Abstract
Basic helix-loop-helix (bHLH) transcription factor DEC2 (bHLHE41/Sharp1) is one of the clock genes that show a circadian rhythm in various tissues. DEC2 regulates differentiation, sleep length, tumor cell invasion and apoptosis. Although studies have been conducted on the rhythmic expression of DEC2 mRNA in various tissues, the precise molecular mechanism of DEC2 expression is poorly understood. In the present study, we examined whether DEC2 protein had a rhythmic expression. Western blot analysis for DEC2 protein revealed a rhythmic expression in mouse liver, lung and muscle and in MCF-7 and U2OS cells. In addition, AMP-activated protein kinase (AMPK) activity (phosphorylation of AMPK) in mouse embryonic fibroblasts (MEFs) exhibited a rhythmic expression under the condition of medium change or glucose-depleted medium. However, the rhythmic expression of DEC2 in MEF gradually decreased in time under these conditions. The medium change affected the levels of DEC2 protein and phosphorylation of AMPK. In addition, the levels of DEC2 protein showed a rhythmic expression in vivo and in MCF-7 and U2OS cells. The results showed that the phosphorylation of AMPK immunoreactivity was strongly detected in the liver and lung of DEC2 knockout mice compared with that of wild-type mice. These results may provide new insights into rhythmic expression and the regulation between DEC2 protein and AMPK activity.
Keywords: DEC2, circadian rhythm, phosphorylation of AMP-activated protein kinase, clock gene
Introduction
Circadian rhythms are important for living mammals and are predominantly regulated by clock genes (1–3). Disturbance of circadian rhythms may be associated with sleep disorder, depression, diabetes and cancer (4–9). A growing number of studies have shown that the abnormalities of clock gene expression are associated with psychiatric disorders, tumor progression, myocardial infarction, metabolic syndrome and immune disorder (10–16).
The transcription factor DEC2 is important in the circadian rhythm, hypoxia response, differentiation and tumor progression (3,10,11,17–24). While DEC1 and DEC2 mRNA indicate a rhythmic expression in the suprachiasmatic nucleus (SCN), peripheral tissues, tumor cells and human mesenchymal stem cells (MSCs) (3,17,18,21,25–29), whether DEC2 protein has rhythmic expression remains unclear. It seems likely that the rhythmic patterns of DEC1 and DEC2 are different in cell types (26), and what influences of DEC2 rhythmic expression under cell culture is poorly understood.
AMP-activated protein kinase (AMPK) is a sensor of cellular energy status (30,31). AMPK activity (phosphorylation of AMPK or pAMPK) is important in glucose metabolism, cell death and tumor progression (32,33). Rhythmic AMPK activity was observed in mice and mouse embryonic fibroblasts (MEFs) and a loss of AMPK disturbed the rhythmic expression of clock genes Bmal1 and Per2 (34,35). DEC1 protein and AMPK activity are known to have rhythmic expression in mouse liver, and DEC1 negatively regulates AMPK activity (36). However, the association between DEC2 and AMPK activity is unclear.
In the present study, we investigated the rhythmic expression of DEC2 protein and AMPK activity in mouse liver, lung and muscle, MCF-7 and U2OS human cancer cell lines, and MEF. These results may contribute to understanding of the biological functions of DEC2 and AMPK activity in various cell types.
Materials and methods
Animals
Six to 8-week-old male C57BL/6 mice were housed as previously described (26). The light in the mouse room was turned on at 7:00 a.m. (ZT 0 corresponds to 7:00 a.m., lights turned on) and turned off at 7:00 p.m. (ZT 12). Mice were entrained on a 12-h light/dark cycle for 2 weeks prior to experiments. Tissue samples were extracted from the liver, lungs and muscles of mice and were subjected to western blot analysis. Animal care and use procedures were approved by the Institutional Animal Care and Use Committee at the UNC at Chapel Hill Animal Care Facility.
DEC2 knockout mice
DEC2−/− mice were generated by inGenious Targeting Laboratory, Inc. (Stony Brook, NY, USA). Briefly, the 2.8-kb BsiEI-AlwNI genomic region of DEC2 that spans exons 1–5, including the entire coding region, was replaced with a Neo cassette. The knockout mice were backcrossed onto a C57BL/6 background. Six to 8-week-old mice were housed as above, and tissues were prepared for immunohistochemistry.
