Abstract
Metastases to the pituitary gland are rare; cancers that most commonly metastasize to the pituitary are breast and lung cancers. No specific computed tomography or magnetic resonance imaging features reliably distinguish primary pituitary masses from metastases. A combination of a detailed clinical assessment together with specialist endocrine and neuroradiology support is essential to make the rare diagnosis of a pituitary metastasis. We present the case of a man with metastatic lung cancer, initially presenting as hypopituitarism. Subtle features in the history, together with neuroimaging findings atypical for pituitary adenomas, provided clues that the diagnosis was one of the pituitary metastases. Treatment of diabetes insipidus (DI) with replacement antidiuretic hormone (ADH) was complicated by extreme difficulties in achieving a satisfactory sodium and water balance. This was the result of coexistent DI and syndrome of inappropriate ADH secretion perpetuated by the patient's primary lung cancer, a phenomenon not previously described in the literature.
INTRODUCTION
Post-mortem studies have shown that the pituitary gland is a rare site for metastases of malignant tumours. Only 1–3.6% of malignant tumours have been found to metastasize to the pituitary [1]. Of those tumours which do spread to the pituitary, breast cancer in women and lung cancer in men are commonest, although others (prostate, renal cell carcinoma, gastrointestinal, lymphoma, leukaemia, thyroid cancer and plasmacytoma) are also reported to cause pituitary metastases [1]. Rarer still are symptomatic manifestations of pituitary metastases. One series found only 7% of pituitary metastases to be symptomatic [2]. Most frequently patients present with symptoms of diabetes insipidus (DI) or anterior pituitary dysfunction, ophthalmoplegia or visual disturbances, or headaches [1, 3].
We present a case of a man with metastatic lung cancer, which initially presented as a pituitary mass causing hypopituitarism. Subtle features in the patient's history, together with neuroimaging findings atypical for pituitary adenomas, provided clues that the diagnosis in the patient was one of the pituitary metastases and not a primary pituitary tumour. Treatment of DI with replacement antidiuretic hormone (ADH) was complicated by extreme difficulties in achieving a satisfactory sodium and water balance. Initially, the patient presented with hypernatraemia as a result of pure water loss from a new presentation of DI, probably unmasked by commencing hydrocortisone replacement. Subsequently, he developed hypotonic hyponatraemia which was initially felt to be due to over-treatment with desmopressin. However, several days after stopping the desmopressin, his hyponatraemia worsened. Because the half-life of desmopressin is only 2–3 h, we hypothesized that there must be endogenous inappropriate ADH secretion. Therefore, the difficulties in stabilizing his sodium were probably the result of coexistent DI unmasked by commencing steroids, as a well as syndrome of inappropriate ADH secretion caused by the patient's primary lung cancer, a phenomenon not previously described in the literature.
CASE REPORT
A 77-year-old male smoker presented to our medical assessment unit with a 1-day history of confusion, insomnia and reduced appetite. There was no other past medical or drug history of relevance. Initial examination revealed normal vital signs, but marked confusion [Abbreviated Mental Test (AMT) score of 4/10, where a score of <8 is suggestive of confusion]. There was no focal neurological deficit, visual field defect or ophthalmoplegia. Initial blood tests were unremarkable other than a mild elevation in his urea and creatinine (Table 1). An urgent computed tomography (CT) brain scan was performed, revealing an enhancing (1.5 × 1.7 cm) suprasellar mass (Fig. 1A). There was also oedema of the overlying optic tract (Fig. 1B), a finding most unusual for primary pituitary adenoma. Further laboratory testing showed hypopituitarism in keeping with anterior pituitary dysfunction (Table 2). Hormone replacement therapy with levothyroxine and hydrocortisone was commenced. There was subsequent improvement in the patient's clinical condition. After discussion with the neurosurgical team, the patient was considered unsuitable for surgical intervention due to his age. He was discharged home with urgent endocrine outpatient follow-up.
Table 1:
Results of admission blood tests at first presentation.
| Na | 135 mmol/l |
| K | 4.6 mmol/l |
| Urea | 8.7 mmol/l |
| Creatinine | 151 umol/l |
| Albumin | 36 g/l |
| ALP | 51 iu/l |
| ALT | 15 iu/l |
| Bilirubin | 10 umol/l |
| WCC | 8.0 × 109/l |
| Hb | 129 g/l |
| Plt | 172 × 109/l |
There is a mild elevation of serum urea and creatinine.
