Abstract
In recent years, there has been considerable interest in the effects of auditory and visual distractions on pedestrian ambulation. A fundamental temporal characteristic of ambulation is the temporal fluctuation of the stride interval. In this paper, we investigate the stationarity of stride interval time series when people are exposed to different forms of auditory and visual distractions. An increase in nonstationary behavior may be suggestive of divided attention and more frequent central modulation of locomotion, both of which may have ramifications on pedestrian vigilance and responsiveness to environmental perturbations. One group of fifteen able-bodied (6 females) young adult participants completed a music protocol (overground walking with and without music). A second group of fifteen (7 females) did a television protocol (treadmill walking while watching TV with and without sound). Three walking trials, each 15 minutes in duration, were performed at each participant’s comfortable walking speed, with force sensitive resistors under the heel of each foot. Using the reverse arrangements test, the vast majority of time series were nonstationary, with a time-varying mean as the principal source of nonstationarity. Furthermore, the television trial with sound had the greatest number of nonstationarities followed by overground walking while listening to music. We discuss the possibility that these conditions measurably affect gait dynamics through a subconscious synchronization to external rhythms or a cyclic distraction followed by a period of increased conscious correction of gait timing. Our findings suggest that the regulation of stride timing is particularly susceptible to constant, time-evolving auditory stimuli, but that normal pacing can be restored quickly upon stimulus withdrawal. These kinds of sensory distractions should thus be carefully considered in studies of pedestrian ambulation.
Keywords: Gait, stride intervals, stationarity, music, television, fractal analysis
1. Introduction
Walking is the most common physical activity among adults (Owen et al., 2004). One of the fundamental temporal characteristics of walking is the stride interval, that is, the time between successive heel strikes. Walking is a complicated task governed by the hierarchical control of the motor cortex, spinal pattern generators and feedback from the vestibular system (Dietz, 2002; West and Griffin, 1999). Due to these varying levels of conscious and autonomic control, interstride times fluctuate in a very complex manner (West and Griffin, 1999; Hausdorff et al., 1999, 2001). These temporal variations of stride intervals have been shown to differentiate between persons with and without pathologies, young and older adult gait, and overground and treadmill walking (Hausdorff et al., 1997; Chau and Rizvi, 2002).
The stationarity of these complex fluctuations may reveal the magnitude with and time scale over which the nervous system makes adjustments as a person walks. Using recurrence plots, a treadmill study reported stationary joint angles and body accelerations while walking and attributed this temporal invariance to the constant speed imposed on the participant (Ding-well and Cusumano, 2000). However, a subsequent pediatric treadmill study found nonstationarities via the reverse arrangement test in stride interval time series despite speed constraints (Fairley et al., 2010), implicating the children’s evolving comfort level with the treadmill and varying attentiveness to the task as possible sources of nonstationarity. The temporal variability of stride intervals may thus be connected to the physical and cognitive capability to dynamically adapt one’s gait and more generally, to respond to changes in the environment.
This study aimed to determine whether or not stride interval time series remain stationary when subjects are exposed to different audio and visual stimuli. In particular, we considered the stationarity of the stride interval time series while subjects walked overground when listening to music or while they walked on a treadmill and watched television. The effects of these sensory stimuli on gait are of concern given the increasing pedestrian use of personal music players (Bungum et al., 2005), handheld gaming devices, text messaging applications, portable video players, and pedestrian navigation systems (Torres-Solis and Chau, 2010). Additionally, video and music are becoming important tools in rehabilitation research and practice (Sveistrup, 2004; Fung et al., 2006; Schauer and Mauritz, 2003).
2. Methodology
2.1. Participants
Two groups of participants were recruited. The first group consisted of 15 able-bodied participants (6 females; mean age 25.9 ± 2.8 years). The second group consisted of 15 able-bodied participants (7 females; mean age 25.4 ± 2.7 years). All participants were recruited from the Bloorview Research Institute and provided written informed consent in accordance with Bloorview Research Ethics Board. Subjects met the inclusion criteria of having normal or corrected-to-normal vision, intact hearing and right-foot dominance. Participants were excluded if they had any history of neurological pathology that would have compromised natural bipedal ambulation. For both protocols, participants were instructed to wear comfortable walking shoes.
2.2. Data collection
Data were collected using two different protocols: music and television. The first group completed the music protocol, which comprised two identical sessions, separated by at least 24 hours, and each consisting of three 15 minute trials in the following sequence: 1. overground walking (OW-NoMusic1), 2. overground walking with music (OW-Music), and 3. overground again (OW-NoMusic2). Unconstrained overground walking was performed at a self-selected pace on an indoor rectangular path (width ~ 2.5 m, length ~ 100 m) in sparsely populated hallways with linoleum flooring. Participants were instructed to “walk at a comfortable pace”. An investigator walked slightly behind the subject during the test to record any stumbles or falls which might skew the stride time distribution. Songs were selected from a “Top 40s” list for that month and remained consistent for each participant. However, the order of song presentation was randomized for each participant.
