Abstract
Neuromyelitis optica (NMO) is characterized by attacks of optic neuritis and longitudinally extensive transverse myelitis. Cases positive for aquaporin 4 antibodies are classified to NMO spectrum disorder (NMOSD) which includes cases with optic neuritis, transverse myelitis, or with brain lesions typical of NMO. Our three cases with NMO/NMOSD revealed five imaging features: (i) extensive transverse cord lesions, extending more than three vertebral segments, partially persisting as cavitation; (ii) periependymal lesions; (iii) lesions of the corticospinal tracts; (iv) extensive and confluent hemispheric white matter lesions reflecting vasogenic edema and partially involving the cerebral cortices and basal ganglia; and (v) two patterns of serial hemispheric white matter lesions: one is cavitation and another is partial regression or disappearance. Cavitations, in the upper spinal cord and hemispheric white matter, are considered to be caused by severe vasogenic edema and are likely to be one of the characteristic findings in NMOSD.
Keywords: Neuromyelitis optica spectrum disorder, aquaporin-4 (AQP4), white matter lesions, extensive transverse myelitis, vasogenic edema, apparent diffusion coefficient
Introduction
Neuromyelitis optica (NMO) is characterized by severe optic neuritis and/or longitudinally extensive transverse myelitis (1). Nearly 90% of patients with NMO are female and brain lesions that can be detected with magnetic resonance imaging (MRI) occur in 60% of patients with NMO (2). NMO had been considered as a subtype of multiple sclerosis (MS). In 2005, it was proved that a disease-specific autoantibody, that is NMO-immunoglobulin G (NMO-IgG), binds selectively to aquaporin-4 (AQP4) (3). This discovery distinguished NMO as a distinct disease from MS. AQP4 is a water channel protein in the central nervous system (CNS) and plays a major role in fluid homoeostasis of the CNS. AQP4 is mainly expressed on astrocytic foot processes at the blood–brain barrier and subpial and subependymal regions (4). Histologically, severe injury of astrocytes is seen in NMO and demyelination is considered to be secondary change to astrocytes injury in NMO (5). The criteria for a diagnosis of NMO require that the patient has both optic neuritis and transverse myelitis (6). However, it has been found that anti-AQP4 antibodies can also be detected in patients with NMO-like symptoms that do not fulfill the criteria to be diagnosed NMO. NMO spectrum disorder (NMOSD), which was proposed in 2007, includes a proportion of patients with recurrent, isolated, longitudinally extensive myelitis or optic neuritis as well as patients with longitudinally extensive myelitis or optic neuritis associated with systemic autoimmune disease or with brain lesions typical of NMO (2).
NMO/NMOSD are usually treated with steroid pulse therapy and plasma exchange therapy in the acute phase. Maintenance therapy is also required to avoid further attacks and it is based on low-dose steroids and non-specific immunosuppresive drugs like azathioprine. New therapy strategies using monoclonal antibodies like rituximab have been tested in NMO/NMOSD. Both interferon beta-1a and fingolimod used in the treatment of MS, are ineffective in NMO/NMOSD and these drugs may exacerbate the disease (7,8). MRI has an increasingly important role in differentiating NMOSD from other inflammatory disorders of the CNS, particularly from MS (9). We present three cases of NMO/NMOSD and describe their clinical setting and imaging features with special attention to MRI.
Case reports
Case 1
A 34-year-old woman presented with diplopia, visual disturbance, and gait impairment in 1995. The patient was diagnosed with MS and had received treatment in another hospital, but the details were unclear. She had pain, numbness, and paralysis in the entire left side of her body at 10, 11, and 12 years after the first onset. Steroid pulse therapy and therapy with intramuscular interferon beta-1a were performed. Ten years following the first onset, MRI images of the head showed signal changes in the right portion of the splenium. The lesion showed linear hyperintensity surrounding lateral ventricle on fluid attenuation inversion recovery (FLAIR) image and diffusion-weighted image and isointensity on apparent diffusion coefficient (ADC) map. No enhancement was seen there. The lesion disappeared on her follow-up MRI obtained 1 year later. She was diagnosed with an autoimmune hepatitis and Sjögren’s syndrome 12 years after the first onset. On T2-weighted (T2W) images obtained 13 years after the onset, lower cervical and upper thoracic cord lesions were seen, with swelling and contiguous hyperintensity, including more than five vertebral segments, although these lesions had almost subsided 15 years after the first onset (Fig. 1a–c). The cerebrospinal fluid (CSF) was negative for oligoclonal bands and showed a normal myelin basic protein level of 40.4 pg/mL (normal range, <102 pg/mL). The serum sample examined at 13 years after the onset was positive for anti-AQP4 antibody. She was diagnosed with NMO, considering her myelopathy and visual disturbance at the first onset. Steroid pulse therapy was performed. However, relapsing-remitting myelopathy was observed; and the myelopathy relapsed at 18 and 20 years after the first onset. T2W images obtained 18 years after the onset showed cord lesions without swelling but with contiguous hyperintensity which included almost the same segments as those 13 years after the onset (Fig. 1d). Furthermore, T2W images obtained 20 years after the onset revealed the cord lesions with atrophy and with intermittent hyperintensity including almost the same segments as those obtained 13 years after the onset (Fig. 1e). Currently, she is being treated with a combination of predonine (15 mg/day) and azathioprine (75 mg/day) as maintenance therapy.