Figure 1:
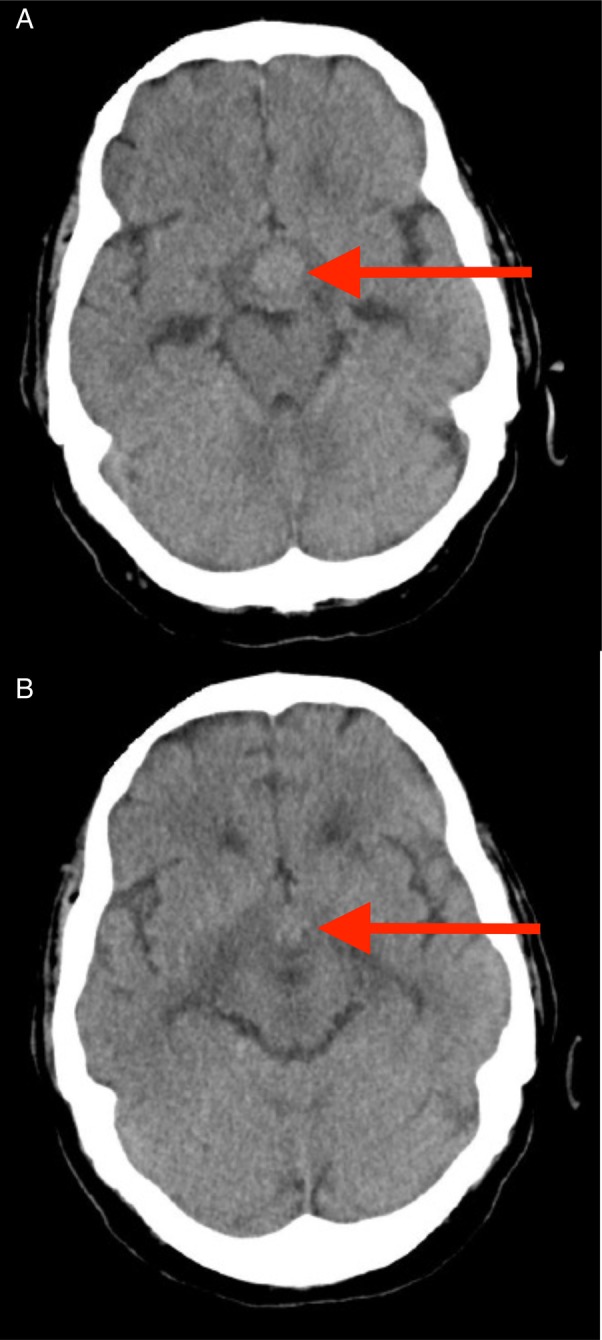
CT head scan images. An enhancing mass is seen in the pituitary fossa (Arrow, A). There is also overlying optic tract oedema (Arrow, B), not typically seen with pituitary adenomas.
Table 2:
Results of pituitary profile blood tests.
| Free thyroxine | 4.8 pmol/l |
| TSH | 0.99 miu/l |
| LH | <0.5 |
| FSH | <0.5 |
| Prolactin | 95 miu/l |
| Testosterone | <0.3 |
| Cortisol | 44 nmol/l |
| SHBG | 32 nmol/l |
There is panhypopituitarism, with low thyroid function tests, sex hormones and cortisol levels.
