Abstract
Accelerometry (ACC) shows promise as an easily implemented clinical measure of balance. The purpose of the study was to estimate test–retest reliability of ACC measures and determine the relationship between ACC measured at the pelvis and underfoot center of pressure (COP) measures during sensory organization test (SOT) conditions. Eighty-one subjects were recruited from the community with no known orthopedic or vestibular deficits (19–85 years). Subjects completed three consecutive, ninety second trials for each of the six SOT conditions, while wearing the accelerometer. ACC and COP time series were described by calculating the normalized path length, root mean square (RMS), and peak-to-peak values. The test–retest reliability of the three measures within each SOT condition was estimated over three trials using the intraclass correlation coefficient. ACC and COP test–retest reliability were similar, ranging from 0.63 to 0.80 using ACC and 0.42 to 0.81 using COP for the measure of normalized path length. Linear regression between ACC and COP measures showed significant correlation under almost every SOT condition using both single and average measures across trials. The degree of association between COP and ACC was equivalent when using the first trial or the 3-trial average, suggesting that one trial may be sufficient. The use of accelerometry may have value in estimating balance function and minimizing clinical evaluation time.
Keywords: Posturography, Accelerometer, Sway, Balance, Measurement
1. Introduction
Postural sway is often used to measure clinical changes in balance over time such as in persons with head injury [1], in older adults [2], [3], [4] and [5], or in patients with neurologic disorders such as Parkinson's disease [6]. The widespread need to assess balance has generated need for a reliable, inexpensive, and quantifiable clinical measure. Several protocols exist that are technologically and task-based. The most widely available of the technological options include computerized dynamic posturography (CDP) and force plates, which measure subjects’ center of pressure (COP). COP has commonly been used to characterize postural sway [7] and studies have indicated that COP correlates with poor balance and risk of falls [8]. CDP has proven useful clinically due to its ability to stimulate specific sensory systems [9]. However, with force plates and CDP being relatively immobile and expensive, finding other less expensive methods has been of interest.
Numerous task-based balance tests also exist with good reliability such as the Berg balance scale (BBS) [10] and [11] or timed “up and go” test (TUG) [12]. Recently, O'Sullivan et al. have reported a significant relationship between accelerometry (ACC) measures and the TUG and BBS in older adults [13]. However, these task-based tests suffer from floor [11] and [14] and ceiling [11] and [15] effects that prevent full characterization of balance. Studies have also shown only moderate correlation between subjective tests and COP indicating that different aspects of balance were being measured [11] and [16]. COP and ACC were moderately correlated in healthy young people during single leg standing [17]. These findings suggest that ACC may be a valid quantitative measure of postural sway that is more strongly related to task-based measures.
Postural sway has been tested clinically with CDP using the sensory organization test (SOT). The SOT consists of six conditions designed to separate the sensory effects of vision, proprioception, and vestibular input during standing balance. The protocol consists of the following conditions: feet together on a (1) solid support surface with eyes open, (2) solid support surface with eyes closed, (3) solid support surface, sway referenced surround with eyes open, (4) sway referenced support surface with eyes open, (5) sway referenced support surface with eyes closed, and (6) sway referenced support surface, sway referenced surround with eyes open. Several studies have reported moderate to good reliability of the SOT using CDP in young subjects and older adults [18] and [19]; Dickin and Clark recently reported that within day test–retest reliability of SOT equilibrium scores using the estimated G coefficient ranged between 0.51 (SOT condition 4) and 0.64 (SOT condition 5) for healthy young adults [18]. Others have reported that composite SOT score test–retest reliability was 0.67, with significant changes in the composite and equilibrium scores for SOT conditions 4, 5 and 6 over 5 repetitions when testing young people [20]; this suggests that though the SOT is a commonly used clinical measure, learning effects are present.
