Abstract
Helicobacter pylori infects ~50% of the world population, causing chronic gastritis and other forms of cellular damage. The present study assessed the influence of H. pylori on the mRNA expression levels of nuclear factor-κB1 (NFKB1), p38α and tumor necrosis factor-α (TNF-α) in human gastric mucosa in a southern Brazilian population. Human gastric tissue was collected by upper endoscopy and H. pylori diagnosis was performed using a rapid urease test and histological analysis. Total RNA was extracted and purified for subsequent cDNA synthesis and analysis by quantitative polymerase chain reaction (qPCR). The gastric tissue samples were divided into four groups as follows: Normal, inactive chronic gastritis, active chronic gastritis and intestinal metaplasia. The SDHA gene was classified as the most stable when compared with ACTB, GAPDH, B2M and HPRT1 genes, and was therefore selected as the reference gene for qPCR data normalization. TNF-α mRNA expression was significantly higher in samples that were positive for H. pylori and with active chronic gastritis. However, no difference was detected in the mRNA expression levels of NFKB1 and p38α between the groups. The present study concluded that the presence of H. pylori is associated with TNF-α upregulation in human gastric mucosa, but had no effect on NFKB1 and p38α mRNA expression levels.
Keywords: Helicobacter pylori, inflammation, gastritis, gene expression, quantitative polymerase chain reaction
Introduction
Helicobacter pylori (H. pylori) are Gram-negative bacteria that adhere to the surface of gastric mucosa and cause inflammation, but do not invade gastric epithelial cells (1). H. pylori infect ~50% of the world population and the main risk factors include age, ethnicity, gender, geographic location and socioeconomic status (2). Infection with H. pylori is considered the major cause of active chronic gastritis and also serves an notable role in peptic ulcers (3).
Since the discovery of H. pylori, it has been strongly associated with the development of gastric cancer (4). H. pylori was the first bacterial species to be recognized by the International Agency for Research on Cancer as a group I carcinogen (5). The development of cancer in H. pylori infected individuals may be through the following possible mechanisms: i) The production of mutagenic radicals as an inflammatory response to H. pylori infection; ii) the reduction of antioxidants in mucosa; and iii) the induction of a hyperproliferative state (6,7). However, only a small percentage of infected individuals will develop neoplasia (1–3%) due to specific interactions between the host and pathogen, which are dependent on specific bacteriological factors and/or inflammatory responses regulated by host genes (8,9).
H. pylori-induced gastritis can lead to other types of cellular damage (10). The most prevalent form of gastric neoplasia is intestinal-type adenocarcinoma, which evolves through a series of events initiated by the transition of normal mucosa to superficial chronic gastritis, which subsequently results in atrophic gastritis, intestinal metaplasia, and eventually to dysplasia and neoplasia (11). Various inflammatory biomarkers, including the tumor necrosis factor-α (TNF-α), nuclear factor-κB (NF-κB) and interleukins (ILs), can be used to track the progress of diseases, as well as assist in the development of novel anti-inflammatory drugs for the treatment and prevention of cancer (8). Pathogenic stimuli induce the expression of TNF-α, which in turn induces proteases and other mediators responsible for inflammatory responses. TNF-α has been associated with the various steps involved in tumorigenesis, including cell transformation, invasion, proliferation, survival, promotion, angiogenesis and metastasis (8). The activation of TNF receptor 1 (TNFR1) by TNF-α can result in the activation of NF-κB as a result of cellular responses (12).
NF-κB serves an essential role in inflammation and innate immunity, and it is becoming increasingly recognized for its crucial role in the initiation and progression of cancer, operating in numerous cell signaling pathways (13). NF-κB regulates the expression of various substances associated with inflammation and is inactive in the majority of cells, but it exists in an activated form in cancer cells (14). This activation is induced by a wide variety of carcinogens and inflammatory stimuli (14,15). In the classic NF-κB signaling pathway, lipopolysaccharides, TNF-α or IL-1 activate TNFR and IL-1 receptors (13).
p38 is a mitogen-activated protein kinase (MAPK) involved in the regulation of inflammatory cytokine biosynthesis (16) and stress responses, such as heat shock or infection (17,18). Protein kinases are the main regulators in pathways of embryogenesis, cell differentiation, proliferation and cell death (19). The p38 MAPK family consists of four identified isoforms, including p38α, p38β, p38γ and p38δ, encoded by the genes MAPK14, MAPK11, MAPK12 and MAPK13, respectively. In human tissues, the most abundant MAPK is p38α (16,20). Although the activation of p38α is normally associated with anti-proliferative functions, studies indicate that it has the ability to upregulate cell proliferation in human tumors and cancer cell lines (20,21).
The use of quantitative polymerase chain reaction (qPCR) to compare mRNA levels between biopsies from different individuals and disease states requires meticulous normalization. For the correction of results from different samples and experimental conditions, the use of an endogenous reference is essential (22). In a number of studies, it has been demonstrated that although common reference genes are considered to be stable and secure in various tissues, this is often not the case (23,24). Only one previous study has validated reference genes for gastric samples in a Western population of patients with adenocarcinoma (25).