The second group completed the television protocol, which comprised two identical sessions (at least 24 hours apart) consisting of three 15 minute trials each. These trials invoked the following conditions in randomized order: 1. walking on a treadmill while viewing television (TW-TV), 2. walking on a treadmill while viewing television without sound (TW-TVNoSound), and 3. walking on a treadmill while viewing television with subtitles (no sound) (TW-TVSubtitles). Television programs were presented in random order from a DVD of popular movie “shorts”. Hence, each participant watched the same videos but in different order. At the start of each session, the participant completed a 5 minute warm-up on the treadmill at a self-selected, comfortable speed. Prior to each trial, the participant also did a 2 minute warm-up at his or her preferred speed, subject to the same TV condition (sound, no sound or subtitles) as the corresponding trial. Subsequent to the warm-up, the participant rested for 45 seconds (quiet standing) and then began walking on the treadmill at his or her preferred speed. The preferred speed was determined prior to the trial with the participant walking slowly on the treadmill. The speed was increased in 0.1 mph increments until the participant reported that his or her preferred speed had been reached. The speed was then increased by 0.5 mph and then sequentially decremented by 0.1 mph until the participant reported attaining his or her preferred speed. The mean speed obtained by the above procedure was taken as the preferred walking speed. A motorized treadmill (GaitKeeper; Mobility Research) was used for the treadmill conditions.
Force-sensitive resistors (Model no. 406, Interlink Electronics) under the insole of each shoe of the subject were used to measure heel strikes. A change in voltage indicating a heel contact with the walking surface was sampled at 200 Hz. These data were recorded by a custom-made datalogger, constructed by mounting a programmable processor (R-Engine-A, Tern Inc.) inside an enclosure. The datalogger was carried by the participant in a waist pouch. The FSRs fed into the datalogger via two wires along the length of the lateral side of each leg. The examiner ensured that the wires and waist pouch did not impede natural gait.
2.3. Stationarity and reverse arrangements test
Suppose x (n) represents observations made during the time interval 0 ≤ n ≤ N − 1, where N represents the length of the signal. From the time series point of view, we can consider x (n) to be a discrete-time series since the observations are made at time intervals from the discrete set ϒ of times (with N representing the cardinality of ϒ). The time series can then be considered a realization of the family of real-valued random variables {χn, n ∈ ϒ} that constitute a stochastic process defined on a probability space (Brockwell and Davis., 1991).
Stationarity is a property of a time series in which the probability distribution of values of the series are independent of time translations. In other words, a time series is strictly stationary if the cumulative distribution function of the joint distribution, Fχn1, …, χnN (x1, x2,…, xN), is invariant to a shift in the origin, i.e.,
| (1) |
for all positive τ. This is also referred to as strong stationarity as opposed to weak or wide-sense stationarity, in which only the first two moments of the series are required to be time-invariant:
| (2) |
| (3) |
The non-parametric reverse arrangement test (RAT) has often been used to test the wide-sense stationarity of a time series (Bendat and Piersol, 2000; Alves and Chau, 2008; Chau et al., 2005; Cao et al., 1997). The test searches for monotonic trends in the mean square values calculated within nonover-lapping intervals of a particular signal of interest. The steps of the reverse arrangement test are as follows (Bendat and Piersol, 2000; Chau et al., 2005):
-
Divide the time series into K nonoverlapping segments, with the assumption that the data within each segment is independent. If a priori knowledge about the length of a segment exists, then the number of segments can be calculated as:
(4) L where L is the desired segment length and ⌊•⌋ represents the greatest integer function. It is clear that N is not necessarily an integer multiple of K. Hence, some of the data points must be omitted. Previous research on stride intervals time series showed that there are no significant statistical differences between stationarity estimates from different data trimming approaches (Fairley et al., 2010). Therefore, the data were trimmed from both sides as suggested in Fairley et al. (2010), and the trimmed version of the signal is denoted by xt(m) where 0 ≤ m ≤ M − 1 and M = KL ≤ N.
- Form a vector y ∈ ℝK whose points are assigned as follows:
(5) -
A reverse arrangement occurs when y(a) > y(b) for a < b. Hence, using this simple rule, for y(k) form an indicator, i(k, d) as follows:
(6) for 1 < d ≤ D where D = K − k − 1. Therefore, the number of reverse arrangements for the kth time step is given by(7) and the total number of reverse arrangements is given by(8) -
For a stationary process, the distribution of IT is approximately normal and its expected value is given by
(9) and its variance by(10) Therefore, the null hypothesis is that IT comes from a normal distribution with its mean and variance given by (9) and (10), respectively. The null hypothesis is rejected at a significance level α if IT falls outside the corresponding critical values.