Though CDP appears to be the best widely accepted measure, drawbacks in the use of COP exist [21]. Relating COP to the measure of balance depends heavily on the single link assumption, where motion of the COP is assumed to be directly proportional to the movement of the center of mass (COM), which is not the case when different balance strategies are employed [22]. Alternatively, accelerometry was suggested over a decade ago [3], where an accelerometer place around the waist can better approximate the COM. As Winter argued, the horizontal acceleration of COM should be proportioned to the difference between COP and COM, making it a better measure of postural sway since the difference is believed to be an error signal within the human postural control system [23]. Kamen et al. reported the intraclass correlation coefficient to be R > 0.75 for standing balance tasks using an accelerometer [3]. However, the cost, poor low-frequency response, and low sensitivity have been a hindrance in moving accelerometers into the clinic. The low frequency response of the accelerometer should be minimally 0.1 Hz. As shown by Schumann et al. postural sway during quiet standing of subjects with vestibular deficits contains a low frequency component between 0.1 and 0.2 Hz not seen in their healthy counter parts [24]. Additionally, it has been shown that sensitivity on the order of 0.001G's is required to differentiate between eyes open and eyes closed conditions [13] and [25]. With the advent of fast wireless technology and low cost accelerometers with excellent low frequency response and sensitivity, accelerometers to record postural sway is now feasible and efficient. The purpose of the study was to estimate test–retest reliability of accelerometry during the clinically relevant SOT protocol and to determine the relationship between acceleration measured at the pelvis and COP for each of the SOT conditions.
2. Methods
2.1. Subjects
A total of 81 subjects (51 females) participated in the research study with ages ranging from 19 to 85 years old (age 47.8 ± 21.2 years; height 66.3 ± 3.7 inches). Subjects were recruited from the community with no known orthopedic or vestibular deficits. They were screened on the phone and on site to ensure vestibular and neurological normality. All subjects provided written informed consent and the protocol was approved by the University of Pittsburgh institutional review board. Subjects were asked to complete an extended SOT protocol, which consisted of three consecutive trials of each of the six conditions (18 total trials). Subjects were outfitted with a safety harness and an accelerometer; instructions were standardized and each trial was ninety seconds in length.
2.2. Instrumentation
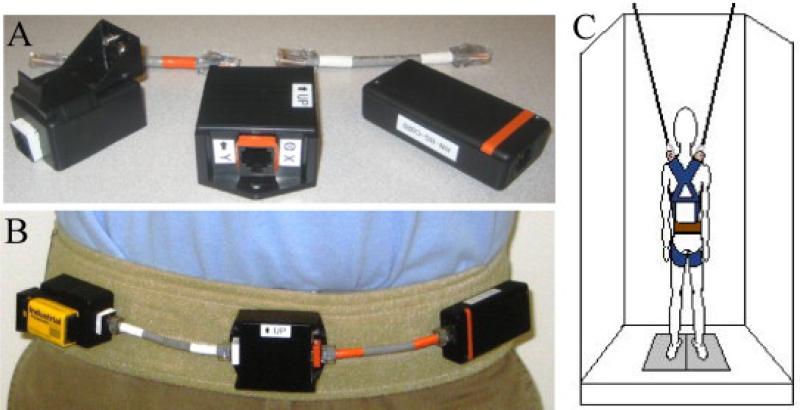
Pelvic accelerations were recorded using a custom accelerometer system. The system was composed of three modules: battery, accelerometer, and Bluetooth transmitter; the modules were connected using CAT6 cables. The system was powered by a 9 V battery with regulated 6 V power to the accelerometer. The accelerometer was a dual-axis accelerometer (ADXL213AE, ±1.2 g, Analog Devices, Inc.) mounted on a breakout board (SparkFun Electronics) and oriented to capture mediolateral and anteroposterior accelerations. The Bluetooth module, Roving Networks BlueSentry (model RN-800S), wirelessly transmitted each axis with 16-bit accuracy at 100 Hz. The accelerometer system was affixed to a gait belt using Velcro (Fig. 1). The gait belt was secured snugly around the subject's pelvis across the subject's anterior superior iliac spine and posterior superior iliac spine bony landmarks. A custom-written LabVIEW (National Instruments Corporation) program was used to acquire the data. COP data were collected using an EquiTest® machine (NeuroCom SMART EquiTest®) at 100 Hz as well.
Fig. 1.