Five days after discharge, the patient re-presented to hospital, this time with worsening confusion, reduced mobility, thirst, polyuria and polyphagia. His wife remarked that she had to conceal food from the patient in light of his new symptoms of increased appetite. These unusual changes in symptoms were not in keeping with those features ordinarily experienced by patients with a pituitary adenoma. The symptoms of hunger and confusion, in particular, raised suspicions that there was involvement of surrounding brain tissue, particularly of the hypothalamus. Examination now revealed a clinically dehydrated man with an AMT score of 2/10. His 24-h fluid intake was 1600 ml, with an output of 4000 ml. There was still no focal neurological deficit. Repeat blood tests showed mild hypernatraemia, raised serum osmolality and a further elevation of the patient's urea (Table 3), in keeping with his dehydration. Repeat thyroid function testing revealed an improvement in serum free thyroxine (9.0 pmol/l, reference range 9.0–25.0 pmol/l). After intravenous fluid rehydration, an urgent magnetic resonance imaging (MRI) brain scan was organized. This showed the known pituitary mass, but now larger with marked hypothalamic extension (Fig. 2). A chest X-ray also uncovered a suspicious left pseudo-nodular shadow at the level of the aortic arch (Fig. 3) and a subsequent CT thorax confirmed a left lung mass lesion with impingement into the left main pulmonary artery (Fig. 4).
Table 3:
Results of blood tests taken at patient's second presentation to hospital.
| Na | 148 mmol/l |
| K | 4.4 mmol/l |
| Urea | 11.0 mmol/l |
| Creatinine | 104 umol/l |
| Serum osm. | 308 mmol/kg |
| Urine osm. | Unavailable |
There is mild hypernatraemia, raised serum urea and creatinine, and an elevated serum osmolality.
Figure 2:
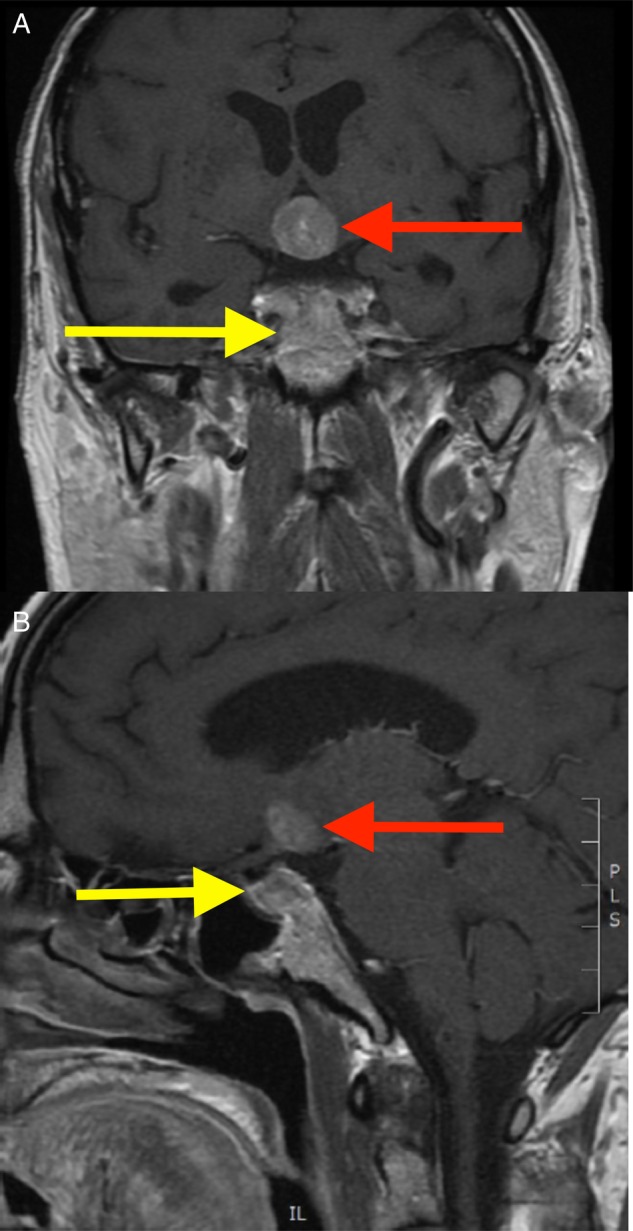
MRI brain scan, coronal (A) and sagittal (B) views are shown. The known pituitary mass is seen (red arrow), but now with marked hypothalamic extension (yellow arrow). The pituitary and hypothalamic masses were deemed to be continuous after detailed review by a specialist neuroradiologist, although this is not easily apparent on any one image.