Immunohistochemistry
pAMPK immunoreactivity in mouse liver and lung tissues were evaluated using serial deparaffinized sections. Immunohistochemistry was performed using a Discovery auto-stainer machine with automated protocols (Ventana Medical Systems, Inc., Tucson, AZ, USA; Roche, Mannheim, Germany). Sections were reacted with pAMPK antibody (1:100) for 2 h.
Cell culture and treatment
The MCF-7 human breast cancer and U2OS human osteosarcoma cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Primary MEF were prepared as previously described (37). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma Chemical Co., St. Louis, MO, USA) or in DMEM without glucose (Gibco-Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium change (fresh medium) was performed at 0 h, and the cells were cultured for 48 or 60 h without further medium change.
Antibodies
The following commercial antibodies were purchased: actin (mouse monoclonal, Ab-1; Millipore Corp., Billerica, MA, USA), DEC1 (rabbit polyclonal, NB100-1800; Novus Biologicals, Littleton, CO, USA), DEC2 (mouse monoclonal, E-4; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), pAMPK (rabbit monoclonal, 40H9) and AMPK (rabbit monoclonal, 23A3) (both from Cell Signaling Technology, Inc., Danvers, MA, USA).
Western blotting
The cells treated with a medium change, or glucose-depleted medium were lysed using 0.5% NP-40 lysis buffer for western blot analysis. The total cell lysates were run on 12.5% SDS-polyacrylamide gel followed by western blotting using the standard procedure. The WesternBright ECL and WesternBright Sirius kits (BioExpress, Kaysville, UT, USA) were used for antibody detection.
Data quantification
The intensities of the bands detected in Western blotting were quantified using Java based free software ImageJ (NIH, Bethesda, MD, USA).
Results
Rhythmic expression of DEC2 protein and pAMPK in mouse liver, lung and muscle
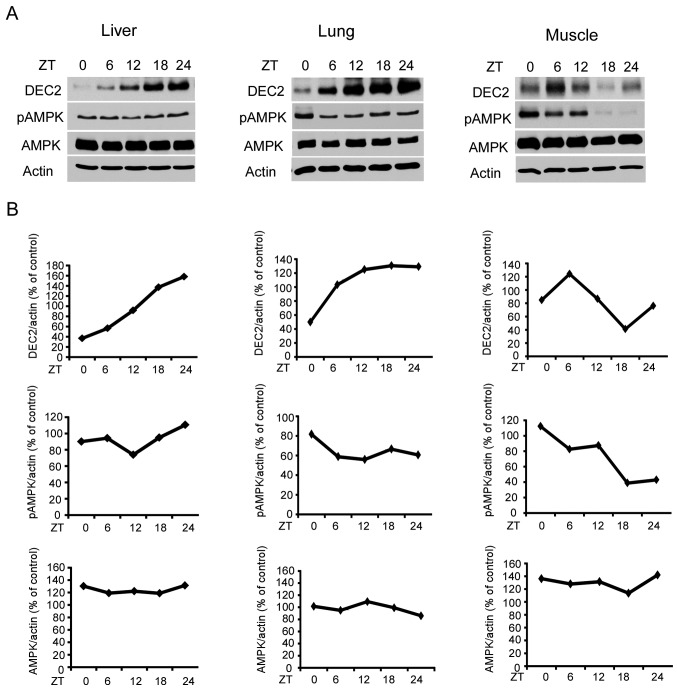
To investigate whether DEC2 protein and pAMPK showed a rhythmic expression in vivo, we performed western blot analysis using samples prepared from mouse liver, lungs and muscles at indicated ZTs. The levels of DEC2 protein and pAMPK showed a rhythmic expression (Fig. 1). A high level of total AMPK was detected in the liver, lungs and muscles at each time-point. These results suggested that DEC2 protein and AMPK activity have a rhythmic expression ubiquitously in various tissues.
Figure 1.
Rhythmic expression of DEC2 protein in mice. (A) Mice were housed under light-dark conditions as described in Materials and methods. Liver, lung and muscle samples were collected from the mice and subjected to western blotting for DEC2, pAMPK, AMPK and actin levels. One representative sample from two independent experiments with similar results is shown. (B) The intensities of DEC2, pAMPK and AMPK bands were quantified and divided by that of actin. AMPK, AMP-activated protein kinase.