In this paper, the test statistic defined as
| (11) |
is used, with the assumption that zT ~ 𝒩 (0, 1). The critical values at significance level α are then z1−α/2 and zα/2 where z is a standard normal variate, and for a 5% significance level these are given by zα/2 = − 1.96 and z1−α/2 = 1.96. The values of the test statistics, zT, can fall within one of the three possibilities:
zT ≤ zα/2 → There are fewer reverse arrangements than expected of a stationary signal, implying the presence of an upward trend in the mean square sequence.
zT ≥ z1−α/2 → There are more reverse arrangements than expected of a stationary signal, implying the presence of a downward trend in the mean square sequence.
zα/2 < zT < z1−α/2 → The null hypothesis that a time series is (weakly) stationary can be accepted.
2.4. Data analysis
2.4.1. Stride Interval Analysis
Stride intervals were calculated using an automatic stride interval extraction algorithm (Chau and Rizvi, 2002). From the set of probabilistic stride intervals, strides that fell outside the 0.01 and 99.99% of a gamma distribution fit were removed as these stride times were considered unphysiologically long or short. It should be also mentioned that stride interval time series were trimmed to 15 minutes duration. This was done by removing the first 59 seconds of data and only including data up to the 959th second. This trimming was done to remove any extraneous static portions of the recordings and to exclude any effects due to acceleration towards the preferrred walking speed at the beginning of the trial and the deceleration at the end of the walking trial.
2.4.2. Stationarity Testing
Due to the sensitivity of the RAT to window size, we tested stationarity at a range of window sizes (10 to 45 strides), at increments of 5 strides. The minimum window size was constrained to at least 10 stride intervals and the maximum window size set such that a minimum of 10 windows were available for analysis. This maintained an adequate number of data points as required to estimate a single statistical parameter (Chau et al., 2005) when calculating both the mean squared value within each interval and the total number of reverse arrangements. As the time series lengths were not exact multiples of the chosen window sizes, both ends of the time series were trimmed, as justified earlier.
We investigated the sources of nonstationarity in all trials that violated the hypothesis of stationarity. We divided each stride interval time series into the chosen window length and the summary statistics of mean and variance were computed for each window. The null hypothesis of time invariance of each summary statistic was tested via regression analysis. A 5% significance level was used throughout. Left and right foot data were considered separately.
3. Results
3.1. Effect of window size
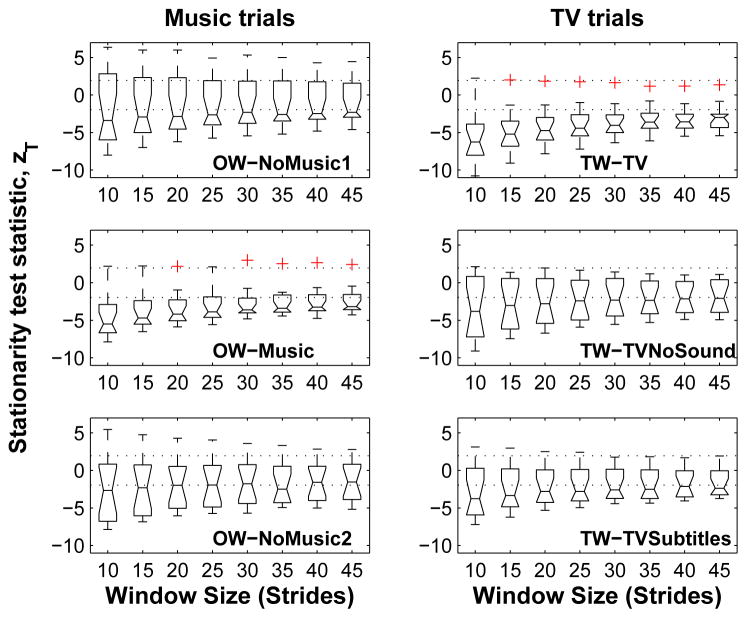
The boxplot in Figure 1 shows the variation in the stationarity test statistic, zT, with different window sizes for the first music and TV sessions, for the right foot. Similar plots were observed for all other walking trials, and for the left foot. A few general observations are in order.
Figure 1.
The effect of window size on the stationarity test statistic, zT, for the first music (left panel) and TV (right panel) sessions, right foot.
