Abstract
Dengue is a common acute viral febrile illness in the tropics. Although the usual presentation is that of a self-limiting illness, its complications are protean. We report a 29-year-old man who presented with an acute febrile illness and was diagnosed with dengue hemorrhagic fever. Despite appropriate supportive therapy, the patient initially improved, but subsequently had clinical deterioration. Evaluation revealed features of hemophagocytic lymphohistiocytosis. He was successfully treated with glucocorticoids and had an uneventful recovery. This case adds to the limited adult cases of virus-associated hemophagocytic syndrome in the literature and the need for prompt recognition and treatment of this rare complication.
INTRODUCTION
Dengue fever, caused by a mosquito-borne virus of the Flaviviridae family, is endemic to India and can present with a wide range of clinical manifestations. The disease presentation can range from a self-limiting febrile illness to life-threatening shock [1, 2]. In recent years, there has been an increase in reports of atypical manifestations, some of which may be severe [3, 4].
Hemophagocytic syndrome is an aggressive disease characterized by excessive immune activation. It can occur in the setting of autoimmune disease, hematological malignancy and infections [5]. Many infections are known to cause hemophagocytosis, and this is often mistaken for sepsis and multiorgan dysfunction and carries a high mortality [6]. Infection by dengue virus is being recognized as a trigger in the recent years [7].
We describe the case of a 29-year-old man who had dengue hemorrhagic fever complicated by the development of hemophagocytosis. This report adds to the limited adult cases of dengue-associated hemophagocytic syndrome described in the literature.
CASE REPORT
A 29-year-old farmer from rural South India, who was previously well, presented to the emergency services with high-grade, intermittent fever for 4 days, which was associated with chills and rigors. He also complained of headache with retro-orbital pain, myalgia and right upper abdominal pain. On examination, he was afebrile with a pulse rate of 112/min, a blood pressure of 100/60 mmHg and a respiratory rate of 28/min. He had a diffuse, blanching erythematous rash over the trunk with no eschar. There was evidence of third space fluid loss, in the form of bilateral pleural effusions and ascites, with tender hepatomegaly. There was no neck stiffness, and fundus examination was negative for papilloedema. Clinical differentials of dengue, rickettsial illness, malaria, leptospirosis and viral hepatitis were considered. The patient's biochemical and hematological investigations during the hospital admission and follow-up are listed in Table 1. Initial evaluation revealed hemoconcentration, thrombocytopenia, elevated white cell count, elevated liver enzymes and renal dysfunction. As the patient had significant hepatic dysfunction, ischemic hepatitis, toxin-induced and viral hepatitis were also considered and ruled out. A diagnosis of dengue hemorrhagic fever was made as serological tests were positive for IgG and IgM antibodies to dengue virus.
Table 1:
Day of illness with corresponding hematological and biochemical parameters of the patient
| Day of illness | 5 | 6 | 7 | 10 | 15 | 17 | 20 | 25 | 30 | 40 |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dl) | 17 | 13.8 | 12.7 | 12 | 10.2 | 8.7 | 8.3 | 10.9 | 11.1 | |
| White blood cell count/mm3, N | 41 800 N-52 |
36 900 N-82 |
16 300 N-63 |
8200 N-64 |
2900 N-56 |
1900 N-36 |
3900 N-60 |
8400 | 8500 N-77 |
11 300 N-80 |
| Platelet count/mm3 | 19 000 | 32 000 | 33 000 | 73 000 | 2.21 L | 2.7 L | 3.24 L | 2.84 L | 2.18 L | 2.26 L |
| Sodium/potassium (mmol/l) | 132/5.2 | 133/4.9 | 137/4.3 | 141/4.4 | 135/4.5 | 131/4.4 | 133/4.5 | |||
| Urea/creatinine (mg%) | 75/1.58 | 77/1.44 | 62/1.1 | 56/1.26 | 38/0.96 | 55/1.05 | /0.61 | 44/0.6 | 48/0.73 | |
| LDH (U/l) | 20 620 | 8870 | 4727 | |||||||
| Total/direct bilirubin (mg/dl) | 1.9/1.3 | 2.3/1.8 | 2.3/1.4 | 1.6/1.3 | 1.5/1.1 | 1.1/0.7 | ||||
| Protein/albumin (g/dl) | 6/2.8 | /2.4 | 7/2.9 | 7.7/3.3 | 7.6/3.4 | 7.1/4 | ||||
| Aspartate/alanine transaminase (U/l) | 7350/3000 | 3322/1562 | 1096/701 | 741/431 | 379/188 | 170/153 | 73/117 | 61/111 | 36/73 |
N, neutrophil; LDH, lactate dehydrogenase.