Figure 3:
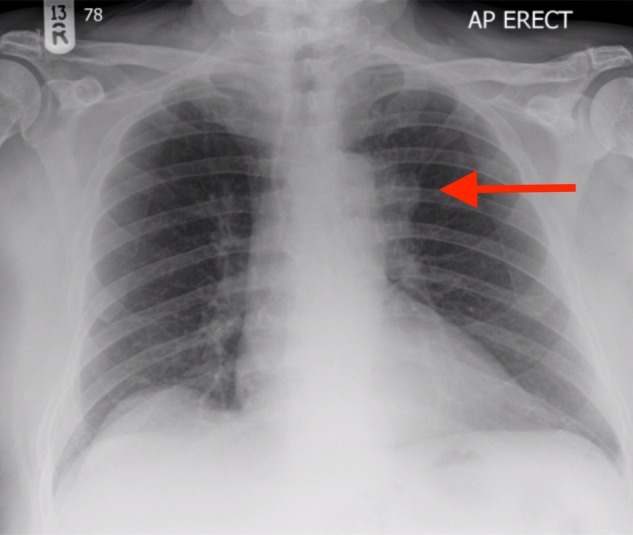
Patient's chest radiograph. There is a pseudo-nodular shadow visible at the left lung hilum (arrow).
Figure 4:
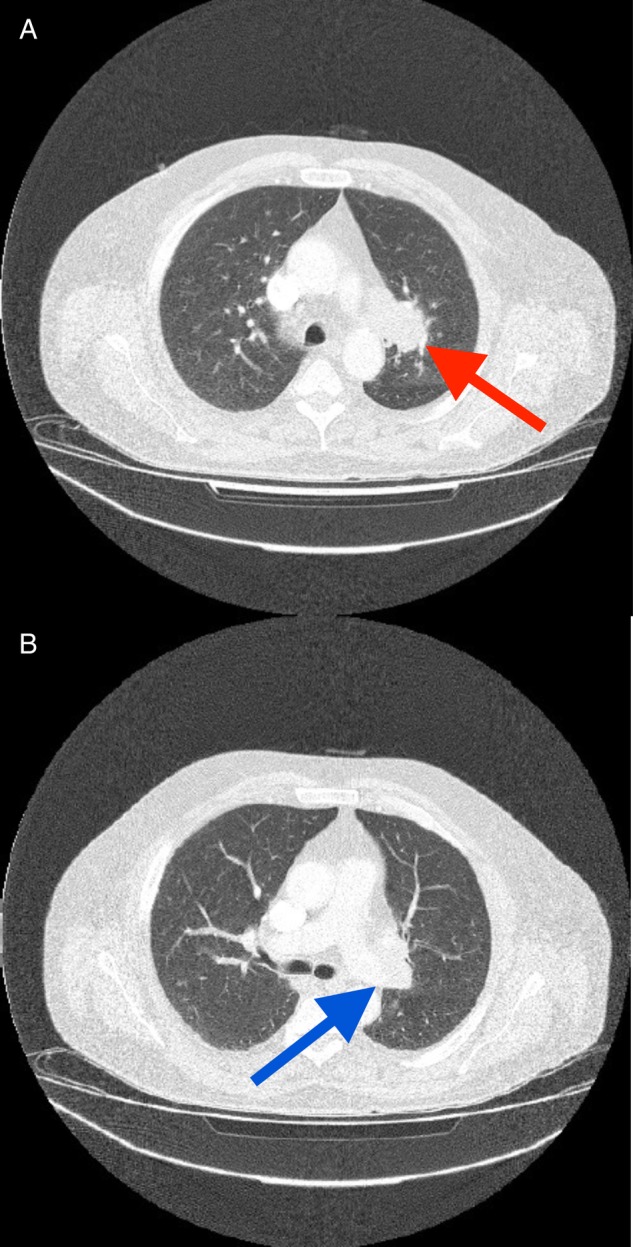
Patients CT scan thorax. A left-sided pulmonary mass is visible (A, red arrow). There is invasion into the left main pulmonary artery (B, blue arrow).