The stride interval time series were overwhelmingly nonstationary. The median values of the stationarity test statistic generally fell outside the stationary range at the 5% level of significance (denoted by the dashed lines), i.e., usually med|zT| > 1.96. Two trials exhibited weakly stationary stride time series: (i) OW-NoMusic2 in music session 1, at window sizes greater than 20 in the right foot and all window sizes for the left foot (not shown), and (ii) OW-NoMusic1 in music session 2 at all window sizes for both feet (not shown). These two time series constituted only 8.3% of the 24 unique combinations of session (music or TV), trial (1,2 or 3) and side (left or right).
The stationarity test statistic alone did not suggest a preferred window size. A Kruskal-Wallis test found that the mean ranks for the stationarity test statistic were not significantly different among window sizes (p ≥ 0.95) in all cases other than the two time series identified above.
The number of nonstationary time series in each walking condition tended to decrease with increasing window size. At larger window sizes, a time series is divided into fewer segments; thus, a shorter mean square sequence is created, resulting in fewer comparisons between subsequent mean square values, and thereby reducing the number of opportunities for detecting a reverse arrangement.
The variation in the stationarity statistic decreased with increasing window size. This trend is apparent in the progressive shortening of the boxplot whiskers in Figure 1.
A reduction in the number of nonstationary stride series with larger window sizes is intuitively correct because even a slow varying trend would appear stationary at a sufficiently large window size. Hence the reverse arrangements test is least reliable at larger window sizes where nonstationarities due to fast varying trends may go undetected. This results in a trade-off between maintaining an adequate number of stride intervals within each window and generating a sufficiently long mean square sequence for identification of non-stationarities; we chose an intermediate value of 25 stride intervals for the window size for all further analysis. This resulted in 263 nonstationarities in total: 124 in session 1 (31 per side for music and TV stimuli), and 139 in session 2 (music: 34 (right), 33 (left); TV: 35 (right), 37 (left)).
3.2. Between sessions and between sides
Gait speeds were consistent among all trials (p > 0.21, Kruskal-Wallis Test) and between sessions (p > 0.12, Mann-Whitney U-test) in the music protocol. Similarly, in the TV protocol, no differences in gait speed among trials (p > 0.51, Kruskal-Wallis Test) or between sessions (p > 0.5, Mann-Whitney U-test) were observed. At the chosen window size of 25 and a given side (left or right), there were no statistical differences in the stationarity test statistic (zT) values between sessions 1 and 2 for all corresponding music (p > 0.36, Mann-Whitney U-test) trials. The same held true for all corresponding TV (p > 0.58, Mann-Whitney U-test) trials. Further, zT values did not differ between left and right sides within any music (p > 0.76, Mann-Whitney U-test) or TV trial (p > 0.89, Mann-Whitney U-test).
3.3. Effect of music or TV on stride interval stationarity
As seen in Figure 2, OW-Music (overground walking while listening to music) generally had the highest number of nonstationarities out of the three walks in the music sessions. A one-way ANOVA confirmed that the number of nonstationarities were different across conditions for both sessions (p < 10−4). Post-hoc pairwise comparisons revealed that in session 1, the music and second no music walks differed significantly using a Bonferroni-corrected adjusted significance level of 0.0167 (p = 0.0017, T-test) while in session 2, the music and first no music walks were significantly different (p = 10−4). TW-TV (TV with sound) had the most nonstationarities of all walking conditions. The trial (either TW-TVNoSound or TW-TVSubtitles) with the least amount of nonstationarities varied with window size and session. A one-way ANOVA verified significant differences across TV trials for both sessions (p < 6.2×10−5) while post-hoc pairwise comparisons identified significant differences between the TV with sound walk and the other two TV walks (p < 0.002).
Figure 2.