(A) Close up view of the accelerometer system; (B) anterior view of accelerometer system worn by subjects; (C) schematic of accelerometer system (brown belt) worn by subject on EquiTest® machine with harness (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.3. Data analysis
Both ACC and COP data were processed with a 4th order, low-pass Butterworth filter with a cut-off frequency of 1.25 Hz. The initial orientation of the accelerometer within each trial was subtracted throughout to remove the initial tilt with respect to gravity. Data from the EquiTest® machine and accelerometer were synchronized programmatically in post-collection using Matlab® by calculating the cross-correlation between the two variables and finding the maximum correlation value within an eleven second window (+1 s to −10 s), since the start up delay of the Bluetooth (negative delay) was typically on the order of −3 to −5 s.
The time series of the COP and ACC were processed to find the normalized path length (NPL), root mean square (RMS), and peak-to-peak (P2P). The first five seconds of the time series was discarded, and the following forty seconds were used. The RMS and NPL were calculated as follows:
| Equation(1) |
| equation(2) |
where t is the time duration, N is the number of time samples, pavg is the average across the time series, and pj is either ACC or COP data at time sample j.
Test–retest reliability was estimated for the NPL, RMS, and P2P values between the three trials of each SOT condition for ACC and COP. Intraclass correlation coefficient (ICC) and 95% confidence intervals were used to determine the ratio of between-subject to total variance in ACC and COP measures for the six SOT testing conditions. Each ICC was derived using two-way mixed effects analysis of variance with a model comprised of subject and trial number. The test–retest reliability was categorized into four groups: poor (0–0.4), fair (0.4–0.59), good (0.6–0.74), and excellent (0.75–1).
The strength of the association between ACC and COP was estimated through linear regression using NPL, RMS, and P2P values with COP as dependent variable, ACC as the univariate predictor, and age as a covariate for each measure separately. The linear regression was performed for a combination of individual trials and 2 and 3-trial averages for each condition. Beta coefficients for ACC and age were tested in each sensory condition against a Type I error rate of p < 0.05. Coefficients of determination (R2) were reported as the amount of variance in COP estimated by ACC and age for conditions showing a significant association. All statistical analyses were conducted using Statistical Package for the Social Sciences, release 16.0.1 (SPSS Inc., 2007, Chicago, IL).
3. Results
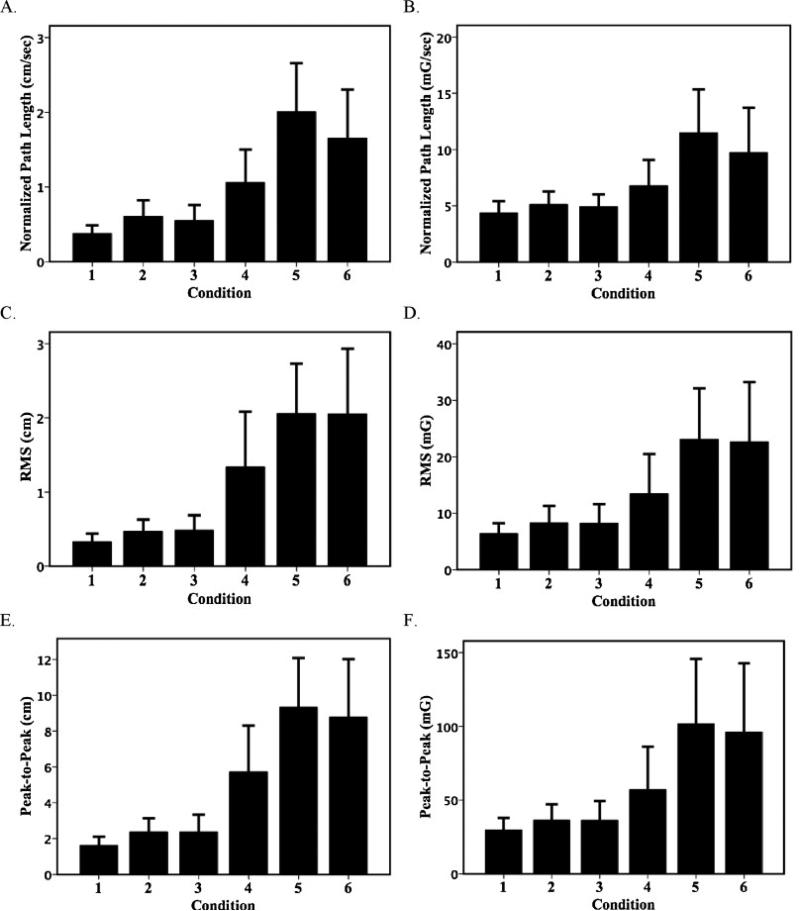
The ACC and COP summary measures during SOT produced similar results. Fig. 2 shows the NPL, RMS, and P2P of COP and ACC averaged across the three trials for all six SOT conditions. Completion rates across three trials ranged from 100% for conditions 1–2, to 79% on condition 5. On average, non-completing subjects were older than those subjects able to complete all three trials of a given condition. The age differences on conditions 4, 5, and 6 were 12, 15, and 9 years, respectively. This age difference was significant on condition five only (p < 0.03). The greatest COP and ACC movement and between subject variability were seen in condition 5.
