioneer factor’ that binds to chromatinized DNA, and opens the chromatin to enhance the combination of ER and target genes (29). While functioning as an inhibitor, FOXA1 expression has been shown to block metastatic progression by influencing the expression of the breast cancer susceptibility gene (BRCA1)-associated cell cycle inhibitor, p27, and promoting E-cadherin expression (30,31). These observations indicate that FOXA1 plays an important role in regulating the growth and activity of cancer cells. In previous years, a number of studies have demonstrated differential expression of FOXA1 in breast cancer, and that FOXA1 plays an important role in the occurrence, development and prognostics of breast cancer, which has attracted further studies (7,32,33). Certain studies have suggested that there is an association between FOXA1 expression and prognostic factors of breast cancer, such as ER, PR, HER-2, lymph node metastasis and histological grade (5–9), which has subsequently lead to the investigation of whether FOXA1 may be a significant prognostic factor. The expression of certain receptors in breast cancer is associated with a variety of factors, of which FOXA1 expression may be one (5–12). The results, however, remain controversial. The majority of studies indicate that the ER and PR status are significantly associated with FOXA1 expression in breast cancer; however, there is no association with the HER-2 status (5,7,8,10–12). By contrast, Wolf et al (6) found that there was no association between FOXA1 expression and PR status in breast cancer, while Albergaria et al (9) reported that FOXA1 expression was associated with the HER-2 status. In the present meta-analysis, the results were consistent with the majority of studies in that the expression of FOXA1 in the ER positive or PR positive groups was significantly higher compared with that in the ER negative or PR negative groups. However, no statistically significant difference was observed between the HER-2 positive and HER-2 negative groups. With regard to the breast cancer process (lymph node metastasis and histological grade), the result for FOXA1 expression at different stages was also divided. Albergaria et al (9) found that FOXA1 expression was associated with lymph node metastasis; however, this was inconsistent with the results observed by Ijichi et al (11) and Thorat et al (8). Albergaria et al (9) and Habashy et al (7) hypothesized that FOXA1 expression was associated with histological grade, while Jiang et al (12) demonstrated no correlation between FOXA1 expression and histological grade. In the current meta-analysis, the combined results indicated that FOXA1 expression was associated with lymph node metastasis and histological grade. The expression levels of FOXA1 in the lymph node negative or low histological grade groups were significantly higher compared with that in the lymph node positive or high histological grade groups, which demonstrated that FOXA1 expression was associated with the development, cell differentiation and prognosis of breast cancer.
Limitations of the present analysis should be acknowledged. Firstly, the cut-off values in the present study were not consistent. Four studies defined a score of 0–3 as FOXA1 expression negative, one study defined a score of 0–2 as FOXA1 expression negative, whereas different thresholds (median) were used in the two additional studies. The remaining study did not discuss cut-off values. Secondly, heterogeneity is a potential problem that may have affected the interpretation of the results of the meta-analysis. When investigating the correlation between FOXA1 expression and the ER status or HER-2 status, high heterogeneity was observed in selected articles. The sources of heterogeneity may be attributed to differences in the ethnicity and histological type. Thirdly, the study was restricted to papers published in English and Chinese, which is likely to have introduced bias. Despite a number of limitations in the present study, to the best of our knowledge, this is the first meta-analysis focusing on the association between FOXA1 expression and the clinical characteristics of breast cancer.
In conclusion, the results of the meta-analysis demonstrated a significant association between FOXA1 expression and the clinical characteristics of breast cancer, which may be valuable to the diagnosis, treatment and prognosis of breast cancer. However, due to the aforementioned limitations, future studies evaluating the significance of FOXA1 expression on the clinical characteristics of breast cancer are strongly recommended.
Acknowledgements
This project was sponsored by the Scientific Research Foundation for Returned Overseas Chinese Scholars by the State Education Ministry. This study was supported by grants from the National Natural Science Foundation of China (nos. 30873044 and 81272372).
