Abstract
Background:
Recent clinical investigations have identified inadequate autograft hamstring graft diameter (<8 mm) to be predictive of failure after reconstruction of the anterior cruciate ligament (ACL).
Purpose/Hypothesis:
The objective of this study was to determine the utility of preoperative magnetic resonance imaging (MRI) variables of the hamstring tendons for the prediction of graft diameter at the time of surgery. The hypothesis was that cross-sectional area (CSA) of the hamstring tendon measured on MRI could accurately predict graft diameter, and threshold measurements could be established to predict graft diameter at the time of surgery.
Study Design:
Cohort study (diagnosis); Level of evidence, 2.
Methods:
A total of 84 consecutive skeletally mature patients prospectively enrolled in our ACL reconstruction patient registry were identified for study purposes. Patients were included if they underwent an MRI of the affected knee at our institution prior to ACL reconstruction with hamstring (HT) autograft. Graft preparation was performed via a standard quadrupled hamstring technique after harvesting both the gracilis and semitendinosus (4-GST). The smallest diameter end of the HT autograft was then utilized for measurement analysis. Total CSA was calculated for both hamstring tendons using the “region of interest tool” on the corresponding proton density–weighted axial image of the knee at the widest condylar dimension. Three independent reviewers measured the MRI scans so that intra- and interrater reliability of the measurements could be determined. A trend analysis was then undertaken to establish correlations between the MRI CSA and graft diameter. Predictive analysis was then performed to establish threshold MRI measurement values for specific graft diameters and determine whether any patient-specific factors would affect graft diameter (age, sex, and body mass index).
Results:
Mean patient age at the time of surgery was 36 years (range, 11-57 years). Intra- and interrater reliability measurements achieved near-perfect agreement for CSA measurements, with intraclass correlation coefficients (ICCs) of 0.994 and 0.932, respectively. Trend analysis demonstrated that increasing CSA correlated well with increasing overall diameter of the graft (P < .001). Receiver operating characteristic (ROC) curves were generated to evaluate threshold CSA measurements for various graft diameters. Maximum sensitivity values of 21.64, 25.25, and 28.256 mm2 were established for the respective graft diameters of 8, 9, and 10 mm in the 4-GST group. Independent patient factors of younger age, shorter stature, and female sex were significantly associated with graft diameter (P = .019, .034, and .028, respectively).
Conclusion:
Preoperative MRI can be used to accurately predict quadrupled hamstring autograft diameter at the time of surgery. A total cross-sectional area of >22 mm2 can reliably provide a graft diameter of >8 mm at the time of surgery.
Keywords: anterior cruciate ligament, autograft, hamstring graft, preoperative planning
Anterior cruciate ligament (ACL) rupture remains one of the most common knee injuries.6 Numerous tendon graft choices exist for successful ligament reconstruction, with autogenous hamstring being a commonly employed option for those with functional instability of the knee in the setting of ACL insufficiency.13 Recurrent instability after ACL reconstruction can be a devastating problem, and an abundance of clinical and biomechanical studies have investigated the mechanisms behind the failure of ACL surgery.10 An emerging concept involving autogenous hamstring autograft surrounds final graft diameter at the time of reconstruction, with diameter sizes of less than 8 mm possibly having a higher propensity toward failure.3,8,9
At the time of harvest, the size of the hamstrings has been reported to be quite variable,7 with various studies examining different methods to adequately predict hamstring size at the time of surgery. These methods have utilized both anthropomorphic measurements and preoperative imaging techniques for prediction algorithms.3 Techniques that have employed preoperative imaging have been limited secondary to sample size,4 clinical relevancy of the graft size,2,12 and imaging techniques that are not often used in the setting of ACL insufficiency.5,14
The aim of the current study was multifaceted. First, we investigated the use of current magnetic resonance imaging (MRI) techniques to accurately predict the size of clinically significant hamstring graft diameter sizes at the time of surgery, with the use of a simple cross-sectional area (CSA) measurement technique. Second, we attempted to investigate the relevance of this measurement technique for the 4-stranded gracilis + semitendinosus graft (4-GST). Third, we sought to determine the reliability and universality of this measurement technique by establishing both intra- and interrater reliability between both orthopaedic surgeons and radiologists. A secondary aim was to determine whether there were any patient factors that could predict graft diameter. Our hypotheses were that our method of measurement would be reproducible, reliable, and accurate for the prediction of graft diameter at the time of surgery and specific patient demographic factors could predict graft diameter.