Fig. 2.
Center of pressure and pelvic acceleration (mean, S.D.) by test condition for (A) COP normalized path length, (B) ACC normalized path length, (C) COP RMS, (D) ACC RMS, (E) COP peak-to-peak, and (C) ACC peak-to-peak: bars represent group mean of three-trial averages for each SOT condition.
Test–retest reliability of ACC was as good as or better than COP for all three summary measures. (Table 1) Test–retest reliability estimates for NPL of ACC measurements taken across the three repetitions ranged from good (0.63–0.72) for condition 1–4 to excellent (0.77–0.80) for conditions 5–6. Test–retest reliability for ACC exceeded the reliability for COP during condition 1, and otherwise was similar. RMS measures exhibited slightly lower ICC values, with ACC ICC values ranging from fair to good (0.46– 0.68) for conditions 2–6 and poor (0.16) for condition 1; however, the ACC ICC values were better than or consistent with those estimated for COP. P2P reliability was similar to NPL with a slightly larger range of ICC values from fair to excellent (0.47–0.79). The ICC estimates for ACC exceeded COP for conditions 1, 3, and 6 for both RMS and P2P measures and were otherwise consistent.
Table 1.
Test-retest reliability of three trials for six testing conditions: intraclass correlation coefficients and 95% confidence intervals calculated for normalized path length, root mean squared (RMS), and peak-to-peak parameters for both ACC and COP.
| Condition | n | Intraclass correlation coefficient (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| Normalized path length | RMS | Peak-to-peak | |||||
| ACC | COP | ACC | COP | ACC | COP | ||
| 1 | 81 | 0.72 (0.62–0.78) | 0.42 (0.28–0.55) | 0.16 (0.03–0.31) | 0.14 (0.11–0.29) | 0.47 (0.34–0.60) | 0.44 (0.30–0.57) |
| 2 | 81 | 0.72 (0.63–0.80) | 0.77 (0.69–0.84) | 0.46 (0.33–0.59) | 0.46 (0.33–0.59) | 0.54 (0.41–0.65) | 0.54 (0.42–0.66) |
| 3 | 80 | 0.63 (0.52–0.73) | 0.63 (0.51–0.73) | 0.57 (0.38–0.63) | 0.45 (0.32–0.58) | 0.58 (0.46–0.69) | 0.56 (0.44–0.68) |
| 4 | 78 | 0.67 (0.56–0.76) | 0.61 (0.49–0.72) | 0.68 (0.57–0.77) | 0.68 (0.58–0.77) | 0.72 (0.62–0.80) | 0.75 (0.66–0.82) |
| 5 | 64 | 0.80 (0.71–0.86) | 0.76 (0.66–0.84) | 0.55 (0.41–0.68) | 0.68 (0.54–0.76) | 0.71 (0.61–0.80) | 0.62 (0.49–0.73) |
| 6 | 69 | 0.77 (0.68–0.84) | 0.81 (0.73–0.87) | 0.66 (0.54–0.76) | 0.64 (0.52–0.74) | 0.79 (0.71–0.86) | 0.75 (0.66–0.83) |
ACC was found to be significantly associated with COP for every SOT condition in almost all combinations of single and averaged trials. Table 2 presents the estimates of the coefficients of determination for COP predicted by ACC for various combinations of trials for each condition with age as a covariate. Normalized path length displays the greatest coefficients of determination. For NPL, single first trial measures of ACC predicted the greatest amount of variance in COP in conditions 2–5; for condition 6, the second and 3-trial and 2-trial averages of ACC sway showed a stronger association with COP (91–92%) when compared with first trial measures (70%). Within NPL measures, age was shown to be a significant covariate (p < 0.05) with ACC in predicting COP most consistently in conditions 1 and 3, where the correlation coefficients were lower.