Currently, the association of inflammation, innate immunity and cancer is widely accepted (26); however, the cellular and molecular mechanisms that mediate these processes remain unknown. The present study aimed to evaluate the mRNA levels of TNF-α, NFKB1 and p38α in human gastric mucosa samples, and to investigate the influence of H. pylori on the expression of these genes in a southern Brazilian population. In order to perform gene expression analysis, the suitability of five reference genes were assessed to determine their suitability for use in qPCR normalization. Although the five genes have been widely studied (27,28), there are presently no studies evaluating their association with H. pylori infection and human gastric inflammation.
Materials and methods
Sample acquisition
Human gastric tissues were obtained by upper endoscopy from 79 adult patients, including 49 women and 30 men (aged 47.33±14.31 years), who were admitted to the Endoscopy Service at Hospital Bruno Born (Lajeado, Brazil) between October 2013 and April 2014 with symptoms of gastritis, including epigastric pain, nausea, vomiting, bloating, belching, heartburn, halitosis and flatulence (29). The present study was approved by the local ethics committee (Univates, Lajeado, Brazil; CEP 353.624) and written informed consent was obtained from each patient prior to sample collection. Exclusion criteria included coagulation disorders (including patients with problems preventing gastric biopsy), and the use of anti-inflammatory drugs, antibiotics or proton pump inhibitors.
Two biopsy samples (~3 mm) were obtained from the lesser curvature of the gastric antrum, one of which was used for the rapid urease test (RNA Laboratórios, Cascavel, Brazil) and the other was placed in RNAlater Stabilization Solution (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for posterior RNA extraction, cDNA synthesis and gene expression analysis. In addition, two further samples of ~3 mm were collected by the gastroenterologist for routine histological analyses. Briefly, the samples were formalin-fixed, paraffin-embedded (both Allkimia, Campinas, SP, Brazil) and stained with Giemsa (Quimica Especializada Erich Ltda, São Paulo, SP, Brazil) for H. pylori detection under the Leica DM500 microscope (Leica Microsystems GmbH, Wetzlar, Germany).
H. pylori infection diagnosis and histological analysis
The rapid urease test is based on the principle that the enzyme urease produced by H. pylori hydrolyses urea into ammonia (30). A biopsy sample was introduced immediately after collection into a tube containing the rapid urease test. The consequent rise in the pH of the medium in the tube as a result of ammonia production was detected by a phenol red indicator, changing the medium color and indicating the presence of H. pylori. When the color change occurred during the first 24 h, the test was considered positive (31). H. pylori diagnosis was performed based on the results of the rapid urease, and the samples were considered positive following confirmation with the modified Giemsa staining procedure (32). The samples were classified according to a histological analysis and observation under the Leica DM500 microscope, using the Sydney classification system (33). The samples were divided into groups according to the severity of changes observed in the histological examination, as follows: i) normal (absence of inflammation); ii) inactive chronic gastritis (inflammation without neutrophils); iii) chronic gastritis (inflammation with neutrophils); and iv) the presence of intestinal metaplasia (a precancerous lesion).
Total RNA extraction
Samples were processed in a Turrax-like tissue homogenizer (Ultra Stirrer; Stanhope-Seta, Chertsey, Surrey, UK) with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The total RNA extraction method by TRIzol was an adaptation from the original method described by Chomczynski and Sacchi (34). Following extraction, the RNA was purified using the RNAspin Mini kit (GE Healthcare Life Sciences, Little Chalfont, UK) according to the manufacturer's instructions and stored at −80°C. The concentration and purity of the RNA was assessed by L-Quant spectrophotometer (Loccus Biotecnologia, Cotia, Brazil) at 260 and 280 nm wavelengths. A ratio of ~2.0 was considered appropriate for RNA purity.
Synthesis of cDNA
cDNA was synthesized from 1 µg total RNA using the Superscript III First Strand Synthesis System SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.) and oligo-dT primers, according to the manufacturer's instructions, and stored at −20°C.
Primer design
The primers used for the amplification of cDNA fragments specific for p38α, TNF-α and NFKB1 were designed using the published sequence of each gene and the online tool Primer3 (35). To select a reference gene, five reference genes commonly used in gene expression studies were examined to identify the most appropriate gene for the current experiment. The primers were supplied by Laboratory of Molecular Biology, Endocrinology and Tumors (Federal University of Rio Grande do Sul, Porto Alegre, Brazil) (27). Each primer was synthesized by Invitrogen Brazil, Ltd. (São Paulo, Brazil). A dissociation curve was created for each pair of primers in order to confirm reaction specificity. The melting temperature (Tm) is specific to each amplicon. The primer sequences, product length and mean Tm of each gene are presented in Table I.
Table I.