Methods
After approval from our institutional review board, skeletally mature patients who underwent both ACL reconstruction with 4-GST along with preoperative MRI at our institution were reviewed for possible inclusion in this study. Information garnered for this study was reviewed in a retrospective fashion and is part of a larger ongoing prospective ACL registry that is being performed at our center. Patient age, sex, height, and body mass index (BMI) were also collected and reviewed for secondary analysis. Only those patients with full-thickness ACL ruptures, confirmed at the time of surgery, were included in the study.
MRI Technique
All studies were performed with either a 1.5- or 3.0-T superconducting magnet (Signa; GE Healthcare) using a knee- specific coil. Knee positioning was standardized to slight flexion of the joint at the time of image acquisition (10° to 20° with a positioning device at the ankle). Imaging slices were 3.5-mm thick without any interslice gap, and a 14- to 16-cm field of view with a 512 × 256-384 matrix was utilized. Sequences gathered at the time of imaging consisted of our institutional knee protocol, including sagittal fat-suppressed fluid-sensitive images, and 3 orthogonal planes of proton density (PD)–weighted images. Only the axial PD-weighted sequence was used for this investigation for its resolution of anatomical structures (repetition time [TR], 4000 ms; echo time [TE], 24 ms).
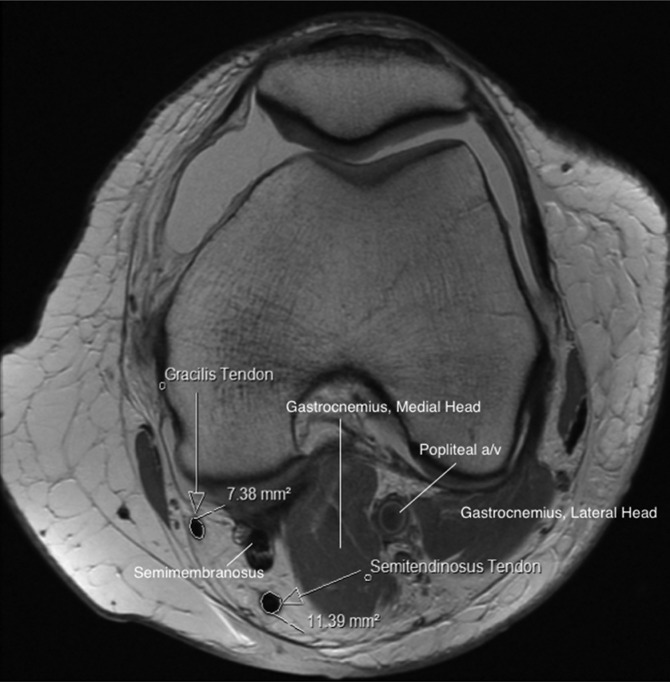
The CSA of both the gracilis tendon and the semitendinosus tendon was determined for each patient via simple modification of the technique described by Bickel et al.2 This modification consisted of using solely the axial image to determine the widest portion of the distal femur and then using this specific slice to quantify respective CSAs of the hamstring tendons. After the slice was determined, the image was then magnified once (providing a 2× view) for CSA measurement. This 2× view was utilized so that only the tendon proper would be measured (ie, eliminating the chance for measurement of tissue with signal consistent with either muscle, vincula, or other soft tissue). The CSA was calculated using the region of interest tool that is located in our PACS software (Centricity Enterprise Web V3.0; GE Healthcare) (Figure 1). This tool automatically determines the CSA (mm2) after manually tracing the area of interest on the preselected image and is found on most PACS systems. The tool itself is approved by the US Food and Drug Administration to compute areas in any plane or volume.2
Figure 1.
Measurement technique utilized to evaluate the cross-sectional area of the hamstring tendons.
Each CSA was measured once independently by 1 of 3 reviewers (B.M.G., P.N.W., or A.B.) to establish interrater reliability values. Thirty consecutive MRIs were then remeasured by each reviewer at a separate 2-week time interval to establish intrarater reliability. Furthermore, the slice selected by each reviewer was recorded and analyzed to establish overall validity to our technique modification. Reviewers consisted of a senior orthopaedic surgery resident and an orthopaedic surgery sports medicine fellow (B.M.G. and P.N.W.) as well as 1 radiologist (A.B.). Each reviewer was blinded to the final graft size generated at the time of surgery as well as the technique used to reconstruct the ACL (4-GST).