Table 2.
Linear regression coefficients of determination of COP values predicted by ACC with age as a covariate for each condition by various trial parameters.
| COP outcome | ACC predictor | Condition coefficients of determination (R2) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Normalized path length | |||||||
| 1st trial | 1st trial | 0.24* | 0.54 | 0.57** | 0.94 | 0.79 | 0.70** |
| 2nd trial | 2nd trial | 0.33 | 0.43 | 0.34** | 0.68* | 0.78** | 0.91 |
| Average | 1st trial | 0.13* | 0.36 | 0.42 | 0.89 | 0.76** | 0.68 |
| Average | Average | 0.18* | 0.43 | 0.43* | 0.87* | 0.77* | 0.92 |
| 2-Trial avg | 2-Trial avg | 0.21* | 0.47 | 0.47** | 0.87* | 0.79 | 0.91* |
| RMS | |||||||
| 1st trial | 1st trial | 0.06 | 0.50* | 0.56 | 0.55 | 0.63 | 0.64 |
| 2nd trial | 2nd trial | 0.17 | 0.18 | 0.17 | 0.57 | 0.40 | 0.52 |
| Average | 1st trial | 0.01 (ns) | 0.39* | 0.53 | 0.48 | 0.43 | 0.56 |
| Average | Average | 0.15 | 0.45 | 0.59 | 0.61 | 0.53 | 0.64 |
| 2-Trial avg | 2-Trial avg | 0.11 | 0.36 | 0.45 | 0.59 | 0.51 | 0.65 |
| Peak-to-peak | |||||||
| 1st trial | 1st trial | 0.12 | 0.54* | 0.63 | 0.62 | 0.65 | 0.71 |
| 2nd trial | 2nd trial | 0.06 | 0.26 | 0.32 | 0.51 | 0.63 | 0.66 |
| Average | 1st trial | 0.05 (ns) | 0.47 | 0.52 | 0.48 | 0.53 | 0.54 |
| Average | Average | 0.21 | 0.57 | 0.57 | 0.62 | 0.61 | 0.71 |
| 2-Trial avg | 2-Trial avg | 0.10 | 0.52 | 0.51 | 0.58 | 0.64 | 0.78 |
ns: not significant.
Age effect as covariate p < 0.05.
Age effect as covariate p < 0.01.
Coefficients of determination for RMS and P2P were lower than those for NPL, predicting less variance in the corresponding COP parameters. The largest predicted variance for RMS and P2P was found comparably in the first trial and average comparison. Additionally, age did not appear as a significant covariate with the RMS and P2P measures. Age was found to be a significant covariate in the relationship between ACC and COP for the RMS parameter with the first trial and the 3-trial average of condition 2 (firm surface/eyes closed). Similarly, age was a significant covariate for the P2P measures only in condition 2 (firm surface/eyes closed) using the first of the three trials.