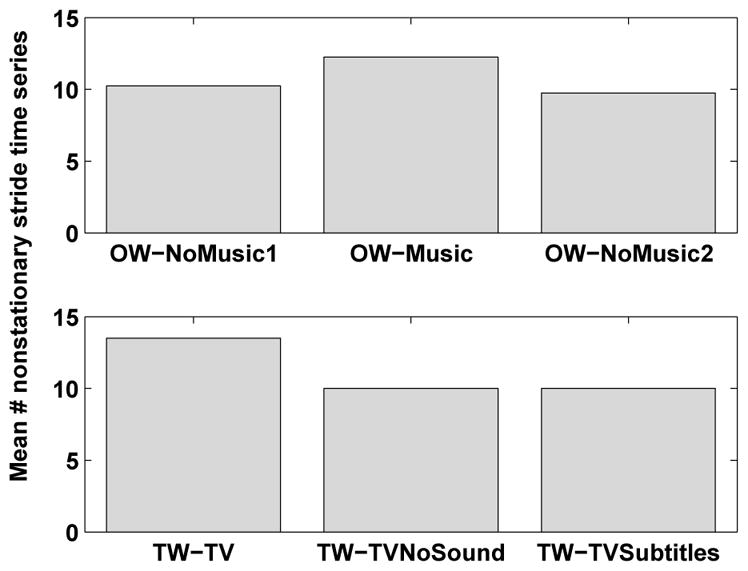
Average number of nonstationary time series per condition
3.4. Sources of non-stationarity
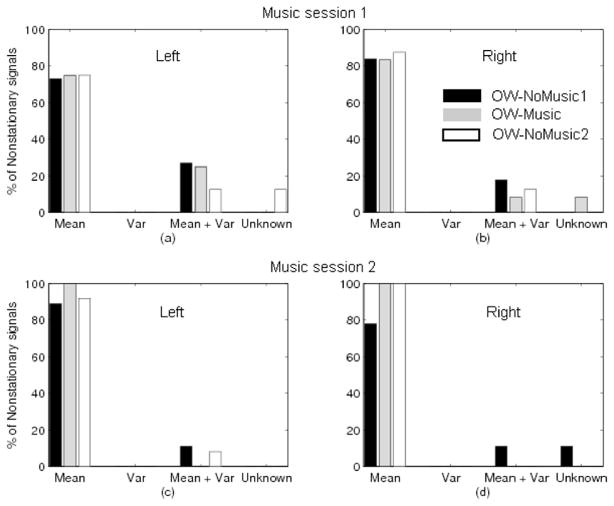
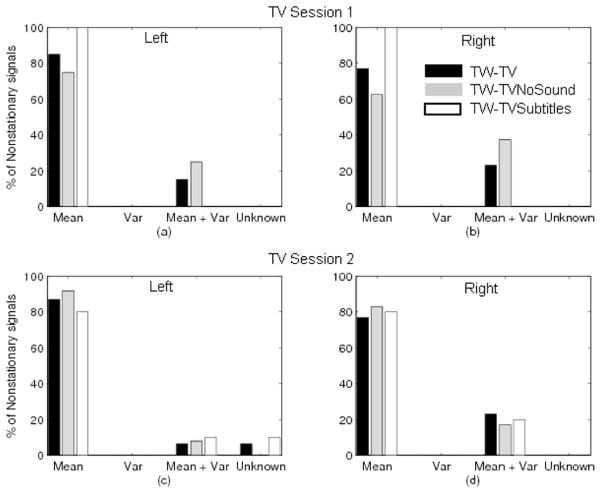
As depicted by Figures 3 and 4, nonstationarities were mainly due to variations in the mean stride interval over time. No stride series demonstrated time-dependent variance alone, though 10–20% of nonstationarities could be attributed to changes in both mean and variance in at least one walk in all sessions. Also, in any one session, no more than one walk would have nonstationarities due to unknown causes, and this was always less than 10% of the nonstationarities for that walk. Hence, for all walking conditions, the major cause of nonstationarity was a time-varying mean.
Figure 3.
Sources contributing to nonstationarity of the time series during music sessions, as a percentage of the nonstationarities identified within each particular walking condition: left foot during first (a) and second (c) sessions; right foot during first (b) and second (d) sessions.
Figure 4.
Sources contributing to nonstationarity of the time series, as a percentage of the nonstationarities identified within each particular walking condition: left foot during first (a) and second (c) sessions; right foot during first (b) and second (d) sessions.
4. Discussion
4.1. Locomotor perspective of nonstationarity
The nonstationarities uncovered for each walking condition in this experiment are suggestive of the complexities inherent to gait. Even during overground walking at one’s self-selected comfortable walking speed, time-varying changes in the stride interval time series occur within a 15 minute walk and are largely due to a time-dependent mean. These changes in time may be the result of fatigue, loss or modification of concentration, anticipation of task completion or boredom (Hausdorff et al., 1999). On the other hand, these nonstationarities may also be a result of intrinsic nonlinearities present in stride dynamics. Studies have shown that the healthy adult locomotor system possesses memory, that is, the change from one stride to the next contains a temporal structure that has been associated with statistical persistence (Hausdorff et al., 1999; Ghafari et al., 2009). Nonstationarities may also arise, at least in part, as a consequence of the integration of multiscale information from various sensory inputs such as the visual, vestibular and proprioceptive systems (Dietz, 2002; West and Griffin, 1999). Furthermore, gait is achieved via the neuronal control system (Ghafari et al., 2009), requiring at least 30 major muscles working in concert, both temporally and kinetically (Kwak, 2007). These interactions between the neuronal and musculoskeletal systems can be affected by tactile, auditory and visual cues (Schauer and Mauritz, 2003; Prokop et al., 1997).