The case was discussed at our local pituitary and lung cancer multidisciplinary team meetings, where a diagnosis of a primary lung cancer with metastasis to the pituitary was made, complicated by cranial DI. The atypical features in the patient's clinical history and neuroimaging were the key factors that contributed to the diagnosis of pituitary metastases than a typical pituitary adenoma. Only symptomatic treatment was considered appropriate for the patient's ongoing management. Replacement ADH therapy in the form of demopressin was commenced with serial blood tests performed for monitoring of serum sodium and paired serum/urine osmolalities (Table 3). There was initial improvement in the patient's biochemistry following ADH replacement, after which increased doses were required to maintain an adequate sodium and water balance. This was followed by a substantial fall in serum sodium and osmolality, which persisted even after discontinuing ADH replacement (Table 4). Although initially it was felt that over-treatment with desmopressin was the likely cause of the hypotonic hyponatraemia, sodium levels continued to fall to a nadir of 122 mmol/l 5 days after stopping desmopressin (Table 4). Because the half-life of desmopressin is 2–3 h, it was felt that there must be endogenous secretion of ADH from a source other than his pituitary gland. Because of his underlying new diagnosis of lung cancer, it was felt that the likely diagnosis was the syndrome of inappropriate ADH secretion (SIADH) from this tumour. The challenging sodium and water balance in this case represented the interplay between coexisting DI as a consequence of a pituitary metastasis and SIADH from a lung primary.
Table 4:
Serial blood tests results following commencement of ADH replacement therapy (desmopressin).
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 15 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na (mmol/l) | 149 | 147 | 142 | 148 | 146 | 137 | 137 | 152 | 145 | 144 | 139 | 130 | 127 | 122 | 122 | 122 |
| Serum osmolality (mmol/kg) | 314 | 313 | 293 | 298 | 283 | 284 | 319 | 305 | 398 | 290 | 261 | 249 | 255 | |||
| Urine osmolality (mosmol/kg) | 594 | |||||||||||||||
| Urine in/out (ml) | 1600/4000 | 2100/3300 | ||||||||||||||
| Urea (mmol/l) | 5.5 | 6.6 | 7.8 | 7.1 | 11.3 | 10.6 | 8.7 | 8.6 | 7.9 | 6 | 6.9 | 6.9 | 6.2 | |||
| Creatinine (umol/l) | 87 | 84 | 80 | 86 | 130 | 110 | 95 | 85 | 82 | 73 | 67 | 74 | 77 | |||
| Estimated GFR (MDRD, ml/min) | 78 | 82 | 86 | 79 | 49 | 60 | 71 | 80 | 84 | >90 | >90 | >90 | 90 | |||
| Desmopressin dose | 10 mcg BD | 10 mcg BD | 10 mcg BD | 10 mcg BD | 10 mcg BD | 10 mcg BD | 10 mcg BD | 30 mcg BD | 30 mcg BD | 30 mcg BD | 10 mcg BD | Stopped | Stopped | Stopped | Stopped | Stopped |
Despite an initial improvement in the patient's sodium and osmolalities with desmopressin, the patient later developed hyponatraemia that persisted even after discontinuing therapy. Note that from Days 11 to 21, the patient's hyponatraemia worsened despite receiving no replacement ADH therapy. Renal function remained relatively unaffected throughout. BD, twice daily..
Sadly, the patient's clinical and biochemical conditions deteriorated. After review by the palliative care team, it was decided that serial blood test monitoring be discontinued and palliative care be initiated. The patient was discharged home, where he died soon after.
DISCUSSION
Metastases to the pituitary gland are rare; three large post-mortem series' reported prevalence rates of 1–3.6% [4–6]. Those cancers which most commonly metastasize to the pituitary gland are breast cancers in women and lung cancers in men [2, 7], as seen in this case.
The vast majority of pituitary metastases are clinically silent, with only 7% reported as symptomatic in one series [2]. When symptoms do occur, these are usually those of DI (polydipsia and polyuria), headaches, visual field defects and ophthalmoplegia [6–8].
Neuroimaging is essential for the diagnosis of pituitary lesions. No specific CT or MRI features can reliably distinguish primary pituitary masses from pituitary metastases. Thickening of the pituitary stalk, extension to surrounding areas and loss of signal from the posterior pituitary have been claimed to be suggestive of pituitary metastases [1]. In this case, overlying optic tract oedema and hypothalamic extension of the pituitary mass were indicative of an atypical pituitary mass. Clearly, the combination of a detailed clinical history together with specialist endocrine and neuro-radiology support is essential to make the rare diagnosis of a pituitary metastasis.