4. Discussion
Acceleration measured at the pelvis and center of pressure were found to be well correlated across the SOT conditions. Additionally, the test–retest reliabilities of the ACC measures were as good as or better than the COP in almost every SOT condition; this includes peak-to-peak, which is currently used in CDP. The correlation of ACC and COP is not surprising, since the accelerations measured in the experiment are not unlike COP. Since the accelerometer used in this case was a dual-axis accelerometer, this made extraction of the gravitational acceleration component due to tilt impossible. Thus, the measured accelerations represent a changing combination of both the tilt and acceleration with respect to the pelvis. The dominating term in very small movements, (conditions 1 and 2) is the pelvic accelerations, while the gravitational acceleration due to tilt dominates during large movements (conditions 5 and 6). The trade off of these terms arises due to the differential magnitudes of these values. Typical pelvic or torso accelerations fall on the order of 0.001–0.01G's as demonstrated by O'Sullivan et al. [13] and Moe-Nilsson et al. [25] while using a triaxial accelerometer with the removal of the gravitational effects; this compares to the gravitational magnitudes of 0.1–0.2G's when the tilt angle falls near 10°. A similar coupling exists in COP. COP is the position of the resultant ground reaction force; this force arises from gravitational torques as well as active and passive torques generated by the body. However, the advantage of using acceleration over COP arises for the position of the measurements. Relative acceleration and orientation of the pelvis more closely approximates the motion of the COM from which balance is defined.
Throughout the study, age was found to be a covariate with the association between ACC and COP; particularly for normalized path length. Since ACC and COP are measured at the same time for all subjects, this suggests that the relationship between NPL of ACC and COP changed with an increase in subject age. The variation in the relationship may be due to changing strategies of balance in the older adults. If a subject were to switch from a continuous ankle strategy to a larger hip strategy that is common of older adults [22], the change in overall motion would be present in NPL. Increased hip strategy leads to greater accelerations of the pelvis in the anteroposterior direction, resulting in increased path length of acceleration but not necessarily COP. As seen in Table 2, condition 3 had the most measures with age as a significant covariate in NPL. Other researchers have also found changes in balance strategy specific to condition 3 of the SOT protocol. Speers et al. reported a change in coordination at the ankles and hips in older adults. Speers et al. argued that older adults may have undergone sensory reweighting in condition 3 not seen in younger patients, leading to an age effect [26]. However, the covariate age effect was not seen across all conditions and measures; this could be due to the stability of the subject population. The older subjects in this study were very healthy; if older adults who were less stable had been included, the greater variability in sway and balance strategies might have made age significant throughout.
The strongest association between ACC and COP for all variables was observed during conditions 4, 5 and 6. The increased association also corresponds with increased sway and variability, since all subjects swayed the most during conditions 5 and 6; similar to what has been reported in the literature [27] and [28]. Previous literature also confirms that acceleration variability is a reliable measure [5] and [29] and can differentiate between healthy and frail older adults while walking [5]. Given that NPL, RMS, and P2P variables are also a measure of variability, it was not surprising that they showed good to excellent reliability on almost all the SOT conditions. Accelerometry was also shown to be better than or consistent with COP in all conditions, indicating that accelerometer may be able to track balance changes just as well as CDP.
No matter which of the three variables was used, it appears that one trial is sufficient to measure sway using accelerometry. As can be seen by the regression coefficients, the first trial is comparable to the three trial average in predicting COP; this indicates that the subjects were consistent with their motor performance and it is unnecessary to introduce repetition that may lead to fatigue or magnified learning effects. The ability to record sway over one trial would lead to increased efficiency, suggesting that time could be minimized if the accelerometry device were used clinically.
Accelerometry also may provide more accurate classification of a patient's balance than CDP. CDP uses the peak-to-peak values of COG (filtered COP data) as the basis for each condition's equilibrium score. The range of COG is anthropometrically related to the anterior–posterior lean angle by approximating height of COG as 55% of the subject's height [30]. Due to the nature of the P2P measure, a single value can be given for several different balance conditions [31]. For example, a patient with a calculated maximum anterioposterior lean of 5° and a minimum of 0° compared with a patient displaying a maximum of 3° of anterior lean and a minimum of −2° during a trial would receive the same score despite the higher degree of instability caused by greater anterior lean in the first case. Due to this limitation as well as others, previous research suggests that SOT is useful but limited in its ability to determine pathology [28], [32] and [33].