4.2. The effect of music
The results of the music sessions suggest that music does in fact alter one’s stride interval dynamics. Overground walking with music led to more nonstationary stride interval time series than overground walking without music. During interviews after each session, nine of the fifteen participants commented that the music altered their strides. These participants reported that they walked to the tempo of the music or that the music caused them to lose concentration during the task. Indeed, previous studies have shown that music can increase walking speed and induce synchronization of walking pace to the beat of the music (Styns et al., 2007). The music in these sessions consisted of a random mix of popular songs, each with a different tempo. Participants may have altered their stride rhythm to align with each tempo, thus leading to more nonstationarities in the stride intervals.
Multiple studies have investigated the effect of rhythmic auditory stimulation (RAS), that is, the effect of rhythmic stimuli, such as rock music on human gait dynamics (Thaut et al., 1999). RAS is a neurologic technique that exploits the physiological effects of an auditory rhythm on the motor system in order to improve the control of movement (Prassas et al., 1997). In fact, RAS has been shown to improve gait performance in Parkinson’s disease (PD), Huntington’s chorea and hemiparesis (Schauer and Mauritz, 2003; Hayashi et al., 2006). By entrainment through the reticulospinal pathway, RAS can act as an internalized time keeper in rhythmic patterned movement (Kwak, 2007; Prassas et al., 1997), intuitively, producing more stationary gait. Contrary to this notion, nonstationarities during music listening increased in our study, presumably because gait is highly susceptible to rhythmic sensory cuing (Thaut et al., 1999) and rhythmic changes in the music occurred throughout the walk.
The magnification of nonstationary behaviour during the music walk (OW-Music) may also be connected to a “distraction effect” that music can cause during exercise at submaximal intensities (Yamashita et al., 2006). Distraction may arise with increasing comfort level of the person exercising, or a decrease in fatigue-induced stress (Yamashita et al., 2006) over time. This distraction effect may also be attributed to a narrowing of one’s attention to focus only on the music and a reduction in the awareness of bodily sensations such as fatigue (Karageorghis et al., 2009). Consequently, the person perceives lower exertion during exercise (Karageorghis et al., 2009), an effect linked to a reduction in the metabolic cost of exercise achieved by promoting greater neuromuscular efficiency (Yamashita et al., 2006). The fact that participants became cognizant of their distracted state implies that they may have attempted to consciously correct their gait rhythm from time to time, exacerbating the overall nonstationarity of the stride interval time series. In such sense, the distraction might be more aptly described as cyclic or intermittent rather than constant.
The combined effects of rhythmic synchronization and cyclic distraction, combined with the distributed effect of music on the brain and motor control system (Karageorghis et al., 2009; Emery et al., 2003) potentially heightened the nonstationarities observed in the walking with music trials through increased central modulation of gait.
4.3. The effect of TV and treadmill
Intuitively, one might think that treadmill walking would result in more stationary gait due to the constant speed constraint. However, our results show the opposite to be true. Increased incidence of nonstationary stride interval time series during treadmill walking may be the result of many factors. The treadmill modality constrains walking by forcing the subject to maintain a relatively constant speed as well as restricting the area of movement and the subject’s stride length, all factors shown to influence human locomotor control (Wall and Charteris, 1981). When subjects first start the trial on the treadmill, they must adjust their speed and stride length to that permitted by the moving belt. In particular, it has been found that stride intervals increase over time while walking on a treadmill (Wall and Charteris, 1981), which may in part explain the time-varying mean. During the television trials, participants visually attended to the TV program while attempting to walk at a steady pace. Studies have shown that the stride length is in-fluenced by visual distractions such as changes in optic flow (Prokop et al., 1997). Most of the participants (87%) subjectively acknowledged that watching television altered their gait. Many stated that the TV diminished their focus on walking, resulting in near stumbles on multiple occasions. Others thought the television helped them to concentrate on their walking, so as to mitigate the risk of falling.
One of the television trials involved listening to the program. Like the walking with music trial, listening to the television further diverted the participant’s attention from the walking task at hand, possibly causing nonstationarities through the distraction effect introduced above. Based on evidence from a mobile phone study (Murray-Smith et al., 2007), it is also possible that people walked in step to conversations on the television. Whether television caused increased concentration, distraction or synchronization, the combination of visual and auditory distractions seems to have led to additional central modulations of the locomotor system, resulting in more nonstationarities in the stride interval time series.