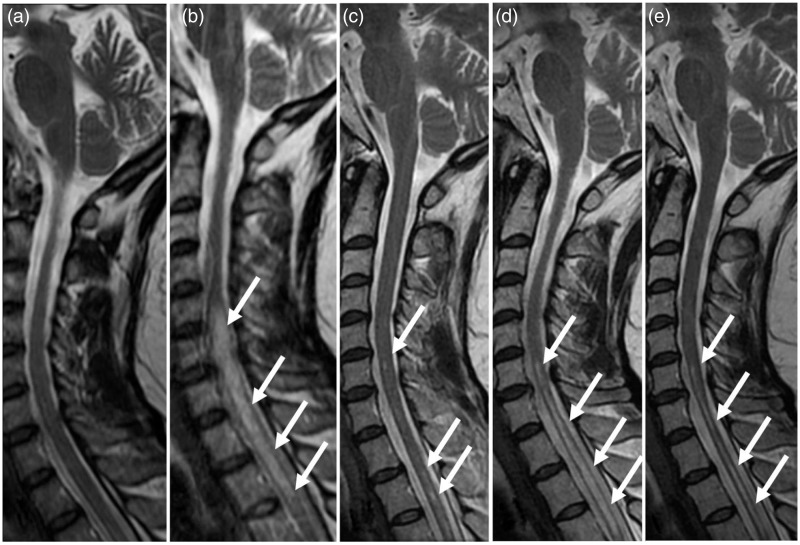
Fig. 1.
Serial changes of the spinal cord lesions in Case 1. Sagittal T2W images obtained 12 years (a), 13 years (b), 15 years (c), 18 years (d), and 20 years (e) after the first onset. (a) No apparent cord lesion is seen. (b) Cord lesions (arrows), with swelling and contiguous hyperintensity, extend more than five vertebral segments. (c) The cord lesions seen in (b) have almost subsided. Small hyperintense lesions (arrows) are scattered. (d) Cord lesions (arrows), without swelling and contiguous hyperintensity, extend almost the same segment as (b). (e) Cord lesions (arrows), with atrophy and intermittent hyperintensity, extend almost the same segment as (b).
Case 2
A 38-year-old woman presented with weakness of the left upper limb and dysarthria in 2011. FLAIR and T2W images showed hyperintensity lesions of the right corticospinal tracts, involving longitudinal fasciculs, cerebral peduncle, and internal capsule. No lesions were seen in the spinal cord and optic nerves. The CSF was negative for oligoclonal bands but showed an increased myelin basic protein level of 717.6 pg/mL. She was diagnosed with MS. Steroid pulse, plasma exchange, and intramuscular interferon beta-1a were given. Various relapsing remitting symptoms such as aphasia, nystagmus, recent memory disturbance, finger agnosia, dysphagia, cerebellar ataxia, truncal ataxia, and lower limb paralysis appeared. She switched from intramuscular interferon beta-1a to oral fingolimod 1 year and 5 months after the first onset. In spite of fingolimod treatment, various relapsing remitting symptoms occurred. The treatment with fingolimod was stopped after 3 months of treatment.