Primer sequences, product lengths and mean Tm of the studied genes.
| Gene symbol | Primer sequence (5′-3′) | NCBI no. | Product length (bp) | Mean Tm (°C) |
|---|---|---|---|---|
| NFKB1 | ||||
| Sense | ACACCGTGTAAACCAAAGCC | NM_003998.3 | 209 | 82.71 |
| Antisense | CAGCCAGTGTTGTGATTGCT | |||
| p38α | ||||
| Sense | CAGTGGGATGCATAATGGCC | NM_001315.2 | 243 | 82.12 |
| Antisense | GCATCTTCTCCAGCAAGTCG | |||
| TNF-α | ||||
| Sense | CCCTGGTATGAGCCCATCTATC | NM_000594.3 | 120 | 84.82 |
| Antisense | AAAGTAGACCTGCCCAGACTCG | |||
| SDHA | ||||
| Sense | TGGTTGTCTTTGGTCGGG | NM_004168.2 | 85 | 81.69 |
| Antisense | GCGTTTGGTTTAATTGGAGGG | |||
| ACTB | ||||
| Sense | CTGGAACGGTGAAGGTGACA | NM_001101.3 | 140 | 82.31 |
| Antisense | AAGGGACTTCCTGTAACAATGCA | |||
| B2M | ||||
| Sense | CTATCCAGCGTACTCCAAAG | NM_004048.2 | 165 | 78.88 |
| Antisense | ACAAGTCTGAATGCTCCACT | |||
| GAPDH | ||||
| Sense | CTTTGTCAAGCTCATTTCCTGG | NM_002046.3 | 133 | 84.70 |
| Antisense | TCTTCCTCTTGTGCTCTTGC | |||
| HPRT1 | ||||
| Sense | AGATGGTCAAGGTCGCAAG | NM_000194.2 | 128 | 79.78 |
| Antisense | GTATTCATTATAGTCAAGGGCATATCC |
NFKB1, nuclear factor-κB1; TNF-α, tumor necrosis factor-α; SDHA, succinate dehydrogenase complex, subunit A, flavoprotein; ACTB, β-actin; B2M, β2-microglobulin; HPRT1, hypoxanthine phosphoribosyltransferase 1; NCBI, National Center for Biotechnology Information; Tm, melting temperature.
qPCR conditions
The amplification of cDNA and relative quantification was performed by qPCR with the StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). A Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen; Thermo Fisher Scientific, Inc.) with a total volume of 25 µl, including 12.5 µl SuperMix, 0.5 µl 50 µmol/l Rox reference dye, 0.3 µl of each primer (10 µmol/l forward and 10 µmol/l reverse), 9.4 µl H2O and 2.0 µl 1:20-diluted template cDNA. Duplicate measurements were recorded according to the following protocol: Initial incubation for 3 min at 94°C, followed by 45 cycles of 30 sec denaturation at 94°C, 30 sec annealing at 55°C and 30 sec extension at 60°C. Standard curves were constructed by plotting the cycle threshold values of the qPCR performed on a five-fold dilution series of cDNA standards.
Statistical analysis
Data were tabulated and analyzed with descriptive statistics using SPSS software version 20.0 (IBM SPSS, Armonk, NY, USA). To confirm the normality of gene expression values, the Kolmogorov-Smirnov test was performed. The Mann-Whitney U test was performed to compare two groups, and the Kruskal-Wallis test followed by Dunn's multiple comparison test were performed to compare more than two groups. P<0.05 was considered to indicate a statistically significant difference.
Gene expression variability for reference gene selection was evaluated using NormFinder algorithm, which is a Visual Basic application for Microsoft Excel that calculates the average expression stability of each studied gene (36).
Results
Classification according to H. pylori infection
The samples were classified as positive (HP+) or negative (HP−) for H. pylori infection, according to the rapid urease test and histological exam results (Fig. 1). Table II presents the principal characteristics of the study population with regards to the H. pylori infection groups. The participants were from the region of Taquari Valley, State of Rio Grande do Sul (Brazil) and their mean age was 47.33±14.31 year.
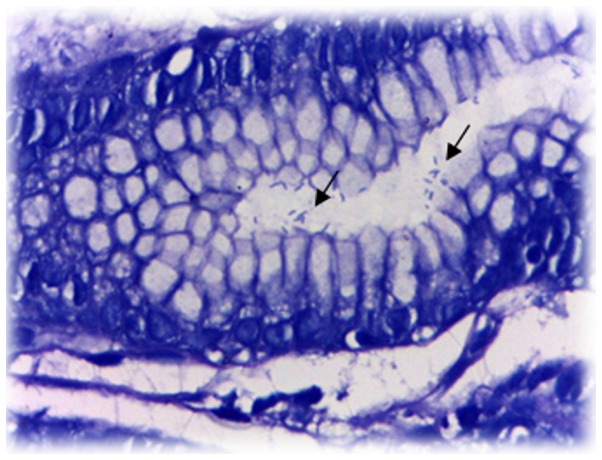
Figure 1.
Representative image of histological examination of human gastric tissue positive for H. pylori, identified by Giemsa staining, highlighted by the black arrows.
Table II.