4.4. Relevance to pedestrian mobility
Interestingly, in a recent study of pedestrian safety while listening to music, Renfroe et al. (2010) noted that self-reported distraction levels were highest when participants listened to music that changed from a slow to fast tempo. Indeed, listening to headphones while walking has been implicated as a distraction that leads to fewer cautionary behaviors (Bungum et al., 2005). As this study deployed mixed musical stimuli with slow to fast tempo transitions, exaggerated nonstationarities may be an indicator of greater distraction, and ultimately reduced safety. Using television as an audio and visual distraction, Malone and Bastian (2010) found that distracted participants exhibited slower adaptation of gait in response to perturbations by a split belt treadmill. Our results offer a potential explanation. With the distraction of television, more stride interval time series became nonstationary, suggesting the occurrence of more locomotor adaptations. It is thus plausible that as the locomotor system becomes preoccupied with adaptations associated with the television distraction, its ability to react to perturbations diminishes.
4.5. Relevance to analysis of statistical persistence
This investigation has determined that the stride interval time series of adults walking to music or watching television are generally nonstationary. Thus when performing further analyses on stride time series, such as the estimation of the fractal scaling exponent, which is beyond the scope of the current manuscript, it is important to use techniques that account for this nonstationary behaviour. In particular, wavelet-based approaches (e.g., Simonsen et al. (1998)) or DFA in some cases (e.g., Peng et al. (1994); Chen et al. (2002)) are particularly suited to the detection of statistical persistence in time series which exhibit nonstationary behaviour (Peng et al., 1994; Chen et al., 2002). However, one should be careful when applying DFA to non-stationary time series since certain nonstationarities such as random spikes or time-evolving correlations can differentially influence the scaling estimate (e.g., introduce cross-overs at certain scales) (Chen et al., 2002).
5. Conclusion
In this paper, we investigated the stationarity of stride intervals in healthy adults while exposed to several conditions of music and television stimuli. Overall, the stride interval time series were nonstationary under these conditions. Nonstationarities, primarily in the form of time-varying means, were particularly abundant when walking on a treadmill while watching television with sound. The heightened number of nonstationarities in trials involving auditory stimuli suggests that natural gait rhythms are especially susceptible to the effects of music and television sounds. The consequence of altered stride dynamics on environmental vigilance (e.g., pedestrian safety) and responsiveness to perturbations (e.g., risk of falls) ought to be investigated in future research.
References
- Alves N, Chau T. Stationarity distributions of mechanomyogram signals from isometric contractions of extrinsic hand muscles during functional grasping. Journal of Electromyography and Kinesiology. 2008;18(3):509–515. doi: 10.1016/j.jelekin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Bendat J, Piersol A. Random Data: Analysis and Measurement Procedures. 3. Wiley; New York: 2000. [Google Scholar]
- Brockwell PJ, Davis RA. Time series : theory and methods. 2. Springer-Verlag; New York, N.Y., USA: 1991. [Google Scholar]
- Bungum T, Day C, Henry J. The association of distraction and caution displayed by pedestrians at a lighted crosswalk. Journal of Community Health. 2005;30(4):269–279. doi: 10.1007/s10900-005-3705-4. [DOI] [PubMed] [Google Scholar]
- Cao H, Ellis BR, Littler JD. The use of the maximum entropy method for the spectral analysis of wind-induced data recorded on buildings. Journal of Wind Engineering and Industrial Aerodynamics. 1997;72(1–3):81–93. [Google Scholar]
- Chau T, Chau D, Casas M, Berall G, Kenny DJ. Investigating the stationarity of paediatric aspiration signals. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005;13(1):99–105. doi: 10.1109/TNSRE.2004.841384. [DOI] [PubMed] [Google Scholar]
- Chau T, Rizvi S. Automatic stride interval extraction from long, highly variable and noisy gait timing signals. Human Movement Science. 2002;21(4):495–514. doi: 10.1016/s0167-9457(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ivanov PC, Hu K, Stanley HE. Effect of nonstationarities on detrended fluctuation analysis. Physical Review E. 2002;65(4):1–15. doi: 10.1103/PhysRevE.65.041107. [DOI] [PubMed] [Google Scholar]