On follow-up T2W and FLAIR images, hyperintense periependymal lesions surrounding the fourth ventricle and lateral ventricles, and hyperintense lesions of the corticospinal tracts and of the hemispheric white matter were seen. Hemispheric white matter lesions showed extensive and/or confluent white matter lesions, partially involving cerebral gray matters (Fig. 2). No enhancement was seen in those lesions. White matter lesions observed at acute manifestation showed serial two patterns: (i) hypointensity on diffusion-weighted images and hyperintensity on FLAIR images and ADC maps at acute manifestation (Fig. 3a–c) and cavitation on follow-up MRI (Fig. 3d–f); and (ii) hyperintensity on diffusion-weighted images and FLAIR images, and iso- to hyperintensity on ADC maps at acute manifestation (Fig. 4a–c), and partial regression or disappearance on follow-up MRI (Fig. 4d–f). The serum sample, examined at 1 year and 9 months after the first onset, was positive for anti-AQP4 antibody. She was diagnosed with NMOSD. Her various symptoms subsided with steroid pulse therapy and plasma exchange therapy. Currently, she receives a combination of predonine (15 mg/day) and azathioprine (75 mg/day) as maintenance therapy.
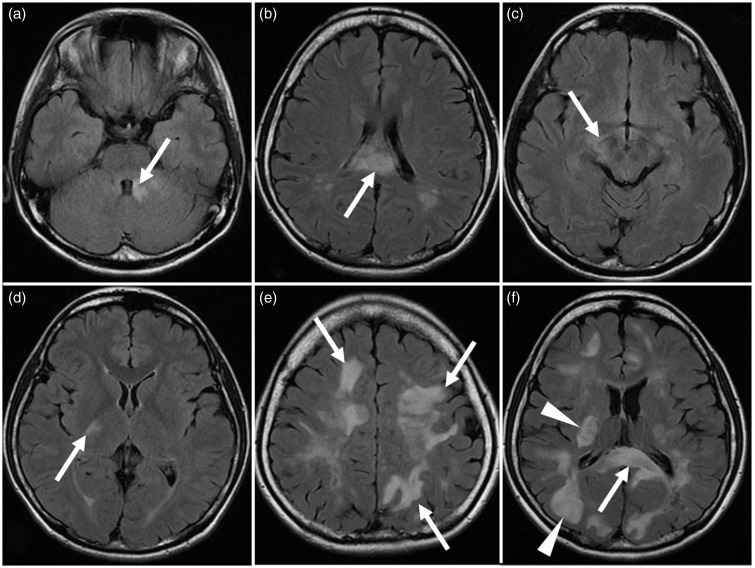
Fig. 2.
Characteristic brain abnormalities on FLAIR images in Case 2. (a, b) Periependymal lesions (arrows) surround the fourth ventricle (a) and lateral ventricle (b). (c, d) Lesions of the corticospinal tracts (arrows) involve cerebral peduncle (c) and internal capsule (d). (e) Extensive and confluent hemispheric white matter lesions (arrows) are seen. (f) Involvement (arrowheads) of the cerebral cortex and the basal ganglia is shown. Periependymal lesion involving corpus callosum (arrow) is also seen.
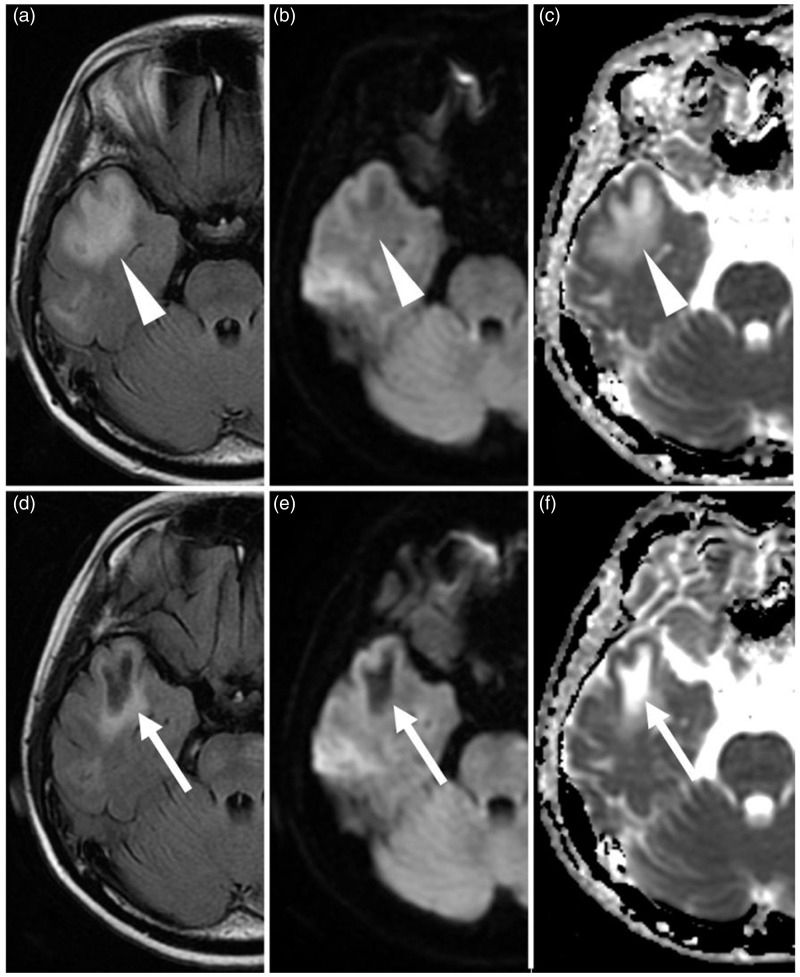
Fig. 3.