The patient was admitted to the high-dependency unit where he was managed conservatively. He was given isotonic intravenous fluids cautiously, with close monitoring of the hematocrit and urine output. At admission, he had deranged coagulation parameters, with no overt bleeding, and was given fresh frozen plasma and platelet concentrates. He responded well to the above measures and was shifted to the ward as his clinical condition improved. His fever resolved, and there were a rise in platelet count, fall in hematocrit and improvement of biochemical and renal parameters.
However, while in the ward, the patient had recurrence of high-grade fever, and he developed features of systemic inflammatory response syndrome. He also developed worsening ascites and pleural effusions, the analysis of which was suggestive of an exudate (Fig. 1). There were no features of fluid overload. Initially, a hospital-acquired infection was suspected, and the patient was evaluated thoroughly for the same. Catheter-related blood stream infection, urinary tract infection, hospital-acquired pneumonia, infected pressure sores, deep-seated collections and drug fever were also looked for and ruled out. Serial cultures failed to isolate any organism. Broad-spectrum antibiotics were initiated, but the fever persisted despite the same. At this stage, a drop in white cell count and hemoglobin was noted, with a resolution of thrombocytopenia (Day 15, Table 1). Occult and overt blood losses were looked for, and hemolysis was ruled out. Hemodilution was unlikely as the patient had been off IV fluids. Further evaluation revealed elevated ferritin (17 386 ng/ml) and triglycerides (297 mg/dl), whereas lactate dehydrogenase persisted to be elevated (Table 1). The fibrinogen levels dropped from 171 to 124 mg/dl, and C-reactive protein was elevated (41.1 mg/l). Despite renal parameters and liver function tests showing an improving trend, the patient appeared toxic with features of hemodynamic instability. In view of the clinical picture, along with bicytopenia and elevated inflammatory markers, hemophagocytosis was considered.
Figure 1:
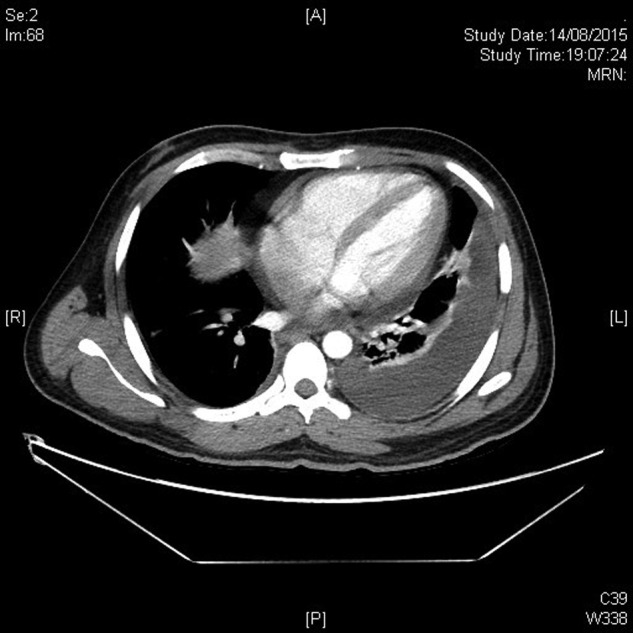
Computed tomography of the abdomen showing ascites.
The patient underwent a bone marrow biopsy on Day 15 of the illness, which showed foci of hemophagocytosis with no evidence of malignancy or infiltrative disease (Fig. 2a and b). The patient thus fulfilled clinical, laboratory and histopathological diagnostic criteria for reactive hemophagocytic lymphohistiocytosis (HLH-2004 trial), with dengue infection being the trigger. As the patient was acutely ill with deteriorating respiratory and hemodynamic parameters, a decision was made to initiate him on steroid therapy. On Day 17, he was started on intravenous corticosteroids, as per the HLH-2004 protocol (dexamethasone 10 mg/m2). There was a dramatic response to the same with resolution of fever and improvement of blood cell counts (Day 20, Table 1). The pleural effusions and ascites gradually improved. In view of the good response, no second-line chemotherapeutic agent was added, and the steroids were tapered over a course of 8 weeks. At 1-month follow-up, the patient was doing well with no further signs of reactivation.