- Dietz V. Proprioception and locomotor disorders. Nature Review Neuroscience. 2002;3(10):781–790. doi: 10.1038/nrn939. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10(4):848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- Emery CF, Hsiao ET, Hill SM, Frid DJ. Short-term effects of exercise and music on cognitive performance among participants in a cardiac rehabilitation program. Heart and Lung. 2003;32(6):368–373. doi: 10.1016/s0147-9563(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Fairley J, Sejdić E, Chau T. An investigation of stride interval stationarity in a paediatric population. Human Movement Science. 2010;29(1):125–136. doi: 10.1016/j.humov.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Fung J, Richards CL, Malouin F, McFadyen BJ, Lamontagne A. A treadmill and motion coupled virtual reality system for gait training post-stroke. CyberPsychology and Behavior. 2006 Apr;9(2):157–162. doi: 10.1089/cpb.2006.9.157. [DOI] [PubMed] [Google Scholar]
- Ghafari AS, Meghdari A, Vossoughi GR. Forward dynamics simulation of human walking employing an iterative feedback tuning approach. Proceedings of the Institution of Mechanical Engineers. 2009;223(I3):289–297. [Google Scholar]
- Hausdorff JM, Askenazy Y, Peng CK, Ivanov PC, Stanley HE, Goldberger AL. When human walking becomes random walking: fractal analysis and moedling of gait rhythm fluctuations. Physica A. 2001;302(1–4):138–147. doi: 10.1016/s0378-4371(01)00460-5. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. Jounral of Applied Physiology. 1997;82(1):262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Zemany L, Peng C, Goldberger AL. Maturation of gait dynamics: stride-to-stride variability and its temporal organization in children. The American Physiological Society. 1999;86(3):1040–1047. doi: 10.1152/jappl.1999.86.3.1040. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Mizuno Y. Music therapy in Parkinson’s disease: Improvements of parkinsonian gait and depression with rhythmic auditory stimulation. Parkinsonism & related disorders. 2006;12:S76. [Google Scholar]
- Karageorghis CI, Mouzourides DA, Priest DL, Sasso TA, Mor-rish DJ, Walley CL. Psychophysical and ergogenic effects of synchronous music during treadmill walking. Journal of Sports & Exercise Psychology. 2009;31(1):18–36. doi: 10.1123/jsep.31.1.18. [DOI] [PubMed] [Google Scholar]
- Kwak EE. Effect of rhythmic auditory stimulation on gait performance in children with spastic cerebral palsy. Journal of Music Therapy. 2007;44(3):198–216. doi: 10.1093/jmt/44.3.198. [DOI] [PubMed] [Google Scholar]
- Malone L, Bastian A. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. Journal of Neurophysiology. 2010;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Smith R, Ramsay A, Garrod S, Jackson M, Musizza B. Gait alignment in mobile phone conversations. 9th International Conference on Human Computer Interaction with Mobile Devices and Services; Association for Computing Machinery; 2007. pp. 214–221. [Google Scholar]
- Owen N, Humpel N, Leslie E, Bauman A, Sallis JF. Understanding environmental influences on walking. American Journal of Preventive Medicine. 2004;27(1):67–76. doi: 10.1016/j.amepre.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of dna nucleotides. Physical Review E. 1994;49(2):1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- Prassas S, Thaut M, McIntosh G, Rice R. Effect of auditory rhythmic cuing on gait kinematic parameters of stroke patients. Gait and Posture. 1997;6(3):218–223. [Google Scholar]
- Prokop T, Schuberet M, Berger W. Visual influence on human locomotor: Modulation to changes in optic flow. Experimental Brain Research. 1997;114(1):63–70. doi: 10.1007/pl00005624. [DOI] [PubMed] [Google Scholar]
- Renfroe M, Stavrinos D, Mizzell J, de Jong D, Schwebel D. Pedestrian safety while listening to music. Annals of Behavioral Medicine. 2010;39(Suppl 1):147. [Google Scholar]
- Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clinical Rehabilitation. 2003 Jul;17(7):713–722. doi: 10.1191/0269215503cr668oa. [DOI] [PubMed] [Google Scholar]
- Simonsen I, Hansen A, Nes OM. Determination of the hurst exponent by use of wavelet transforms. Physical Review E. 1998 Sep;58(3):2779–2787. [Google Scholar]
- Styns F, van Noorden L, Moelants D, Leman M. Walking on music. Human Movement Science. 2007;26(5):769–785. doi: 10.1016/j.humov.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Sveistrup H. Motor rehabilitation using virtual reality. Journal of NeuroEngineering and Rehabilitation. 2004;1(1):10. doi: 10.1186/1743-0003-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function: Implications for therapy of movement disorders. IEEE Engineering in Medicine and Biology Magazine. 1999;18(2):101–108. doi: 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- Torres-Solis J, Chau T. Wearable indoor pedestrian dead reckoning system. Pervasive and Mobile Computing. 2010;6(3):351–361. [Google Scholar]
- Wall J, Charteris J. A kinematic study of long-term habituation to treadmill walking. Ergonomics. 1981;24(7):531–542. doi: 10.1080/00140138108924874. [DOI] [PubMed] [Google Scholar]
- West BJ, Griffin L. Allometric control, inverse power laws and human gait. Chaos, Solitons & Fractals. 1999;10(9):1519–1527. [Google Scholar]
- Yamashita S, Iwai K, Akimoto T, Sugawara J, Kono J. Effects of music during exercise on RPE, heart rate and the autonomic nervous system. The Journal of Sports Medicine and Physical Fitness. 2006;46(3):425–430. [PubMed] [Google Scholar]