Rhythmic expression of DEC2 protein by medium change in U2OS and MCF-7 cells
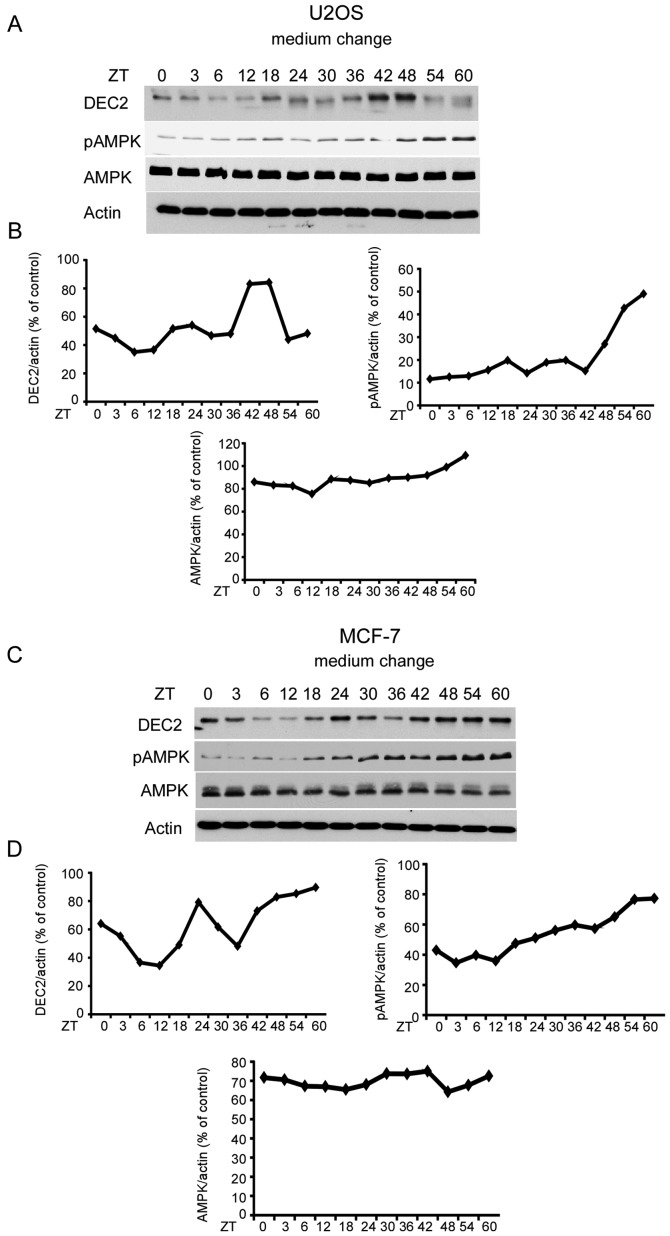
To determine whether medium change affected the rhythmic expression, we examined DEC2 protein levels in U2OS and MCF-7 cells. DEC2 protein levels in U2OS cells increased slightly at 18 and 24 h and the highest levels were observed at 42 and 48 h after medium change (Fig. 2A and B). DEC2 protein levels in MCF-7 cells strongly increased at 24 h and at 42–60 h after medium change (Fig. 2C and D). These results suggested that DEC2 protein is produced in a rhythmic manner by medium change. In addition, pAMPK in U2OS and MCF-7 cells increased in a time-dependent manner until 60 h. A high level of total AMPK was observed in the MCF-7 and U2OS cells with a little alteration by medium change at each time-point. No rhythmic patterns were observed in pAMPK and total AMPK in the two cell lines.
Figure 2.
Rhythmic expression of DEC2 protein in U2OS and MCF-7 cells by the medium change. (A and B) U2OS and (C and D) MCF-7 cells were cultured and subjected to a medium change. The cells were sampled at 0 and 3 h and then every 6 h until 60 h. Samples were subjected to western blotting for DEC2, pAMPK, AMPK and actin levels. One representative sample from two independent experiments with similar results is shown. The intensities of DEC2, pAMPK and AMPK bands were quantified as described above. AMPK, AMP-activated protein kinase.