Hamstring Harvest
Hamstring harvest was accomplished in a standard fashion through a 4-cm longitudinal incision centered on the tibia midway between its anterior tubercle and posterior osseous border. After incision of the sartorial fascia, each hamstring was identified and carefully isolated. Any fascial bands connected to the medial gastrocnemius were sharply divided. The tendons were then stripped of their proximal muscle bellies using either an open- or closed-ended stripper. Importantly, patients who sustained premature hamstring amputation were excluded from the study. Type of suspensory fixation employed was also left to the discretion of the treating surgeon and consisted of an intramedullary cortical button (Smith & Nephew or Arthrex).
After hamstring graft preparation, diameters were measured and recorded for each end of the graft as well as the midsubstance. Measurement was accomplished with a standard proprietary graft sizing block (0.5-mm increments), and the smallest diameter recorded was chosen for final analysis.
Statistical Analysis
Reliability tests were also performed via an intraclass correlation coefficient (ICC) to calculate intra- and interrater reliability for the various measurements that were made on MRI (including CSA measurements and slice chosen to measure CSA). All analyses were performed for the 4-GST constructs. Simple linear regression models were used to calculate Pearson correlation coefficients to determine whether any correlations existed between CSA and graft diameter at the time of surgery. A linear trend analysis was then performed to investigate whether increasing CSA resulted in an increasing overall graft size.
Univariate analyses included independent-samples t tests and 1-way analysis of variance (ANOVA) models for comparisons of graft diameter between patient sex and BMI groups. Bivariate correlations were conducted to evaluate the correlation between graft diameter versus age, height, weight, and BMI. A multivariable regression model was then generated to discern whether there were any patient-specific factors that could be used to predict eventual graft diameter at the time of surgery.
For a ≥8-mm graft, total CSA was divided into a series of thresholds that would correspond to clinically relevant graft diameter size measurements. Chi-square analyses were then used to calculate the odds ratios for the various CSAs to determine minimum CSA values that would correspond to clinically relevant ≥8-mm graft diameter size. A receiver operating characteristic (ROC) curve was then generated to give a precise cutoff estimate of CSA measurement that was most predictive of ≥8-mm graft diameter size, with reported sensitivity and specificity of 100%. ROC curves were then repeated for 9-mm and 10-mm graft diameters.
Results
A total of 84 consecutive patients prospectively enrolled in our ACL reconstruction patient registry with 4-GST were identified for the study. The mean age at the time of surgery was 36 years (range, 11-57 years), with an equal distribution between males and females (50:50). Mean height of the cohort was 67 inches (range, 54-78 inches), while mean BMI was 24.2 kg/m2 (range, 17.1-34.4 kg/m2). Mean CSA of the gracilis tendon was 8.9 mm2 (range, 4.18-19.63 mm2), and mean CSA of the semitendinosus tendon was 13.95 mm2 (range, 6.15-29.26 mm2). The combined mean CSA of both tendons was 22.88 mm2 (range, 10.33-42.26 mm2). The mean graft diameter at the time of surgery was 8.2 mm (range, 6-11.5 mm) (Table 1). The mean graft diameter of the 4-GST was 7.99 mm (range, 6-10 mm).
TABLE 1.
Patient and Graft Dataa
| Variable | Mean (Range) |
|---|---|
| Age, y | 36 (9-58) |
| Height, in | 67 (54-78) |
| BMI, kg/m2 | 24.2 (17.1-34.4) |
| CSA gracilis, mm2 | 8.9 (4.18-19.63) |
| CSA ST, mm2 | 13.95 (6.15-29.26) |
| CSA GST, mm2 | 22.88 (10.33-42.26) |
| Graft diameter, mm | 8.2 (6-11.5) |
| 4-GST, mm | 7.99 (6-10) |
aBMI, body mass index; CSA, cross-sectional area; GST, gracilis and semitendinosus; ST, semitendinosus.
Reliability of Measurements
Overall, the reliability of the various measurements was excellent, with near perfect agreement within and between raters throughout. Intrarater reliability for measurements of within-rater reproducibility of the CSA measure ranged from 0.898 to 0.993. Interrater reliability between the 3 raters was also high, with an ICC of 0.932. In terms of the method used for slice determination at which the CSA (widest portion of distal femur) was measured, an excellent agreement between all 3 raters was established (ICC = 0.980).