Cost is another consideration in developing a clinical postural measurement system. Most devices that presently record sway, including CPD, are expensive and large, requiring lab or clinical space. Accelerometers are small and have significantly decreased in cost, making them reasonable to purchase and package with software to record sway on a large scale basis. These devices will most likely be used clinically soon, yet significant work must be accomplished in order to determine if acceleration data can be used independent of CDP in determining diagnoses, risk of falling, and sensitivity to change over time.
Further investigation should be directed toward developing a protocol where sensory conditions can be altered, as in CDP, to differentiate patient populations and establish population norms. The use of acceleration shows promise in the clinical field, but a sensitivity analysis needs to be performed to assert its usefulness and value. The current study only examined healthy adults that spanned a large age range (19–85 years). More directed studies should be conducted to target specific populations.
5. Conclusion
Accelerometry measurements correlated well with COP during SOT conditions. The ACC measures showed good to excellent test–retest reliability, comparable if not exceeding those using COP, with NPL of the ACC and COP displaying the best test–retest reliability. It was also evident that one trial of accelerometry data exhibited similar results as the mean of three trials, suggesting that one trial may be as useful as conducting multiple trials in a clinical setting. Thus, accelerometry appears to provide a promising avenue for a reliable, low-cost, portable balance measure.
Footnotes
Conflict of interest statement
None of the authors has any conflict of interest.
References
- 1.Lehmann J, Boswell S, Price R, Burleigh A, DeLateur B, Jaffe K, Hertling D. Quantitative evaluation of sway as an indicator of functional balance in post-traumatic brain injury. Arch Phys Med Rehabil. 1990;71:955–962. [PubMed] [Google Scholar]
- 2.Du Pasquier R, Blanc Y, Sinnreich M, Landis T, Burkhard P, Vingerhoets F. The effect of aging on postural stability: a cross sectional and longitudinal study. Clin Neurophysiol. 2003;33:213–218. doi: 10.1016/j.neucli.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Kamen G, Patten C, Du C, Sison S. An accelerometry-based system for the assessment of balance and postural sway. Gerontology. 2000;44:40–45. doi: 10.1159/000021981. [DOI] [PubMed] [Google Scholar]
- 4.Lord S, Ward J, Williams P. Exercise effect on dynamic stability in older women: a randomized controlled trial. Arch Phys Med Rehabil. 1996;77:232–236. doi: 10.1016/s0003-9993(96)90103-3. [DOI] [PubMed] [Google Scholar]
- 5.Moe-Nilssen R, Helbostad J. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait Posture. 2005;21:164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Rocchi L, Chiari L, Cappello A, Horak F. Identification of distinct characteristics of postural sway in parkinson's disease: a feature selection procedure based on principal component analysis. Neurosci Lett. 2006;394:140–145. doi: 10.1016/j.neulet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Prieto T, Myklebust J, Hoffmann R, Lovett E, Myklebust B. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 2002;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 8.Piirtola M, Era P. Force platform measurements as predictors of falls among older people—a review. Gerontology. 2006;52:1–16. doi: 10.1159/000089820. [DOI] [PubMed] [Google Scholar]
- 9.Furman J. Role of posturography in the management of vestibular patients. Otolaryngol Head Neck Surg. 1995;112:8–15. doi: 10.1016/S0194-59989570300-4. [DOI] [PubMed] [Google Scholar]
- 10.Berg K, Wood-Dauphinee S, Williams J. The balance scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27. [PubMed] [Google Scholar]