Rhythmic expression of DEC2 protein by medium change in MEF
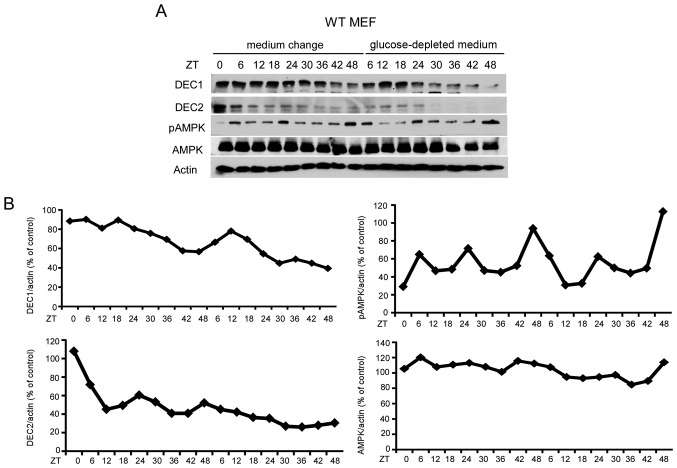
To investigate whether DEC2 protein was produced in a rhythmic manner in MEF, MEF was treated with medium change or glucose-depleted medium. Although DEC2 protein levels in MEF were decreased after medium change or glucose-depleted medium treatment (Fig. 3), rhythmic protein production was observed at 24 and 48 h after medium change. However, an obvious rhythmic pattern of DEC2 protein was not observed under the condition of glucose-depleted medium.
Figure 3.
Rhythmic expression of DEC2 protein in MEF is decreased by medium change. (A) MEF were cultured and subjected to medium change or glucose-depleted medium. The cells were sampled at 0 and 6 h and then every 6 h until 48 h from the time of the medium change (0 h) or glucose-depleted medium. Samples were subjected to western blotting for DEC1, DEC2, pAMPK, AMPK and actin levels. One representative sample from two independent experiments with similar results is shown. (B) The intensities of DEC1, DEC2, pAMPK and AMPK bands were quantified as described above. MEF, mouse embryonic fibroblast; AMPK, AMP-activated protein kinase.
On the other hand, a rhythmic expression of pAMPK was evident in MEF following treatment with medium change and glucose-depleted medium, whereas total AMPK was constitutively produced without a rhythmic pattern under these conditions. In addition, DEC1 protein levels began to decrease gradually ~18 h after treatment with medium change or glucose-depleted medium. Thus, DEC1 in MEF had no rhythmic expression under these conditions.
AMPK activity may be suppressed by DEC2
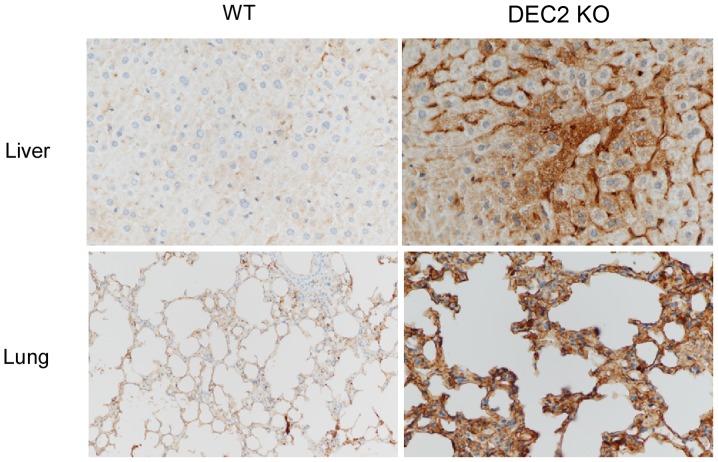
In a recent study, we reported that DEC1 negatively regulates AMPK activity (36). To investigate whether DEC2 regulated AMPK activity, we performed immunohistochemical detection of pAMPK in the liver and lung of DEC2 knockout mice using pAMPK antibody. The representative images of pAMPK immunoreactivity are shown in Fig. 4. Strong pAMPK was detected in the cytoplasm of the hepatocyte and sinusoid, and in the cytoplasm of the alveolar epithelial and stromal cells of DEC2 knockout mice compared with those of wild-type mice. It is therefore possible that DEC2 may suppress AMPK activity.
Figure 4.
pAMPK immunoreactivity was strongly detected in DEC2 knockout mice tissues. Strong pAMPK immunoreactivity was detected in the liver and lung of DEC2 KO mice compared with WT mice. AMPK, AMP-activated protein kinase; KO, knockout; WT, wild-type.