CSA and Graft Diameter
The 4-GST showed a statistically significant correlation between increasing CSA and increasing graft diameter, as measured by the Pearson correlation coefficient. A positive relationship was noted for both measures where an increase in CSA correlated with an increase in overall graft diameter (4-GST: R = 0.416, P < .001). When graft diameters were separated into 4 groups, a positive linear trend was noted for CSA and graft diameter (P = .073). This finding must be interpreted with caution, however, as diameters larger than 9 mm were infrequently encountered at the time of surgery. Based on ROC results, cutoff CSA values were determined to predict specific graft sizes. CSA values of 21.64, 22.25, and 28.26 mm2 accurately predicted graft diameters of 8, 9, and 10 mm, respectively (Table 2). Again, the 10-mm size must be viewed with caution, as there were not many grafts larger than 9 mm.
TABLE 2.
Sensitivity of CSA Measurement Values for Clinically Relevant Graft Diametersa
| Diameter, mm | CSA 4-GST, mm2 |
|---|---|
| 8 | 21.64 |
| 9 | 25.25 |
| 10 | 28.256 |
aCSA, cross-sectional area; GST, gracilis and semitendinosus.
Patient Factors Used to Predict Graft Diameter
Secondary analysis of patient-specific factors and their influence on final graft diameter demonstrated a number of predictive relationships. Females, on average, had a statistically smaller graft diameter (P = .019). Albeit the size difference was not large, this difference did approach clinical significance at 0.5 mm. Patient height and weight were both positive predictors of increased graft size with correlation values of 0.213 (P = .034) and 0.258 (P = .010), respectively. Multivariable regression analysis indicated increasing age and total CSA as predictive of increased graft size (P = .028 and .004, respectively). While not statistically significant, females were still more likely to have smaller grafts after adjustment in the model (P = .056).
Discussion
Here we present an effective, reproducible, and simple method for accurately predicting autologous hamstring graft diameter based on preoperative MRI imaging and subsequent measurement of the cross-sectional area of the tendons on axial sequences. From a clinical perspective, it is most important to note that a combined cross-sectional area of larger than 22 mm2 can be used as minimum cutoff for a graft diameter of 8 mm. A linear relationship between CSA and eventual graft diameter at the time of surgery was found, and our method of measuring CSA was found to be very reliable and reproducible. This study is in agreement with previous investigations that have evaluated the utility of preoperative MRI2,4,5,12,14 for discerning eventual hamstring autograft diameter and adds a number of key points to the growing body of literature on ACL surgical planning. We can also confidently provide updated information regarding the influence of patient-specific factors on hamstring graft diameter and which of these factors may better predict a graft diameter of at least 8 mm. Namely, very young age, shorter stature, and female sex are associated with an increased risk of producing an at-risk graft size of smaller than 8 mm.
Our results are consistent with a recent study performed by Erquicia et al4 who sought to determine the utility of both MRI and ultrasonography measurement of hamstring tendon diameter for prediction of intraoperative graft diameter. In a cohort of 33 patients, they evaluated the effect of magnification of the image on CSA measurement and graft size prediction. For those images with a 2× magnification (similar to the methods presented within), a threshold value of 28.1 mm2 was determined for accurate prediction of a graft greater than 8 mm, whereas those patients with a CSA of 23.7 mm2 or less consistently displayed a graft diameter of smaller than 8 mm. A 4× magnification changed these values to 19.8 and 15.24 mm2, respectively. Our study’s cohort was nearly triple in size and was also able to successfully predict an accurate diameter value (ie, 8 vs 9 mm) rather than simply producing threshold values, which can be advantageous to the operating surgeon when planning graft diameter needs based on patient-specific parameters.
Our study is also consistent with previous investigations that have demonstrated a linear relationship between CSA measurements of the hamstring tendons on MRI and eventual graft diameter.1,2,5,12 Previous studies have focused on the adolescent patient population,2 performed a double-bundle technique,12 utilized complex pixel counting for CSA measurement,5 or used a clinically insignificant graft diameter threshold of 7 mm for prediction purposes.1 Here we have simplified the CSA measurement technique by using only the axial imaging sequence and are the first to report on and demonstrate excellent inter- and intrarater reliability for the applied measurement technique.
Although not the primary intention of our study, we were also able to provide updated anthropomorphic data on which patient-specific factors portend a hamstring graft size of 8 mm or larger. A diameter of 8 mm was chosen as our cutoff diameter for the various predictors as a result of the emerging evidence that is now elucidating the importance of having a minimum soft tissue graft diameter of 8 mm. A recent systematic review demonstrated that a hamstring graft size of 8 mm or larger reduced the failure rate of ACL reconstruction surgery.3 Our findings are in line with a study performed by Treme et al11 that demonstrated patient weight, thigh circumference, BMI, and sex to all be correlated with graft diameter. However, this study utilized a graft diameter threshold of 7 mm.