- 11.Blum L, Korner-Bitensky N. Usefulness of the berg balance scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 12.Morris S, Morris M, Iansek R. Reliability of measurements obtained with the timed “up & go” test in people with parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan M, Blake C, Cunningham C, Boyle G, Finucane C. Correlation of accelerometry with clinical balance tests in older fallers and non-fallers. Age Ageing. 2009;38:308–318. doi: 10.1093/ageing/afp009. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Await E, Carver D, MacKnight C. Feasibility and measurement properties of the functional reach and thetimed up and go tests in the canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2000;55A:M70–M73. doi: 10.1093/gerona/55.2.m70. [DOI] [PubMed] [Google Scholar]
- 15.Boulgarides L, McGinty S, Willett J, Barnes C. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys Ther. 2003;83:328–339. [PubMed] [Google Scholar]
- 16.Frykberg G, Lindmark B, Lanshammar H, Borg J. Correlation between clinical assessment and force plate measurement of postural control after stroke. J Rehabil Med. 2007;39:448–453. doi: 10.2340/16501977-0071. [DOI] [PubMed] [Google Scholar]
- 17.Adlerton A, Moritz U, Moe Nilssen R. Forceplate and accelerometer measures for evaluating the effect of muscle fatigue on postural control during one legged stance. Physiother Res Int. 2003;8:187–199. doi: 10.1002/pri.289. [DOI] [PubMed] [Google Scholar]
- 18.Dickin D, Clark S. Generalizability of the sensory organization test in college-aged males: obtaining a reliable performance measure. Clin J Sport Med. 2007;17:109–115. doi: 10.1097/JSM.0b013e31803bf647. [DOI] [PubMed] [Google Scholar]
- 19.Ford-Smith C, Wyman J, Elswick R. Test–retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76:77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 20.Wrisley D, Stephens M, Mosley S, Wojnowski A, Duffy J, Burkard R. Learning effects of repetitive administrations of the sensory organization test in healthy young adults. Arch Phys Med Rehabil. 2007;88:1049–1054. doi: 10.1016/j.apmr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Visser J, Carpenter M, van der Kooij H, Bloem B. The clinical utility of posturography. Clin Neurophysiol. 2008;119:2424–2436. doi: 10.1016/j.clinph.2008.07.220. [DOI] [PubMed] [Google Scholar]
- 22.Panzer V, Bandinelli S, Hallett M. Biomechanical assessment of quiet standing and changes associated with aging. Arch Phys Med Rehabil. 1995;76:151–157. doi: 10.1016/s0003-9993(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 23.Winter D. Human balance posture control during standing walking. Gait Posture. 1995;3:193–214. [Google Scholar]
- 24.Schumann T, Redfern M, Furman J, El-Jaroudi A, Chaparro L. Time-frequency analysis of postural sway. J Biomech. 1995;28:603–607. doi: 10.1016/0021-9290(94)00113-i. [DOI] [PubMed] [Google Scholar]
- 25.Moe-Nilssen R, Helbostad J. Trunk accelerometry as a measure of balance control during quiet standing. Gait Posture. 2002;16:60–68. doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 26.Speers R, Kuo A, Horak F. Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture. 2002;16:20–30. doi: 10.1016/s0966-6362(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 27.Cohen H, Heaton L, Congdon S, Jenkins H. Changes in sensory organization test scores with age. Age Ageing. 1996;25:39–44. doi: 10.1093/ageing/25.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Furman J. Posturography: uses and limitations. Baillieres Clin Neurol. 1994;3:501. [PubMed] [Google Scholar]
- 29.Henriksen M, Lund H, Moe-Nilssen R, Bliddal H. B Danneskiod-Samsoe Test–retest reliability of trunk accelerometric gait analysis. Gait Posture. 2004;19:288–297. doi: 10.1016/S0966-6362(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 30.Appendix: Principles of Operation. NeuroCom International Inc.; Clackamas, OR: 2007. [Google Scholar]
- 31.Chaudhry H, Findley T, Quigley K, Bukiet B, Ji Z, Sims T, Maney M. Measures of postural stability. J Rehabil Res Dev. 2004;41:713–720. doi: 10.1682/jrrd.2003.09.0140. [DOI] [PubMed] [Google Scholar]
- 32.Di Fabio R. Sensitivity and specificity of platform posturography for identifying patients with vestibular dysfunction. Phys Ther. 1995;75:290–305. doi: 10.1093/ptj/75.4.290. [DOI] [PubMed] [Google Scholar]
- 33.El-Kahky A. Balance control near the limit of stability in various sensory conditions in healthy subjects and patients suffering from vertigo or balance disorders: impact of sensory input on balance control. Acta Otolaryngol (Stockh) 2000;120:508–516. doi: 10.1080/000164800750046018. [DOI] [PubMed] [Google Scholar]