Discussion
In the present study, we used differentiated and undifferentiated cells and tissues to compare the rhythmic expression of DEC2 and AMPK activity, to understand the biological functions of DEC2 in various cell types. DEC2 mRNA is known to show a rhythmic expression in SCN, kidneys, liver, muscles, sarcoma cells and hepatocellular carcinoma HepG2 cells (3,21,25,38,39). However, there are no studies available on the rhythmic expression of DEC2 protein. Therefore, we focused on the expression levels of DEC2 protein. DEC1 was previously shown to have an inverted rhythmic pattern of AMPK activity in mouse liver and heart, binding to the E-box of the LKB1 promoter (36). Thus, DEC1 negatively regulates AMPK activity via LKB1 (36). DEC2 binds to the E-boxes of the DEC1, PER1, PER2, MyoD, sterol regulatory element-binding protein-1c (SREBP-1c) and DNA mismatch repair (MMR) promoter (3,18,40–43). The rhythmic expression of DEC2 in lung had an inverted pattern of pAMPK immunoreactivity. In addition, strong immunostaining of pAMPK was detected in DEC2 knockout mice tissues compared with those of wild-type mice. These results suggest that DEC2 regulates the metabolism of various tissues via AMPK activity.
Medium change in cultured rat-1 fibroblasts induces the rhythmic expression of PER2 and BMAL1, suggesting that medium change resets the circadian clock in cells (44). However, whether DEC2 expression is affected by medium change remains unclear. Therefore, we examined whether DEC2 has a rhythmic expression in cultured cells by medium change. As the endogenous expression of DEC2 was low in fibroblasts, we used two cancer cell-lines (MCF-7 and U2OS), as previously described (21,45,46). We found that DEC2 protein levels showed a rhythmic expression by medium change. These results suggest that medium change induces endogenous DEC2 expression in a rhythmic manner, resetting the circadian clock in U2OS and MCF-7 cells.
We also found that pAMPK increased in cultured cells at a later stage after medium change, although a rhythmic expression was not observed. Considering that cancer cells consume much glucose compared with normal cells, an increase of pAMPK observed in cancer cells may be associated with glucose condition where pAMPK is known to be induced by glucose depletion (32). Of note, pAMPK in MEF showed a rhythmic expression under the condition of medium change or glucose-depleted medium. This result suggests that medium change reset the circadian clock, inducing rhythmic expression. It is possible that the effect on the reset of the circadian clock by medium change may differ in cell types. Future studies are required to clarify the different effects on other types of cells.
The rhythmic expression of DEC2 protein in MEF was not clear compared with that in U2OS and MCF-7 cells. Since MEF are undifferentiated cells, it is possible that DEC2 may show a rhythmic expression in differentiated cells, although the rhythmic expression of DEC2 may decrease in undifferentiated cells.
In conclusion, we have demonstrated a rhythmic expression of DEC2 protein. In addition, pAMPK in MEF has shown the rhythmic expression under the condition of medium change or glucose-depleted medium. These results provide new aspects of DEC2 protein and AMPK activity in culture cells.
Acknowledgements
We would like to thank Shenli Hew from the Department of Clinical Research Center, Wakayama Medical University, for proofreading and editing the manuscript. The present study was supported by grants (CA100302) from the NIH awarded to Y.Z., a Grant-in-Aid for Scientific Research (24590488) from the Ministry of Education, Science, Sport, and Culture of Japan awarded to Y.M., and from the Special Research Project of Wakayama Medical University School of Medicine awarded to F.S.