Cavitation of the white matter lesions in Case 2. (a–c): Obtained 1 year and 8 months after the first onset at acute manifestation. (d–f) Obtained 2 weeks after (a), (b), and (c), respectively. (a–c) The subcortical white matter lesions (arrowheads) with swelling show hyperintensity on FLAIR image (a) and ADC map (c) and slightly hypointensity on diffusion-weighted image (b), whose findings reflect vasogenic edema. (d–f) The lesions remain as cavitation (arrows), showing hypointensity on FLAIR image (d) and diffusion-weighted image (e), and hyperintensity on ADC map (f).
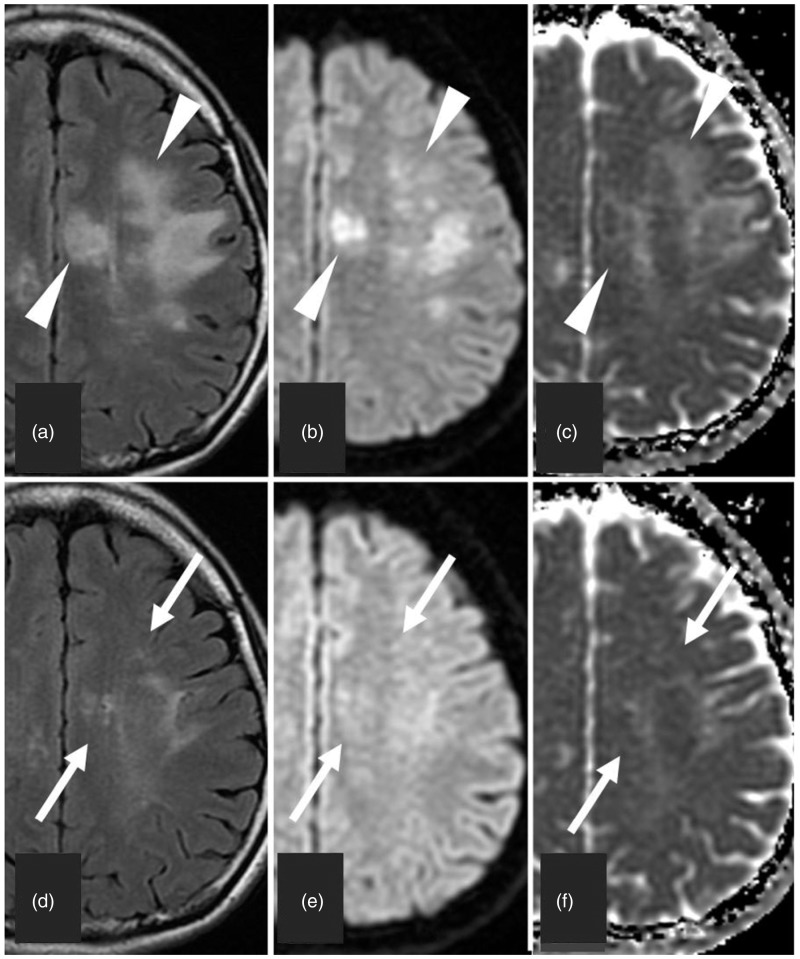
Fig. 4.