Main characteristics of the study population (n=79) according to HP infection.
| Parameter | HP+ (n=27) | HP− (n=52) |
|---|---|---|
| Age (years) | ||
| Mean ± standard deviation | 47.93±12.17 | 47.02±15.40 |
| Range | 22–64 | 19–81 |
| Gendera | ||
| Female (n=49) | 15 (30.61) | 34 (69.39) |
| Male (n=30) | 12 (40.00) | 18 (60.00) |
| Previous HP treatmenta (n=29) | 6 (20.69) | 23 (79.31) |
Values expressed as case numbers (% of the row total). HP, H. pylori.
Classification according to histological analysis
According to the severity of changes observed on histological examination, the samples were classified into the following groups: Normal; inactive chronic gastritis (ICG); active chronic gastritis (ACG); and intestinal metaplasia (IM). The group classification is presented in Table III.
Table III.
Main characteristics of the study population according to histological analysis.
| Parameter | Normal (n=17) | ICG (n=29) | ACG (n=25) | IM (n=8) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± standard deviation | 45.00±15.47 | 46.90±15.95 | 46.80±11.93 | 55.50±11.67 |
| Range | 21–75 | 19–81 | 22–64 | 35–70 |
| Gendera | ||||
| Female | 13 (76.47) | 19 (65.52) | 15 (60.00) | 2 (25.00) |
| Male | 4 (23.53) | 10 (34.48) | 10 (40.00) | 6 (75.00) |
| Previous HP treatmenta | 5 (29.41) | 8 (27.59) | 6 (24.00) | 5 (62.50) |
| HP+ | 0 (0.00) | 0 (0.00) | 25 (100.0) | 2 (25.00) |
Values expressed as case numbers and (% of group total). ICG, inactive chronic gastritis; ACG, active chronic gastritis; IM, intestinal metaplasia; HP, Helicobacter pylori.
All samples classified as ACG were diagnosed as HP+. In the IM group, 62.5% (n=5) of patients received previous HP treatment, 25.0% (n=2) were HP+, while 12.5% of this group (n=1) had no prior report of H. pylori infection. The groups classified as normal and ICG were diagnosed as HP−.
Reference gene selection
For reference gene selection, qPCR was performed on 39 samples (this is the number of samples that had been collected at the time and was deemed sufficient for this analysis), divided into the following groups: Normal (n=11), ICG (n=11), ACG (n=12) and IM (n=5).
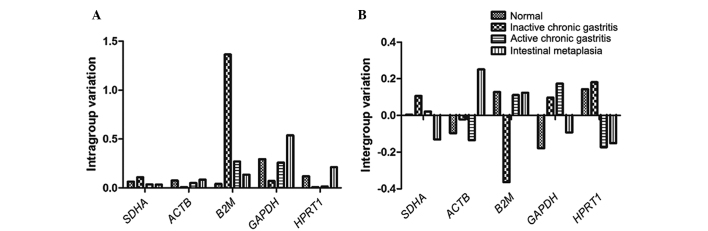
The expression stability of a candidate gene was indicated by its stability value, with a smaller value indicating a more stable gene. The stability values of the candidate genes obtained using NormFinder software are presented in Table IV. NormFinder analysis demonstrated that the succinate dehydrogenase complex, subunit A, flavoprotein (SDHA) was the most stable gene, with the lowest stability value and smallest intergroup variation (Fig. 2), and thus SDHA was selected for normalization of the subsequent data. The most stable combination of the two genes was SDHA plus ACTB (stability value, 0.112). The qPCR assay of each gene was analyzed in the linear phase, and a linear function of the relative fluorescence vs. cycle number was fitted with a typical R2 value of >9.
Table IV.
Candidate reference genes for normalization of quantitative polymerase chain reaction in human gastric non-neoplastic tissues, according to their stability, as calculated by NormFinder software.
| Rank | Gene | Stability value |
|---|---|---|
| 1 | SDHA | 0.149 |
| 2 | ACTB | 0.180 |
| 3 | HPRT1 | 0.231 |
| 4 | GAPDH | 0.265 |
| 5 | B2M | 0.270 |
ACTB, β-actin; B2M, β2-microglobulin; SDHA, succinate dehydrogenase complex, subunit A, flavoprotein; HPRT1, hypoxanthine phosphoribosyltransferase 1.
Figure 2.
(A) Intra- and (B) intergroup variation of five reference genes in human gastric samples as calculated by NormFinder, identifying SDHA as the gene with the smallest, and B2M with the highest, variation. SDHA, succinate dehydrogenase complex, subunit A, flavoprotein; ACTB, β-actin; B2M, β2-microglobulin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPRT1, hypoxanthine phosphoribosyltransferase 1.