References
- 1.Merrow M, Spoelstra K, Roenneberg T. The circadian cycle: Daily rhythms from behaviour to genes. EMBO Rep. 2005;6:930–935. doi: 10.1038/sj.embor.7400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 3.Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- 4.Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol. 2007;22(Suppl 2):S1–S8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- 5.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Jr, Rossner MJ, Nishino S, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen G, Brand SR. Sleep in children with cancer: Case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. 2011;19:985–994. doi: 10.1007/s00520-010-0921-y. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Morohashi S, Kato Y, Kijima H. Basic helix-loop-helix transcription factors DEC1 and DEC2 regulate the paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer cells. Int J Mol Med. 2011;27:491–495. doi: 10.3892/ijmm.2011.617. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada K, et al. The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol. 2012;41:1337–1346. doi: 10.3892/ijo.2012.1559. [DOI] [PubMed] [Google Scholar]
- 12.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Kohsaka A, Das P, Hashimoto I, Nakao T, Deguchi Y, Gouraud SS, Waki H, Muragaki Y, Maeda M. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS One. 2014;9:e112811. doi: 10.1371/journal.pone.0112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Lu B, Li RQ, Flavell RA, Taneja R. Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nat Immunol. 2001;2:1040–1047. doi: 10.1038/ni721. [DOI] [PubMed] [Google Scholar]
- 15.Papagerakis S, Zheng L, Schnell S, Sartor MA, Somers E, Marder W, McAlpin B, Kim D, McHugh J, Papagerakis P. The circadian clock in oral health and diseases. J Dent Res. 2014;93:27–35. doi: 10.1177/0022034513505768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baier PC, Brzózka MM, Shahmoradi A, Reinecke L, Kroos C, Wichert SP, Oster H, Wehr MC, Taneja R, Hirrlinger J, et al. Mice lacking the circadian modulators SHARP1 and SHARP2 display altered sleep and mixed state endophenotypes of psychiatric disorders. PLoS One. 2014;9:e110310. doi: 10.1371/journal.pone.0110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato F, Kawamoto T, Fujimoto K, Noshiro M, Honda KK, Honma S, Honma K, Kato Y. Functional analysis of the basic helix-loop-helix transcription factor DEC1 in circadian regulation. Interaction with BMAL1. Eur J Biochem. 2004;271:4409–4419. doi: 10.1111/j.1432-1033.2004.04379.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto T, Noshiro M, Sato F, Maemura K, Takeda N, Nagai R, Iwata T, Fujimoto K, Furukawa M, Miyazaki K, et al. A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem Biophys Res Commun. 2004;313:117–124. doi: 10.1016/j.bbrc.2003.11.099. [DOI] [PubMed] [Google Scholar]
- 19.Noshiro M, Furukawa M, Honma S, Kawamoto T, Hamada T, Honma K, Kato Y. Tissue-specific disruption of rhythmic expression of Dec1 and Dec2 in clock mutant mice. J Biol Rhythms. 2005;20:404–418. doi: 10.1177/0748730405280195. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki K, Kawamoto T, Tanimoto K, Nishiyama M, Honda H, Kato Y. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J Biol Chem. 2002;277:47014–47021. doi: 10.1074/jbc.M204938200. [DOI] [PubMed] [Google Scholar]
- 21.Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Imaizumi T, Imanaka T, Kondo J, Koyanagi S, Noshiro M, Yoshida H, et al. Basic-helix-loop-helix (bHLH) transcription factor DEC2 negatively regulates vascular endothelial growth factor expression. Genes Cells. 2008;13:131–144. doi: 10.1111/j.1365-2443.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 22.Iwata T, Kawamoto T, Sasabe E, Miyazaki K, Fujimoto K, Noshiro M, Kurihara H, Kato Y. Effects of overexpression of basic helix-loop-helix transcription factor Dec1 on osteogenic and adipogenic differentiation of mesenchymal stem cells. Eur J Cell Biol. 2006;85:423–431. doi: 10.1016/j.ejcb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Sato F, Kawamura H, Wu Y, Sato H, Jin D, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Seino H, et al. The basic helix-loop-helix transcription factor DEC2 inhibits TGF-β-induced tumor progression in human pancreatic cancer BxPC-3 cells. Int J Mol Med. 2012;30:495–501. doi: 10.3892/ijmm.2012.1037. [DOI] [PubMed] [Google Scholar]