References
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 2.Clark GM. Prognostic and predictive factors for breast cancer. Breast Cancer. 1995;2:79–89. doi: 10.1007/BF02966945. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo GM, Keri RA. FOXA1: A transcription factor with parallel functions in development and cancer. Biosci Rep. 2012;32:113–130. doi: 10.1042/BSR20110046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakshatri H, Badve S. FOXA1 in breast cancer. Expert Rev Mol Med. 2009;11:e8. doi: 10.1017/S1462399409001008. [DOI] [PubMed] [Google Scholar]
- 5.Hisamatsu Y, Tokunaga E, Yamashita N, et al. Impact of FOXA1 expression on the prognosis of patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012;19:1145–1152. doi: 10.1245/s10434-011-2094-4. [DOI] [PubMed] [Google Scholar]
- 6.Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: Growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer. 2007;120:1013–1022. doi: 10.1002/ijc.22389. [DOI] [PubMed] [Google Scholar]
- 7.Habashy HO, Powe DG, Rakha EA, et al. Forkhead-box A1 expression in breast cancer and its prognostic significance. Eur J Cancer. 2008;44:1541–1551. doi: 10.1016/j.ejca.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Thorat MA, Marchio C, Morimiya A, Savage K, Nakshatri H, Reis-Filho JS, Badve S. Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J Clin Pathol. 2008;61:327–332. doi: 10.1136/jcp.2007.052431. [DOI] [PubMed] [Google Scholar]
- 9.Albergaria A, Paredes J, Sousa B, et al. Expression of FOXA1 and GATA-3 in breast cancer: The prognostic significance in hormone receptor-negative tumours. Breast Cancer Res. 2009;11:R40. doi: 10.1186/bcr2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Niu Y, Wang SL, Yu Q, Zhang RJ, Liu TJ. Diagnostic and prognostic significance of FOXAl expression in molecular subtypes of breast invasive ductal carcinomas. Zhonghua Yi Xue Za Zhi. 2010;90:1403–1407. (In Chinese) [PubMed] [Google Scholar]
- 11.Ijichi N, Shigekawa T, Ikeda K, et al. Association of double-positive FOXA1 and FOXP1 immunoreactivities with favorable prognosis of tamoxifen-treated breast cancer patients. Horm Cancer. 2012;3:147–159. doi: 10.1007/s12672-012-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang GY, Li H, He WL, Wang NX. Prognostic significance of FOXA1 and BRCA1 expression in triple negative breast cancer. Zhongguo Bing Li Sheng Li Za Zhi. 2012;28:1230–1234. (In Chinese) [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Ademuyiwa FO, Thorat MA, Jain RK, Nakshatri H, Badve S. Expression of Forkhead-box protein A1, a marker of luminal A type breast cancer, parallels low oncotype DX 21-gene recurrence scores. Mod Pathol. 2010;23:270–275. doi: 10.1038/modpathol.2009.172. [DOI] [PubMed] [Google Scholar]
- 16.Kawase M, Toyama T, Takahashi S, et al. FOXA1 expression after neoadjuvant chemotherapy is a prognostic marker in estrogen receptor-positive breast cancer. Breast Cancer. 2013 Jun 16; doi: 10.1007/s12282-013-0482-2. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Imamura Y, Sakamoto S, Endo T, et al. FOXA1 promotes tumor progression in prostate cancer via the insulin-like growth factor binding protein 3 pathway. PloS One. 2012;7:e42456. doi: 10.1371/journal.pone.0042456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badve S, Turbin D, Thorat MA, et al. FOXA1 expression in breast cancer-correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13:4415–4421. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- 19.McCune K, Mehta R, Thorat MA, Badve S, Nakshatri H. Loss of ERα and FOXA1 expression in a progression model of luminal type breast cancer: Insights from PyMT transgenic mouse model. Oncol Rep. 2010;24:1233–1239. doi: 10.3892/or_00000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardo GM, Lozada KL, Miedler JD, et al. FOXA1 is an essential determinant of ERα expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisamatsu Y, Tokunaga E, Yamashita N, Akiyoshi S, Okada S, Nakashima Y, et al. Impact of GATA-3 and FOXA1 expression in patients with hormone receptor-positive/HER-2-negative breast cancer. Breast Cancer. 2014 Jan 11; doi: 10.1007/s12282-013-0515-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Hosoda M, Yamamoto M, Nakano K, et al. Differential expression of progesterone receptor, FOXA1, GATA3, and p53 between pre- and postmenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2014;144:249–261. doi: 10.1007/s10549-014-2867-0. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, Miller CT, Contreras JI, et al. The hepatocyte nuclear factor 3 α gene, HNF3α (FOXA1), on chromosome band 14q13 is amplified and overexpressed in esophageal and lung adenocarcinomas. Cancer Res. 2002;62:5273–5279. [PubMed] [Google Scholar]
- 24.Nucera C, Eeckhoute J, Finn S, et al. FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin Cancer Res. 2009;15:3680–3689. doi: 10.1158/1078-0432.CCR-08-3155. [DOI] [PubMed] [Google Scholar]
- 25.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe Y, Ijichi N, Ikeda K, Kayano H, Horie-Inoue K, Takeda S, Inoue S. Forkhead box transcription factor, forkhead box A1, shows negative association with lymph node status in endometrial cancer, and represses cell proliferation and migration of endometrial cancer cells. Cancer Sci. 2012;103:806–812. doi: 10.1111/j.1349-7006.2012.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sérandour AA, Avner S, Percevault F, et al. Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers. Genome Res. 2011;21:555–565. doi: 10.1101/gr.111534.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Q, Luo Z, Xu T, et al. FOXA1: A promising prognostic marker in breast cancer. Asian Pac J Cancer Prev. 2014;15:11–16. doi: 10.7314/APJCP.2014.15.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Beck S, Sommer P, dos Santos Silva E, Blin N, Gött P. Hepatocyte nuclear factor 3 (winged helix domain) activates trefoil factor gene TFF1 through a binding motif adjacent to the TATAA box. DNA Cell Biol. 1999;18:157–164. doi: 10.1089/104454999315547. [DOI] [PubMed] [Google Scholar]
- 30.Williamson EA, Wolf I, OKelly J, Bose S, Tanosaki S, Koeffler HP. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27 (Kip1) Oncogene. 2006;25:1391–1399. doi: 10.1038/sj.onc.1209170. [DOI] [PubMed] [Google Scholar]
- 31.Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–8290. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RJ, Jain RK, Leung S, Choo J, Nielsen T, Huntsman D, Nakshatri H, Badve S. FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res Treat. 2012;131:881–90. doi: 10.1007/s10549-011-1482-6. [DOI] [PubMed] [Google Scholar]
- 33.Nakshatri H, Badve S. FOXA1 as a therapeutic target for breast cancer. Expert Opin Ther Targets. 2007;11:507–14. doi: 10.1517/14728222.11.4.507. [DOI] [PubMed] [Google Scholar]