Expression of NFKB1, p38α and TNF-α
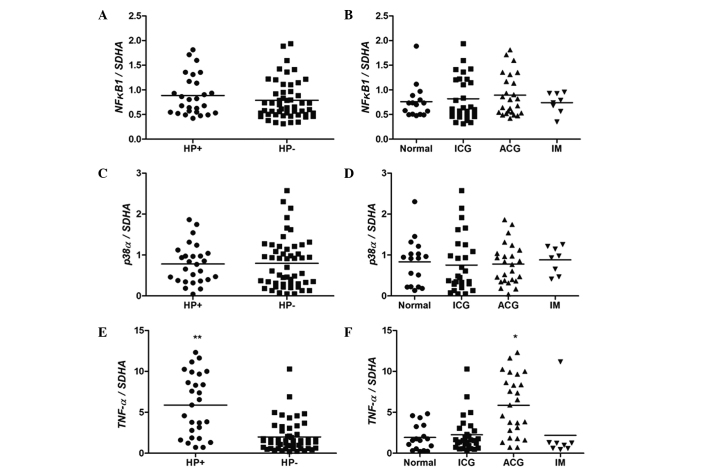
As presented in Fig. 3, no significant differences in NFKB1 and p38α levels were observed among the groups with regards to the H. pylori infection status (P>0.05; Mann-Whitney test; Fig. 3A and C) and histological analysis (P>0.05; Kruskal-Wallis followed by Duncan's multiple comparisons test; Fig. 3B and D). However, a statistically significant difference was observed between the expression of TNF-α in groups with and without H. pylori infection (P<0.0001; Fig. 3E). Considering the results of histological analysis, the ACG group demonstrated higher expression levels of TNF-α when compared with the other groups (P<0.01; Fig. 3F).
Figure 3.
mRNA expression levels of (A and B) NFKB1, (C and D) p38α and (E and F) TNF-α in human gastric mucosa, according to HP infection and histological analysis statuses. *P<0.01 vs. normal (Kruskal-Wallis followed by Duncan's multiple comparisons test), **P<0.0001 vs. HP− (Mann-Whitney U test). ICG, inactive chronic gastritis; ACG, active chronic gastritis; IM, intestinal metaplasia; SDHA, succinate dehydrogenase complex, subunit A, flavoprotein; HP, H. pylori.
Discussion
In the present study, the expression levels of TNF-α were increased in H. pylori-positive samples, regardless of the patient gender. The group classified as ACG, when compared with other groups, also demonstrated an increase in TNF-α expression levels. This may be explained by the fact that all samples in this group tested positive for H. pylori infection. These results are in accordance with the study conducted by Pimentel-Nunes et al (37), who observed increased levels of TNF-α mRNA in gastric biopsies infected with H. pylori. It is known that H. pylori infection leads to inflammation of the stomach, associated with the production of inflammatory cytokines, such as TNF-α, as demonstrated by studies observing increased levels of TNF-α in infected patients (38,39). However, a study by Abbas et al (40) conducted on patients with liver cirrhosis observed no statistically significant difference in the levels of TNF-α expression when comparing positive and negative H. pylori groups, or groups with moderate and severe gastropathy (41).
TNF-α is a key cytokine in tumor promotion; therefore, it is important to clarify how the pro-inflammatory cytokines induced by H. pylori are involved in the development of gastric cancer (42). Previous studies have demonstrated the presence of the gene encoder of the TNF-α inducer protein (Tipα) in the H. pylori genome, which functions as a carcinogenic factor by inducing the gene expression of TNF-α and activating NF-κB (41,42).
In the current study, no significant difference was observed in NF-κB expression levels between groups, which is similar to the results observed in the study of Pimentel-Nunes et al (37). Furthermore, Naito and Yoshikawa (43) reported that H. pylori activates the NF-κB gene in epithelial cells of the gastric mucosa in vitro and in vivo. In addition, Huang et al (44) demonstrated that H. pylori induces phosphorylation of the proteins Iκβα (an NK-κB inhibitor) and RELA (also known as p65) that activate RELA nuclear translocation, making H. pylori an activator of the NF-κB signaling pathway. Considering the results of the present study, it is not possible to determine whether the NF-κB pathway is associated with increased levels of TNF-α, since the methods used could not verify the phosphorylation levels and, consequently, the protein activation.
Ferrand et al (45) demonstrated an increase in TNF-α expression levels in response to H. pylori infection in mouse gastric epithelial cells in vitro. This increase was associated with the activation of NF-κB and nuclear translocation of p65. The same study observed the influence of H. pylori on the NF-κB pathway and its involvement in the migration of mesenchymal stem cells, which may be associated with gastric pathophysiology and carcinogenesis (45). In the current study, a total of 87.5% of the IM samples, which is considered a precancerous lesion, had a history of infection with H. pylori (previous HP treatment or HP+ status), suggesting the involvement of H. pylori in the development of these lesions.
Kim et al (46) demonstrated that H. pylori induces the activation of p38 MAPK in vitro, which reduces the expression of the MucA gene, responsible for the production of mucus, by promoting apoptosis in gastric epithelial cells. Seo et al (47) concluded that MAPKs, such as p38 and ERK, can control the activation of NF-κB in gastric epithelial cells infected by H. pylori. However, these studies were analyzed at the protein level, not the mRNA level. In the present study, no differences in p38 expression were observed. For the activation of p38 translocation to the nucleus and the stimulation of transcription factors, p38 must be phosphorylated. Thus, protein analysis is required to detect this phosphorylation.