Figure 2:
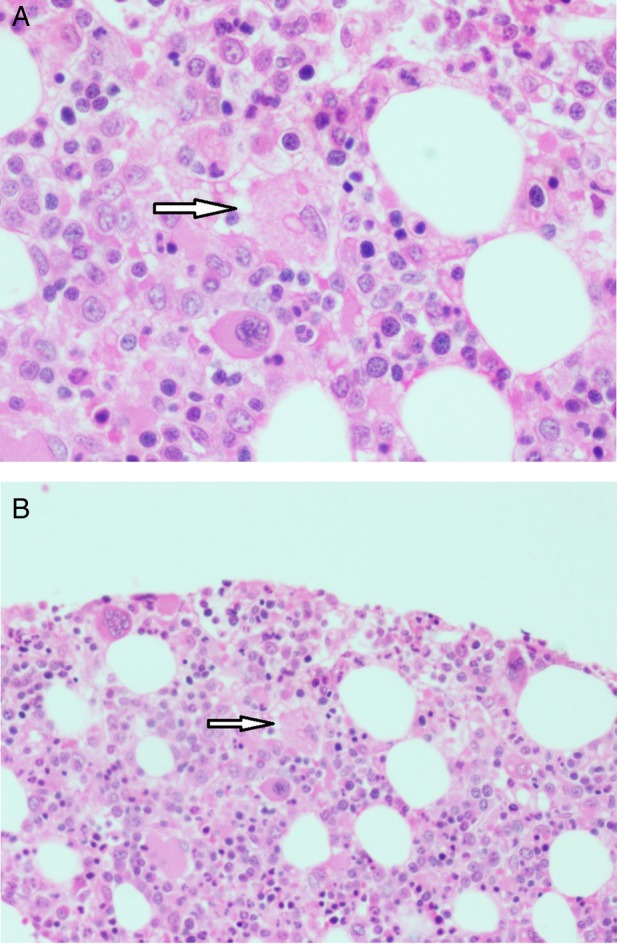
Bone marrow histopathology image showing foci of hemophagocytosis.
DISCUSSION
HLH is a rare, life-threatening disorder characterized by tissue destruction due to abnormal immune activation. Afflicted patients present with fever and multiorgan dysfunction, which is often mistaken for sepsis [5]. Although primary HLH is typically seen in the pediatric age group, secondary HLH can occur in adults and children in association with certain triggers [8]. Common triggers for secondary HLH include infections, solid organ malignancies, lymphoid or myeloid, rheumatological disorders and inherited or acquired immunodeficiency. Infectious triggers are usually viral, and Epstein–Barr virus has been most commonly implicated. HLH can also occur in the setting of Cytomegalovirus, Parvovirus B19, HIV, tuberculosis, bacterial, fungal and parasitic infections [6].
This disorder is characterized by excessive macrophage activation and cytokine release due to a failure in natural killer cell function. This results in immune dysregulation and unchecked inflammation. Patients with HLH are acutely ill with fever, hepatosplenomegaly, effusions and lymphadenopathy. Laboratory findings include bicytopenia, coagulopathy, liver dysfunction, hyperferritinemia and elevated triglycerides and lactate dehydrogenase. Bone marrow infiltration by activated macrophages can be demonstrated, and diagnosis is made based on the HLH-2004 protocol proposed by the Histiocyte Society—HLH can be established in the presence of (i) molecular diagnosis consistent with HLH or (ii) the presence of five out of eight criteria, namely, fever, splenomegaly, cytopenias, hypertriglyceridemia, hypofibrinogenemia, hemophagocytosis in tissue, hyperferritinemia, increase in CD25/IL-2 receptor or reduced NK cell function. The sensitivity and specificity of the same, however, remain untested [5]. Glucocorticoids are used as the initial agent in the treatment of HLH. Other agents include etoposide, intrathecal methotrexate and intravenous immunoglobulin [9].
Dengue fever, caused by a mosquito-borne virus of the Flaviviridae family, is a serious public health problem in the Indian subcontinent. The year 2015 has seen a doubling in the number of dengue cases reported in India. Manifestations range from a self-limiting febrile illness to life-threatening disease with shock and multiorgan dysfunction. The diagnosis of dengue is based on the detection of virus nonstructural protein 1, which has greater sensitivity in primary infection (90%) when compared with secondary infection (60–80%). Alternatively, real-time PCR tests are available for virus detection. Serological testing for IgM and IgG antibodies helps in differentiation between primary and secondary infections [1].