Partial regression of the white matter lesions in Case 2. (a–c) Obtained 2 months after the first onset at acute manifestation. (d–f) Obtained 6 months after (a), (b), and (c), respectively. (a–c) The cerebral white matter lesions (arrowheads) with swelling show hyperintensity on FLAIR image (a) and diffusion-weighted image (b) and iso- to hyperintensity on ADC map (c). (d–f) The lesions (arrows) are partially resolved and remain slightly hyperintensity on FLAIR image (d), diffusion-weighted image (e), and ADC map (f).
Case 3
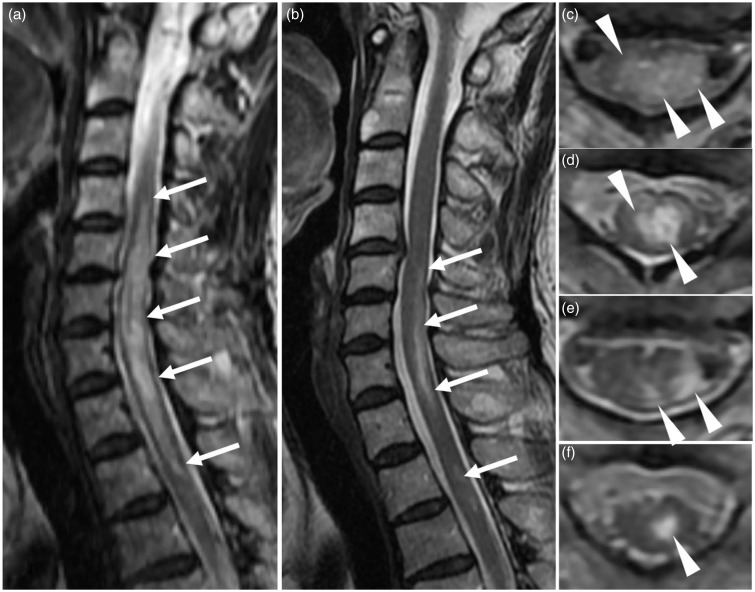
A 59-year-old woman presented with diplopia and ocular motility dysfunction in 2015. T2W and FLAIR images showed periependymal high signal changes around the fourth ventricle and the left inferior horn of the lateral ventricle. No enhancement was seen in those lesions. After 2 weeks of treatment of acute ischemic stroke, paralysis on the left side of her face, ataxic paralysis of her left lower limb, and hypopallesthesia appeared. T2W images of the cervical and upper thoracic spine showed cord lesions with swelling as well as with contiguous hyperintensity, which included more than five vertebral segments. The lesions existed mainly at the central portion of the spinal cord (Fig. 5a, c, and d). No enhancement was seen in the lesions. The CSF was negative for oligoclonal bands and showed an increased myelin basic protein level of 670.4 pg/mL. The serum sample was positive for anti-AQP4 antibody. No optic nerve lesions were detected on the MRI. She was given a diagnosis of NMOSD. Her symptoms subsided with steroid pulse therapy and plasma exchange therapy. On the follow-up brain MRI, no apparent change was seen in the periependymal lesions of the brain. T2W image of cervical and upper thoracic spine showed that the cord lesions had regressed yet still persisted. Also, cavitation was partially seen in the lesions (Fig. 5b, e, and f). She now receives a combination of predonine (15 mg/day) and azathioprine (75 mg/day) as maintenance therapy.
Fig. 5.
Serial spinal cord lesions in Case 3. (a, c, d) Obtained at acute manifestation. (b, e, f) Obtained at symptoms resolved. (a, b) Sagittal T2W images. (c–f) Axial T2W images at the level of C5/6 (c, e) and C7/Th1 (d, f). (a, c, d) Cord lesions, with swelling and contiguous hyperintensity, extend more than five vertebral segments (arrows in (a)) and the lesions exist mainly at the central portion of the spinal cord (arrowheads in (c) and (d)). (b, e, f) The cord lesions are regressed but still persist (arrows in (b)). Cavitation is partially seen in the cord lesions (arrowheads in (e) and (f)).