Among the p38 isoforms, the p38α gene was selected for analysis, since it is the most abundant isoform in tissues and also the most widely studied. However, the isoform p38δ is detected primarily in endocrine tissue (48), which may form the subject of further studies due to its presence in gastric epithelium endocrine cells. O'Callaghan et al (49) reported the importance of studying p38δ as a result of evidence demonstrating that it may act as a promoter and as a tumor suppressor.
The results of the present study demonstrated that infection with H. pylori is associated with active inflammation of the gastric tissue, and 100% of the H. pylori-positive samples were also positive for active gastritis. The most severe type of lesion observed in the samples from the current study was intestinal metaplasia. Of these, 25% tested positive for H. pylori, and only one sample (12.5%) had no history of infection by H pylori. Although H. pylori is recognized as the main cause of chronic gastritis, other factors such as smoking, alcoholism, anxiety, stress, poor diet and lifestyle may contribute to the onset of clinical manifestations (50).
In the present study, five candidate genes were analyzed for their potential to be used as the reference gene in qPCR with human non-neoplastic gastric samples obtained by upper endoscopy. NormFinder was selected to compare candidate genes due to its ability to estimate inter and intragroup variation and consequently calculate a stability value (36). A value closer to zero indicates greater stability of gene expression. Considering that an arbitrary cut-off value of 0.15 indicates acceptable stability of the reference gene (51), the present study concluded that SDHA was the most appropriate gene for qPCR normalization compared with ACTB, B2M, HPRT1 and GAPDH. Furthermore, the combination of SDHA and ACTB demonstrated a lower stability value, suggesting that it is a more stable combination of genes for the normalization of qPCR sample results.
Although ACTB has a low intragroup variation (similar to SDHA), its higher intergroup variation raises the stability value, thus it can not be suggested as a reference gene for the experiments of the present study. ACTB has been traditionally regarded as a reference gene in quantifying expression levels in tumors (23). However, accumulating evidence indicates that ACTB is dysregulated in gastric and a number of other types of cancer (52–54). In the present study, the analysis was focused on non-neoplastic tissues; however, ACTB intergroup variation was observed to be higher in the samples with intestinal metaplasia, which is considered a precancerous lesion (55), thus affecting the gene stability value. The study by Wisnieski et al (25) analyzing normal and adenocarcinoma samples of gastric tissue and cell lines, determined that ACTB was the most appropriate reference gene for all tissues; however, SDHA was not included in their analysis.
GAPDH is one of the most commonly used reference genes and it is considered a ‘classical’ reference gene in the majority of scientific studies (51,56,57). Numerous studies of gene expression in human gastric mucosa use GAPDH as the reference gene (58,59), despite the knowledge that GAPDH is upregulated in stomach cancer (24). The present study demonstrated that GAPDH was highly variable between groups, and was therefore not recommended as a reference gene.
SDHA has been previously investigated as a reference gene in numerous studies with different experimental conditions. These studies determined that SDHA was the best reference gene compared with other frequently used reference genes (27,60,61). According to the results of the present and previous studies, SDHA may be used as a reference gene for qPCR in the conditions described, as a result of its stability. Therefore, the inclusion of SDHA as a candidate gene for further studies of reference gene selection with different conditions and samples must be considered.
Chronic gastritis is a prevalent disease in the world population, and its association with H. pylori infection is well-described (5). The study of H. pylori, its virulence factors and resistance to therapy are crucial in order to improve treatments aiming to eradicate infection with these bacteria. Understanding the genes associated with the inflammatory and proliferative pathways in different populations may facilitate the development of effective treatments and prevention of gastric disease, aid in the reduction of side effects and increase the efficacy of current treatments.
In conclusion, the present study demonstrated that H. pylori infection increases the expression of TNF-α mRNA expression levels in human gastric mucosa, but does not have an effect on the expression of p38α and NFKB1. It also demonstrated that H pylori infection is associated with chronic active gastritis and the presence of intestinal metaplasia in a southern Brazilian population. The SDHA was observed to be the most appropriate reference gene for qPCR in the current study, as a result of its stability. Therefore, the results support the inclusion of SDHA as a candidate gene for further studies of reference gene selection with different conditions and samples. Future studies are required to elucidate the association of NFKB1 and p38α with H. pylori infection.
Acknowledgements
The present study was supported by the Selection FAPERGS/CAPES 14/2012 Masters Scholarship Program. The authors also wish to thank Hospital Bruno Born for collaboration in this research.