Dengue-associated HLH has been described commonly in children, with few reports in adults [10–12]. It has been more commonly noted in patients with dengue hemorrhagic fever, as in our patient [10, 13]. However, there have been reports of HLH in patients with primary dengue fever as well [10, 11]. Dengue virus-infected T cells produce cytokines leading to uncontrolled histiocytic activity [13, 14]. It has been proposed that the increased production of cytokines, interferon-γ and tumor necrosis factor-α plays a role in the pathogenesis of HLH. Till date, only three serotypes of dengue viruses have been attributed to cause HLH (DEN1, DEN3 and DEN4) [13, 15]. Nelson et al. [16], based on postmortem studies, proposed that hemophagocytosis was present in the terminal stages of dengue virus infection. As the febrile period in dengue lasts for 3–7 days, ongoing fever after 8 days with persistence of cytopenia and multiorgan dysfunction should alert the clinician toward a diagnosis of HLH [17]. On review of other cases, dengue-associated HLH usually presented in the second week of illness. There have been two previous reports of HLH occurring at the end of the second week following the onset of dengue fever [13, 18]. In our patient, the diagnosis was established on Day 15 of illness.
Our patient was successfully treated with a tapering course of corticosteroids as per the HLH-2004 protocol and showed good response to the same. At 1-month follow-up, he was doing well with no features of reactivation.
On review of the literature, there have been <30 cases of hemophagocytosis associated with dengue infection in adults. In our patient, initial improvement was followed by clinical worsening leading to a diagnosis of dengue-associated HLH. This case highlights the need for awareness of this challenging disorder as prompt recognition and appropriate management can improve survival of patients.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
There were no sources of funding.
ETHICAL APPROVAL
The work meets ethical guidelines and adheres to local legal requirements.
CONSENT
The patient's informed signed consent was obtained.
GUARANTOR
S.G.H. is the guarantor of this study.
REFERENCES
- 1.Simmons CP, Farrar JJ, Nguyen VC, Wills B. Dengue. N Engl J Med 2012;366:1423–32. [DOI] [PubMed] [Google Scholar]
- 2.Guzman MG, Harris E. Dengue. Lancet 2015;385:453–65. [DOI] [PubMed] [Google Scholar]
- 3.Dengue Challenges India's Health System [Internet]. http://www.sciencedirect.com/science/article/pii/S014067361500313X (17 October 2015, date last accessed). [DOI] [PubMed]
- 4.Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Microvascular and endothelial function for risk prediction in dengue: an observational study. Lancet 2015;385(Suppl 1):S102. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet 2014;383:1503–16. [DOI] [PubMed] [Google Scholar]
- 6.Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis 2000;6:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemophagocytic Syndrome in Severe Dengue Fever: A Rare Presentation [Internet]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4192178/ (17 October 2015, date last accessed). [DOI] [PMC free article] [PubMed]
- 8.Freeman HR, Ramanan AV. Review of haemophagocytic lymphohistiocytosis. Arch Dis Child 2011;96:688–93. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood 2011;118:4041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray S, Kundu S, Saha M, Chakrabarti P. Hemophagocytic syndrome in classic dengue fever. J Glob Infect Dis 2011;3:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Koninck A-S, Dierick J, Steyaert S, Taelman P. Hemophagocytic lymphohistiocytosis and dengue infection: rare case report. Acta Clin Belg 2014;69:210–3. [DOI] [PubMed] [Google Scholar]
- 12.Tan LH, Lum LCS, Omar SFS, Kan FK. Hemophagocytosis in dengue: comprehensive report of six cases. J Clin Virol 2012;55:79–82. [DOI] [PubMed] [Google Scholar]
- 13.Khurram M, Faheem M, Umar M, Yasin A, Qayyum W, Ashraf A et al. . Hemophagocytic Lymphohistiocytosis Complicating Dengue and Plasmodium vivax Coinfection. Case Rep Med 2015;2015:696842 http://doi.org/10.1155/2015/696842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura I, Nakamura-Uchiyama F, Komiya N, Ohnishi K. A case of dengue fever with viral-associated hemophagocytic syndrome. Kansenshogaku Zasshi 2009;83:60–3. [DOI] [PubMed] [Google Scholar]
- 15.Ellis EM, Pérez-Padilla J, González L, Lebo E, Baker C, Sharp T et al. . Unusual cluster in time and space of dengue-associated hemophagocytic lymphohistiocytosis in Puerto Rico. Blood 2013;122:3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson ER, Bierman HR, Chulajata R. Hematologic phagocytosis in postmortem bone marrows of dengue hemorrhagic fever. (Hematologic phagocytosis in Thai hemorrhagic fever). Am J Med Sci 1966;252:68–74. [PubMed] [Google Scholar]
- 17.Pal P, Giri PP, Ramanan AV. Dengue associated hemophagocytic lymphohistiocytosis: a case series. Indian Pediatr 2014;51:496–7. [PubMed] [Google Scholar]
- 18.Rathore R, Daniyal SM, Iqbal A, Butt NF, Mehbob F. Hemophagocytic syndrome—a rare complication of dengue fever. Ann KEMU 2012;18:124–6. [Google Scholar]