Discussion
Spinal cord lesions in NMO/NMOSD frequently present in the cervical and upper thoracic spinal cord segments rather than in the lower thoracic and lumbar cord segments (10). The most distinct manifestation of spinal cord lesions is longitudinally extensive transverse myelitis. The lesions, characterized by hyperintensity on T2W images and by hypointensity on T1-weighted (T1W) images, extend over three or more contiguous vertebral segments (2). Also, the lesions mainly involve the central gray matter; AQP4 is abundant in the gray matter and in glial cell processes adjacent to the ependymal cells of the central canal of the spinal cord (11). Pathologically, there are at least two types of acute NMO lesions (5,12,13). The classic acute NMO lesion is characterized by perivascular demyelination, prominent infiltration of macrophages, severe axonal loss, necrosis of both the gray and white matter of the spinal cord, and pronounced loss of astrocytes, showing longitudinally extensive transverse myelitis. The second acute NMO lesion is characterized by non-demyelinated lesions with vacuolated myelin, given the potential for some NMO lesions to be reversible. Also, chronic NMO lesions are characterized by gliosis, cystic degeneration, cavitation, and atrophy of the spinal cord.
The cord lesions in cases 1 and 3, presenting in the cervical and upper thoracic spine, included more than five vertebral segments and were localized mainly at the central portion of the spinal cord, showing longitudinally extensive transverse myelitis. In both cases, most of the lesions regressed, but cavitation as chronic NMO lesions still persisted. In case 1, MRI evaluations of the spine were serially performed for 8 years. The T2 hyperintense lesions in the cervical and upper thoracic spinal cord relapsed and subsided within the area of the first attack and then sequentially atrophied. The recurrent and subsided T2 hyperintense lesions were considered to reflect edema. Furthermore, the atrophied cord lesions on the follow-up MRI were likely to reflect tissue rarefaction and neuronal loss as a result of intense edema associated with astrocytes injury. Frequent relapses at the same upper spinal cord area in patients with NMOSD were reported as seen in our cases (10,14). Cavitation and frequent relapses at the same upper spinal cord may be characteristic findings of cord lesions in NMOSD.
In our three cases, no apparent optic nerve lesions were detected on MRI. MRI studies have reported optic nerve sheath thickening, optic nerve hyperintensity on T2W images and gadolinium enhancement on T1W images in acute optic neuritis of NMOSD (15). However, these findings also have been reported in optic neuritis of MS and are not considered diagnostic of NMOSD. Recently, the differential MRI findings of the optic nerve lesion between NMOSD and MS have been reported; more posterior involvement of the optic nerve, including chiasm and simultaneous bilateral disease, has also been observed in NMOSD (16,17).
NMO brain lesions pathologically resemble NMO opticospinal lesions; NMO brain and opticospinal lesions are considered to have a shared pathogenesis (5). Detection of brain lesions in NMOSD is based on hyperintensity on T2W or FLAIR images. The lesion is typically localized in the periependymal regions surrounding the lateral ventricles, the third ventricle, cerebral aqueduct, and the fourth ventricle, where AQP4 is highly expressed (4). Also, other NMOSD-characteristic brain lesions involving corticospinal tracts and hemispheric white matters, where AQP4 expression is not particularly high, have been described (18). Hemispheric white matter lesions include extensive and confluent hemispheric white matter lesions such as tumefactive (>3 cm in the longest diameter) lesions, long spindle-like or radial-shape lesions following white matter tracts. Occasionally, cerebral cortical involvement is also seen (19). In all of our cases, periependymal lesions surrounding the lateral ventricles and/or the fourth ventricles were present. In case 2, corticospinal tracts lesions and various above-mentioned hemispheric white matter lesions were seen as well. Also, several hemispheric white matter lesions extended to adjacent basal ganglia or cerebral cortices.
There were no enhanced brain lesions in our three cases. Most brain lesions in patients with NMOSD are not enhanced, although enhanced brain lesions in patients with NMOSD can be seen during the course of the disease (19). The enhancement of the brain lesions appears as a poorly marginated, subtle, and multiple patchy patterns, so-called “cloud-like” enhancement (20), whose findings are considered to reflect vasogenic edema due to blood–brain barrier disruption.
Hemispheric white matter lesions serially often shrink or disappear, although they sometimes remain as cystic lesions (9,19), pathologically reflecting cystic degeneration and cavitation as chronic NMO lesions. These lesions may cause various symptoms such as hemiparesis, encephalopathy, and visual field defects depending on the area they involve (9,19). In case 2, cerebral white matter lesions obtained at acute manifestation showed two serial patterns: (i) hypointensity on diffusion-weighted images and hyperintensity on FLAIR images and ADC maps at acute manifestation, and cavitation on follow-up MRI (Fig. 3); and (ii) hyperintensity on diffusion-weighted and FLAIR images and iso- to hyperintensity on ADC maps at acute manifestation, and partial regression or disappearance on follow-up MRI (Fig. 4). The former pattern is likely related to severe vasogenic edema which can cause severe neuronal loss and result in subsequent cavitation (21). Therefore, this pattern, associated with severe vasogenic edema caused by severe injury of astrocytes, can result in cavitation that may have prognostic implications of the hemispheric white matter lesions in NMOSD.