References
- 1.Clyne M, Drumm B. Adherence of Helicobacter pylori to primary human gastrointestinal cells. Infect Immun. 1993;61:4051–4057. doi: 10.1128/iai.61.10.4051-4057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Gastroenterology Organisation: World Gastroenterology Organisation Global Guideline: Helicobacter pylori in developing countries. J Clin Gastroenterol. 2011;45:383–388. doi: 10.1097/MCG.0b013e31820fb8f6. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89(Suppl):S116–S128. [PubMed] [Google Scholar]
- 4.Suzuki H, Hibi T, Marshall BJ. Helicobacter pylori: Present status and future prospects in Japan. J Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testerman TL, Morris J. Beyond the stomach: An updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781–12808. doi: 10.3748/wjg.v20.i36.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- 7.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19(Suppl 1):S37–S43. [PubMed] [Google Scholar]
- 8.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 10.Hahm KB, Song YJ, Oh TY, Lee JS, Surh YJ, Kim YB, Yoo BM, Kim JH, Han SU, Nahm KT, et al. Chemoprevention of Helicobacter pylori-associated gastric carcinogenesis in a mouse model: Is it possible? J Biochem Mol Biol. 2003;36:82–94. doi: 10.5483/BMBRep.2003.36.1.082. [DOI] [PubMed] [Google Scholar]
- 11.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process - First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 12.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 13.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Shishodia S, Aggarwal BB. Nuclear factor-kappaB: A friend or a foe in cancer? Biochem Pharmacol. 2004;68:1071–1080. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 17.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 19.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 21.Nebreda AR, Porras A. p38 MAP kinases: Beyond the stress response. Trends Biochem Sci. 2000;25:257–260. doi: 10.1016/S0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo C, Liu S, Wang J, Sun MZ, Greenaway FT. ACTB in cancer. Clin Chim Acta. 2013;417:39–44. doi: 10.1016/j.cca.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, Brittner B, Ludwig B, Schilling M. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101–109. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Wisnieski F, Calcagno DQ, Leal MF, dos Santos LC, Gigek CO, Chen ES, Pontes TB, Assumpção PP, de Assumpção MB, Demachki S, et al. Reference genes for quantitative RT-PCR data in gastric tissues and cell lines. World J Gastroenterol. 2013;19:7121–7128. doi: 10.3748/wjg.v19.i41.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza AF, Brum IS, Neto BS, Berger M, Branchini G. Reference gene for primary culture of prostate cancer cells. Mol Biol Rep. 2013;40:2955–2962. doi: 10.1007/s11033-012-2366-5. [DOI] [PubMed] [Google Scholar]
- 28.Santin AP, Souza AF, Brum IS, Furlanetto TW. Validation of reference genes for normalizing gene expression in real-time quantitative reverse transcription PCR in human thyroid cells in primary culture treated with progesterone and estradiol. Mol Biotechnol. 2013;54:278–282. doi: 10.1007/s12033-012-9565-0. [DOI] [PubMed] [Google Scholar]
- 29.Schubert TT, Schubert AB, Ma CK. Symptoms, gastritis, and Helicobacter pylori in patients referred for endoscopy. Gastrointest Endosc. 1992;38:357–360. doi: 10.1016/S0016-5107(92)70432-5. [DOI] [PubMed] [Google Scholar]
- 30.Berry V, Sagar V. Rapid urease test to diagnose Helicobacter pylori infection. JK Science. 2006;8:86–88. [Google Scholar]
- 31.Ornellas LC, Cury M, de Lima VM, Ferrari Junior AP. Evaluation of rapid urease test stored in refrigerator. Arq Gastroenterol. 2000;37:155–157. doi: 10.1590/s0004-28032000000300003. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 32.Wabinga HR. Comparison of immunohistochemical and modified Giemsa stains for demonstration of Helicobacter pylori infection in an African population. Afr Health Sci. 2002;2:52–55. [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 35.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 36.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 37.Pimentel-Nunes P, Gonçalves N, Boal-Carvalho I, Afonso L, Lopes P, Roncon-Albuquerque R, Jr, Henrique R, Moreira-Dias L, Leite-Moreira AF, Dinis-Ribeiro M. Helicobacter pylori induces increased expression of Toll-like receptors and decreased Toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesis. Helicobacter. 2013;18:22–32. doi: 10.1111/hel.12008. [DOI] [PubMed] [Google Scholar]
- 38.Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao C, Lu X, Bu X, Zhang N, Wang W. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer. 2010;10:419. doi: 10.1186/1471-2407-10-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbas Z, Yakoob J, Usman MW, Shakir T, Hamid S, Jafri W. Effect of Helicobacter pylori and its virulence factors on portal hypertensive gastropathy and interleukin (IL)-8, IL-10, and tumor necrosis factor-alpha levels. Saudi J Gastroenterol. 2014;20:120–127. doi: 10.4103/1319-3767.129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suganuma M, Kurusu M, Okabe S, Sueoka N, Yoshida M, Wakatsuki Y, Fujiki H. Helicobacter pylori membrane protein 1: A new carcinogenic factor of Helicobacter pylori. Cancer Res. 2001;61:6356–6359. [PubMed] [Google Scholar]