Sample size, specific graft diameter prediction measurements (ie, 8 vs 9 mm), the use of a more clinically relevant graft diameter threshold (8 mm), investigating multiple graft constructs, reporting on a simplified CSA measurement technique, and the validation of the reliability and reproducibility of CSA measurements are the inherent strengths of the present investigation. However, this study also contains a number of weaknesses that do not necessarily deter from the conclusions that can be drawn from the data. Interesting, in a total of 100 ACL reconstructions using autologous hamstring graft, only 7 patients had hamstring bulk that provided an intraoperative graft diameter of 10 mm or larger. This information can be viewed as germane to the operating surgeon in a number of clinical scenarios and is likely of the most paramount importance when planning for the ACL revision or primary posterior cruciate ligament reconstruction where graft diameter is often viewed as an important factor for securing a successful postoperative outcome.
Conclusion
Preoperative MRI CSA measurement of the hamstring tendons can accurately, reliably, and reproducibly predict hamstring autograft diameter at the time of ACL reconstruction surgery. A total CSA of larger than 22 mm2 will be able to provide a graft diameter of larger than 8 mm. Additionally, surgeons should be aware that female patients, on average, have smaller graft sizes.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Beyzadeoglu T, Akgun U, Tasdelen N, Karahan M. Prediction of semitendinosus and gracilis autograft sizes for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20:1293–1297. [DOI] [PubMed] [Google Scholar]
- 2. Bickel BA, Fowler TT, Mowbray JG, Adler B, Klingele K, Phillips G. Preoperative magnetic resonance imaging cross-sectional area for the measurement of hamstring autograft diameter for reconstruction of the adolescent anterior cruciate ligament. Arthroscopy. 2008;24:1336–1341. [DOI] [PubMed] [Google Scholar]
- 3. Conte EJ, Hyatt AE, Gatt CJ, Dhawan A. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy. 2014;30:882–890. [DOI] [PubMed] [Google Scholar]
- 4. Erquicia JI, Gelber PE, Doreste JL, Pelfort X, Abat F, Monllau JC. How to improve the prediction of quadrupled semitendinosus and gracilis autograft sizes with magnetic resonance imaging and ultrasonography. Am J Sports Med. 2013;41:1857–1863. [DOI] [PubMed] [Google Scholar]
- 5. Hamada M, Shino K, Mitsuoka T, Abe N, Horibe S. Cross-sectional area measurement of the semitendinosus tendon for anterior cruciate ligament reconstruction. Arthroscopy. 1998;14:696–701. [DOI] [PubMed] [Google Scholar]
- 6. Kim HS, Seon JK, Jo AR. Current trends in anterior cruciate ligament reconstruction. Knee Surg Relat Res. 2013;25:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maeda A, Shino K, Horibe S, Nakata K, Buccafusca G. Anterior cruciate ligament reconstruction with multistranded autogenous semitendinosus tendon. Am J Sports Med. 1996;24:504–509. [DOI] [PubMed] [Google Scholar]
- 8. Magnussen RA, Glisson RR, Moorman CT. Augmentation of Achilles tendon repair with extracellular matrix xenograft: a biomechanical analysis. Am J Sports Med. 2011;39:1522–1527. [DOI] [PubMed] [Google Scholar]
- 9. Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy. 2013;29:1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murawski CD, van Eck CF, Irrgang JJ, Tashman S, Fu FH. Operative treatment of primary anterior cruciate ligament rupture in adults. J Bone Joint Surg Am. 2014;96:685–694. [DOI] [PubMed] [Google Scholar]
- 11. Treme G, Diduch DR, Billante MJ, Miller MD, Hart JM. Hamstring graft size prediction: a prospective clinical evaluation. Am J Sports Med. 2008;36:2204–2209. [DOI] [PubMed] [Google Scholar]
- 12. Wernecke G, Harris IA, Houang MT, Seeto BG, Chen DB, MacDessi SJ. Using magnetic resonance imaging to predict adequate graft diameters for autologous hamstring double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1055–1059. [DOI] [PubMed] [Google Scholar]
- 13. West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13:197–207. [DOI] [PubMed] [Google Scholar]
- 14. Yasumoto M, Deie M, Sunagawa T, Adachi N, Kobayashi K, Ochi M. Predictive value of preoperative 3-dimensional computer tomography measurement of semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:259–264. [DOI] [PubMed] [Google Scholar]