In our cases, all cases were positive for anti-AQP4 antibody. Yet there are other cases that are clinically diagnosed with NMOSD and negative for anti-AQP4 antibodies, namely seronegative NMOSD. Approximately 10% of the patients with NMO and less than half of the patients with NMOSD are negative for anti-AQP4 antibody despite the use of the serum samples collected during an acute attack before any treatment. Also, patients with seronegative NMO/NMOSD tend to have different clinical features and different lesion distributions from patients with seropositive NMO/NMOSD (22). Recently, anti-myelin oligodendrocyte glycoprotein (MOG) antibodies have been reported in patients with seronegative NMO (23). Furthermore, there has been a report that patients with anti-MOG antibodies represented about 20% of patients with sereonegative NMO. Compared to patients with seropositive NMOSD, patients with anti-MOG antibodies were more often male, had more frequent bilateral simultaneous optic neuritis, had spinal cord lesions distributed in the lower portion of the spinal cord, and usually demonstrated better functional recovery after an attack (24).
In conclusion, we present three cases of NMO/NMOSD and describe their clinical setting and MRI features. Although many of the MRI lesions in NMO/NMOSD may be non-specific, their characteristic location and configuration are helpful in the diagnosis of NMO/NMOSD. Specifically, cavitations in the upper spinal cord and hemispheric white matter, are considered to be associated with severe vasogenic edema and are likely to be one of the characteristic findings in NMOSD.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–815. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005; 202: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittock SJ, Weinshenker BG, Lucchinetti CF, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006; 63: 964–968. [DOI] [PubMed] [Google Scholar]
- 5.Lucchinetti CF, Guo Y, Popescu BF, et al. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol 2014; 24: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006; 66: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Tanaka K, Komori M. Interferon-beta (1b) treatment in neuromyelitis optica. Eur Neurol 2009; 62: 167–170. [DOI] [PubMed] [Google Scholar]
- 8.Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 2012; 18: 113–115. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 2015; 84: 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassinotto C, Deramod H, Olindo S, et al. MRI of the spinal cord in neuromyelitis optica and recurrent longitudinal extensive myelitis. J Neuroradiol 2009; 36: 199–205. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Miyazawa I, Fujihara K, et al. Preferential spinal cord gray matter involvement in neuromyelitis optica: an MRI study. J Neurol 2008; 255: 163–170. [DOI] [PubMed] [Google Scholar]
- 12.Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 2007; 130: 1224–1234. [DOI] [PubMed] [Google Scholar]
- 13.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 2007; 130: 1194–1205. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto H, Shimizu T, Okabe S, et al. Recurrent spinal cord attacks in a patient with a limited form of neuromyelitis optica. Intern Med 2011; 50: 509–513. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Liu Y, Duan Y, et al. Brain MRI abnormalities in neuromyelitis optica. Eur J Radiol 2011; 80: 445–449. [DOI] [PubMed] [Google Scholar]
- 16.Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol 2012; 32: 216–220. [DOI] [PubMed] [Google Scholar]
- 17.Storoni M, Davagnanam I, Radon M, et al. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: a novel magnetic resonance imaging scoring system. J Neuroophthalmol 2013; 33: 123–127. [DOI] [PubMed] [Google Scholar]
- 18.Kim W, Park MS, Lee SH, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult Scler 2010; 16: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 19.Kim W, Kim SH, Huh SY, et al. Brain abnormalities in neuromyelitis optica spectrum disorder. Mult Scler Int 2012; 2012: 735486–735486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S, Mori M, Makino T, et al. “Cloud-like enhancement” is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol 2009; 66: 425–428. [DOI] [PubMed] [Google Scholar]
- 21.Saiki S, Ueno O, Moritani T, et al. Extensive hemispheric lesions with radiological evidence of blood-brain barrier integrity in a patient with neuromyelitis optica. J Neurol Sci 2009; 284: 217–219. [DOI] [PubMed] [Google Scholar]
- 22.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 2012; 9: 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012; 79: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 24.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014; 82: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]