- 42.Suganuma M, Kurusu M, Suzuki K, Nishizono A, Murakami K, Fujioka T, Fujiki H. New tumor necrosis factor-alpha-inducing protein released from Helicobacter pylori for gastric cancer progression. J Cancer Research Clin Oncol. 2005;131:305–313. doi: 10.1007/s00432-004-0652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323–336. doi: 10.1016/S0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Lv B, Zhang S, Dai Q, Chen BB, Meng LN. Effects of radix curcumae-derived diterpenoid C on Helicobacter pylori-induced inflammation and nuclear factor kappa B signal pathways. World J Gastroenterol. 2013;19:5085–5093. doi: 10.3748/wjg.v19.i31.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrand J, Lehours P, Schmid-Alliana A, Mégraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-κB-dependent pathway. PLoS One. 2011;6:e29007. doi: 10.1371/journal.pone.0029007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Seo JH, Kim KH. The effect of p38 mitogen-activated protein kinase on mucin gene expression and apoptosis in Helicobacter pylori-infected gastric epithelial cells. Ann NY Acad Sci. 2003;1010:90–94. doi: 10.1196/annals.1299.014. [DOI] [PubMed] [Google Scholar]
- 47.Seo JH, Lim JW, Kim H. Differential role of ERK and p38 on NF-κB activation in Helicobacter pylori-infected gastric epithelial cells. J Cancer Prev. 2013;18:346–350. doi: 10.15430/JCP.2013.18.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, Delaney J, Cole CN, Chan-Hui PY, Mantlo N, et al. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:23668–23674. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 49.O'Callaghan C, Fanning LJ, Barry OP. p38δ MAPK: Emerging roles of a neglected isoform. Int J Cell Biol. 2014;2014:272689. doi: 10.1155/2014/272689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ddine LC, Ddine CC, Rodrigues CC, Kirsten VR, Colpo E. Factors associated with chronic gastritis in patients with presence and absence of Helicobacter pylori. Arq Bras Cir Dig. 2012;25:96–100. doi: 10.1590/S0102-67202012000200007. [DOI] [PubMed] [Google Scholar]
- 51.Pérez R, Tupac-Yupanqui I, Dunner S. Evaluation of suitable reference genes for gene expression studies in bovine muscular tissue. BMC Mol Biol. 2008;9:79. doi: 10.1186/1471-2199-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le PU, Nguyen TN, Drolet-Savoie P, Leclerc N, Nabi IR. Increased beta-actin expression in an invasive moloney sarcoma virus-transformed MDCK cell variant concentrates to the tips of multiple pseudopodia. Cancer Res. 1998;58:1631–1635. [PubMed] [Google Scholar]
- 53.Micheva KD, Vallée A, Beaulieu C, Herman IM, Leclerc N. Beta-actin is confined to structures having high capacity of remodelling in developing and adult rat cerebellum. Eur J Neurosci. 1998;10:3785–3798. doi: 10.1046/j.1460-9568.1998.00391.x. [DOI] [PubMed] [Google Scholar]
- 54.Popow A, Nowak D, Malicka-Blaszkiewicz M. Actin cytoskeleton and beta-actin expression in correlation with higher invasiveness of selected hepatoma Morris 5123 cells. J Physiol Pharmacol. 2006;57:111–123. [PubMed] [Google Scholar]
- 55.Miao XP, Li JS, Li HY, Zeng SP, Zhao Y, Zeng JZ. Expression of ornithine decarboxylase in precancerous and cancerous gastric lesions. World J Gastroenterol. 2007;13:2867–2871. doi: 10.3748/wjg.v13.i20.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te Meerman GJ, ter Elst A. Evidence based selection of housekeeping genes. PLoS One. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. Biotechniques. 2000;29:332–337. doi: 10.2144/00292rv02. [DOI] [PubMed] [Google Scholar]
- 58.de Souza CR, Leal MF, Calcagno DQ, Costa Sozinho EK, Borges BN, Montenegro RC, Dos Santos AK, Dos Santos SE, Ribeiro HF, Assumpção PP, et al. MYC deregulavtion in gastric cancer and its clinicopathological implications. PLoS One. 2013;8:e64420. doi: 10.1371/journal.pone.0064420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuk K, Peczek L, Stec-Michalska K, Medrek M, Nawrot B. SATB1 expression in gastric mucosa in relation to Helicobacter pylori infection and family history of gastric cancer. Adv Med Sci. 2012;57:237–243. doi: 10.2478/v10039-012-0049-z. [DOI] [PubMed] [Google Scholar]
- 60.Balogh A, Paragh G, Jr, Juhász A, Köbling T, Törocsik D, Mikó E, Varga V, Emri G, Horkay I, Scholtz B, Remenyik E. Reference genes for quantitative real time PCR in UVB irradiated keratinocytes. J Photochem Photobiol B. 2008;93:133–139. doi: 10.1016/j.jphotobiol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Gur-Dedeoglu B, Konu O, Bozkurt B, Ergul G, Seckin S, Yulug IG. Identification of endogenous reference genes for qRT-PCR analysis in normal matched breast tumor tissues. Oncol Res. 2009;17:353–365. doi: 10.3727/096504009788428460. [DOI] [PubMed] [Google Scholar]