A combined diagnosis of DI and SIADH, as in the case presented here, was not reached without careful consideration of other possible diagnoses. In cases of hypopituitarism, it has been reported that low cortisol levels seen in ACTH deficiency can mask symptoms of DI [9, 10]. Cortisol has a direct inhibitory effect on endogenous ADH secretion; hence, ACTH deficiency increases tonic ADH activity, reducing the capacity for excretion of free water. When patients are rendered steroid replete, the ability to excrete free water is restored, and this may have been the reason our patient presented with classical features of DI soon after starting hydrocortisone.
The unique aspect of our case was the development of hypotonic hyponatraemia 5 days after stopping desmopressin. Because his hyponatraemia continued to worsen long after the presumed clearance of desmopressin, we felt that the likely cause was endogenous ADH secretion from his lung cancer. This provided a management dilemma because it would usually be counter-intuitive to fluid restrict a patient that has recently developed DI due to the risk of severe dehydration and hypernatraemia. However, we felt that he had dual biochemical pathology and that the initial predominant biochemical ‘lesion’ was ADH deficiency, which subsequently became over-ridden by rising levels of ADH from his aggressive lung tumour, leading to endogenous hypotonic hyponatraemia.
Other possible causes of hypotonic hyponatraemia apart from SIADH include adrenal insufficiency and total body salt depletion, but we felt these were clinically unlikely because the patient was on supra-physiological doses of hydrocortisone with no clinical signs of hypoadrenalism or hypovolaemia. In the workup of hyponatraemia, it is usually very important to measure urine sodium, a level of >30 mmol/l suggesting total body salt loss or low effective arterial volume. In our patient, urine sodium was not measured, which was an omission, but salt depletion was unlikely given that he presented with hypernatraemia from DI-associated pure water loss only a few days earlier, and at the time of hyponatraemia, the patient was clinically euvolemic with no signs of low arterial volume.
In summary, we present a very interesting case of lung cancer with pituitary metastasis, which proved a challenging management dilemma as regards his sodium. With the recently published European Guidelines [11], there has been increased prominence of the recommended systematic approach to managing hyponatraemia. This case brings out several interesting aspects of sodium homeostasis including the effect of corticosteroids on ADH, and the likely co-existence of ADH and SIADH in the same patient, which provides a good basis for the discussion of sodium pathophysiology.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ETHICAL APPROVAL
None required.
CONSENT
The patient presented in this report is deceased; therefore, no consent was obtained.
GUARANTOR
G.S.G is the guarantor of this work.
REFERENCES
- 1.Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus 2004;16:1–4. [PubMed] [Google Scholar]
- 2.Teears RJ, Siverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the pituitary gland. Cancer 1974;36:478–80. [DOI] [PubMed] [Google Scholar]
- 3.Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE et al. Tumours metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab 2004;89:574–80. [DOI] [PubMed] [Google Scholar]
- 4.Abrams HL, Spiro R, Golstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer 1950;3:74–85. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs K. Metastatic cancer of the pituitary gland. Oncology 1973;27:533–42. [DOI] [PubMed] [Google Scholar]
- 6.Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981;31:998–1002. [DOI] [PubMed] [Google Scholar]
- 7.Morita A, Meyer FB, Laws ER. Symptomatic pituitary metastases. J Neurosurg 1998;89:69–73. [DOI] [PubMed] [Google Scholar]
- 8.Branch CL, Laws ER. Metastatic tumours of the sella turcica masquerading as primary pituitary tumours. J Clin Endocrinol Metab 1987;65:469–74. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Chou K, Lee P. A case of lymphocytic hypophysitis with masked diabetes insipidus unveiled by glucocorticoid replacement. Am J Kidney Dis 2005;45:197–200. [DOI] [PubMed] [Google Scholar]
- 10.Ohta M, Kimura T, Ota K. Glucocorticoid-induced central diabetes insipidus in a case of malignant lymphoma. Tohuku J Exp Med 2006;163:245–54. [DOI] [PubMed] [Google Scholar]
- 11.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014;170:G1–47. [DOI] [PubMed] [Google Scholar]