- 24.Turley H, Wykoff CC, Troup S, Watson PH, Gatter KC, Harris AL. The hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and its expression in human tissues and tumours. J Pathol. 2004;203:808–813. doi: 10.1002/path.1585. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga N, Inoue M, Kusunose N, Kakimoto K, Hamamura K, Hanada Y, Toi A, Yoshiyama Y, Sato F, Fujimoto K, et al. Time-dependent interaction between differentiated embryo chondrocyte-2 and CCAAT/enhancer-binding protein α underlies the circadian expression of CYP2D6 in serum-shocked HepG2 cells. Mol Pharmacol. 2012;81:739–747. doi: 10.1124/mol.111.076406. [DOI] [PubMed] [Google Scholar]
- 26.Sato F, Sato H, Jin D, Bhawal UK, Wu Y, Noshiro M, Kawamoto T, Fujimoto K, Seino H, Morohashi S, et al. Smad3 and Snail show circadian expression in human gingival fibroblasts, human mesenchymal stem cell, and in mouse liver. Biochem Biophys Res Commun. 2012;419:441–446. doi: 10.1016/j.bbrc.2012.02.076. [DOI] [PubMed] [Google Scholar]
- 27.Sato F, Muragaki Y. Circadian expression of DEC1, SMAD3 and SNAIL in human mesenchymal stem cells. J Stem Cells Res, Rev & Rep. 2014;1:1–3. [Google Scholar]
- 28.Oishi K, Koyanagi S, Ohkura N. Circadian mRNA expression of coagulation and fibrinolytic factors is organ-dependently disrupted in aged mice. Exp Gerontol. 2011;46:994–999. doi: 10.1016/j.exger.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima A, Kawamoto T, Honda KK, Ueshima T, Noshiro M, Iwata T, Fujimoto K, Kubo H, Honma S, Yorioka N, et al. DEC1 modulates the circadian phase of clock gene expression. Mol Cell Biol. 2008;28:4080–4092. doi: 10.1128/MCB.02168-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP kinase signaling determines whether c-Jun N-terminal kinase promotes survival or apoptosis during glucose deprivation. Carcinogenesis. 2009;30:529–537. doi: 10.1093/carcin/bgn259. [DOI] [PubMed] [Google Scholar]
- 34.Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato F, Muragaki Y, Zhang Y. DEC1 negatively regulates AMPK activity via LKB1. Biochem Biophys Res Commun. 2015;467:711–716. doi: 10.1016/j.bbrc.2015.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clegg HV, Itahana Y, Itahana K, Ramalingam S, Zhang Y. Mdm2 RING mutation enhances p53 transcriptional activity and p53-p300 interaction. PLoS One. 2012;7:e38212. doi: 10.1371/journal.pone.0038212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noshiro M, Kawamoto T, Furukawa M, Fujimoto K, Yoshida Y, Sasabe E, Tsutsumi S, Hamada T, Honma S, Honma K, et al. Rhythmic expression of DEC1 and DEC2 in peripheral tissues: DEC2 is a potent suppressor for hepatic cytochrome P450s opposing DBP. Genes Cells. 2004;9:317–329. doi: 10.1111/j.1356-9597.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu T, Ni Y, Zhuge F, Sun L, Xu B, Kato H, Fu Z. Significant dissociation of expression patterns of the basic helix-loop-helix transcription factors Dec1 and Dec2 in rat kidney. J Exp Biol. 2011;214:1257–1263. doi: 10.1242/jeb.052100. [DOI] [PubMed] [Google Scholar]
- 40.Choi SM, Cho HJ, Cho H, Kim KH, Kim JB, Park H. Stra13/DEC1 and DEC2 inhibit sterol regulatory element binding protein-1c in a hypoxia-inducible factor-dependent mechanism. Nucleic Acids Res. 2008;36:6372–6385. doi: 10.1093/nar/gkn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azmi S, Ozog A, Taneja R. Sharp-1/DEC2 inhibits skeletal muscle differentiation through repression of myogenic transcription factors. J Biol Chem. 2004;279:52643–52652. doi: 10.1074/jbc.M409188200. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue S, Fujimoto K, Myung J, Hatanaka F, Kato Y, Takumi T. DEC2-E4BP4 heterodimer represses the transcriptional enhancer activity of the EE element in the Per2 promoter. Front Neurol. 2015;6:166. doi: 10.3389/fneur.2015.00166. doi: 10.3389/fneur.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura H, Tanimoto K, Hiyama K, Yunokawa M, Kawamoto T, Kato Y, Yoshiga K, Poellinger L, Hiyama E, Nishiyama M. Human mismatch repair gene, MLH1, is transcriptionally repressed by the hypoxia-inducible transcription factors, DEC1 and DEC2. Oncogene. 2008;27:4200–4209. doi: 10.1038/onc.2008.58. [DOI] [PubMed] [Google Scholar]
- 44.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem. 2002;277:44244–44251. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Sato F, Kawamoto T, Fujimoto K, Morohashi S, Akasaka H, Kondo J, Wu Y, Noshiro M, Kato Y, et al. Anti-apoptotic effect of the basic helix-loop-helix (bHLH) transcription factor DEC2 in human breast cancer cells. Genes Cells. 2010;15:315–325. doi: 10.1111/j.1365-2443.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 46.Hu T, He N, Yang Y, Yin C, Sang N, Yang Q. DEC2 expression is positively correlated with HIF-1 activation and the invasiveness of human osteosarcomas. J Exp Clin Cancer Res. 2015;34:22. doi: 10.1186/s13046-015-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]