Abstract
It has been suggested that 1,25-dihydroxyvitamin D3 (vitamin D) plays a protective role against inflammation and insulin resistance (IR) in type 2 diabetes mellitus (T2DM). The present study investigate the hypothesis that vitamin D may exert beneficial effects on the liver in a rat model of T2DM by regulating the expression of inflammation-related cytokines and ameliorating IR induced by inflammation. Normal control group rats were fed a basic diet (NC). Experimental rats received a high-fat diet for 8 weeks and were then injected with streptozotocin (STZ) to induce T2DM. Half of the T2DM model rats received vitamin D (0.03 µg/kg/day) for 8 weeks (vitamin D-treated group; VD; n=11), while the other (T2DM group; DM; n=10) and NC group received an equivalent quantity of peanut oil. Following sacrifice, fasting plasma glucose (FPG) and fasting insulin (FINS) were recorded and homeostasis model assessment of IR (HOMA-IR) was calculated. Liver histopathology was examined using hematoxylin and eosin staining. The levels of the inflammatory cytokines C-Jun N-terminal kinase, C-Jun, tumor necrosis factor-α and interleukin-1β were measured using immunohistology, quantitative polymerase chain reaction and western blot analyses. The results revealed that treatment with vitamin D markedly alleviated the pathological alterations of liver and reduced the expression of inflammatory cytokines at the protein and mRNA levels. Furthermore, decreased levels of FPG, HOMA-IR and increased FINS were detected. In conclusion, the results of the present study indicate that vitamin D has therapeutic effects on diabetes-induced liver complications in T2DM model rats, which may involve the modulation of the inflammatory response, attenuating the crosstalk’ between inflammation and IR and ameliorating hyperglycemic state.
Keywords: vitamin D, type 2 diabetes mellitus, inflammation, insulin resistance, liver
Introduction
The World Health Organization calculates that ~171 million individuals worldwide have diabetes, and that its prevalence will reach epidemic proportions by 2030, affecting ~366 million individuals (1). The increasing prevalence of diabetes represents a significant burden to human health due to its numerous and serious complications. It has been suggested that chronic low-intensity inflammation and insulin resistance (IR) are risk factors for type 2 diabetes mellitus (T2DM), and are involved in the initial onset of T2DM and the progression of its complications. Evidence from various directions, including observational and experimental studies, indicates the protective capacity of 1,25-dihydroxyvitamin D3 (active vitamin D) in chronic low-intensity inflammation and IR in T2DM and its complications. Although the underlying mechanism is yet to be clarified, it is recognized that this role may be at least partially associated with the immunological regulation of vitamin D, which is able to downregulate the production of proinflammatory cytokines (2). Previous studies have reported associations between vitamin D insufficiency and diseases related to an amplified inflammatory state, such as diabetes. The effects of vitamin D in downregulating the proinflammatory profile in T2DM patients may be implicated in the association between vitamin D deficiency and impaired glucose tolerance or T2DM that has been observed in humans (3), and in the protective effects of vitamin D supplementation (4).
Diabetes-induced liver complications are characterized by varying degrees of inflammation, fatty degeneration, fibrosis and other pathological changes. Current studies regarding the effects of vitamin D on diabetes-induced liver complications and its potential mechanisms in T2DM are seldom reported. In consideration of the protective role of vitamin D against inflammation and IR in T2DM, we hypothesized that the cytokines or molecules involved in inflammation and IR in T2DM may be targets of vitamin D. The present study was conducted to test the hypothesis that vitamin D may improve the diabetes-induced liver complications by suppressing the expression of inflammation cytokines including C-Jun N-terminal kinase (JNK), C-Jun, tumor necrosis factor (TNF)-α and interleukin (IL)-1β in rat models of T2DM, thus determining the association, if any, between vitamin D, inflammation and diabetes-induced liver complications. Investigations into the targets of vitamin D may provide insight into the potential therapeutic mechanism of diabetes-induced liver complications.
Materials and methods
Animal modeling and grouping
A total of 34 male Sprague-Dawley rats (specific pathogen-free; weight, 280±10 g; age, 8 weeks) were provided by the Xinjiang Center for Disease Control and Prevention (Urumqi, China). The rats were housed in individual cages maintained under ambient temperature (25±2°C) and had ad libitum access to water and food. After a week of acclimation, rats were randomly divided into two groups: Normal control (NC; n=10) and T2DM model (n=24) groups. The T2DM rat model was established by administering a high-fat and high-sugar diet (containing 10% refined lard, 20% sucrose, 2% cholesterol, 8% custard powder and 60% of normal diet; supplied by the Institute of Research in Xinjiang Medical University, Urumqi, China) for 8 weeks. Subsequently, the rats received a peritoneal injection of 35 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) in 0.1 mol/l citrate buffer (pH 4.2; Weber Liyang Chemical Group, Xi'an, China), while the NC group rats were fed the basic diet and received citrate buffer alone. One week later, random non-fasting blood glucose was measured from tail blood samples using a portable glucometer (Accu-Chek, Mannheim, Germany). Diabetes was determined by the presence of hyperglycemia (random non-fasting glucose level, >16.7 mmol/l). Among the initial 24 rats in the T2DM model group, 22 met the hyperglycemia criteria. Then, half of the T2DM model rats (n=12) were randomly allocated to the vitamin D-treated group (VD; n=11), and each rat was administered vitamin D (0.03 µg/kg/day; Shanghai Roche Pharmaceutical Ltd., Shanghai, China) by filling the stomach using a lavage needle (insertion depth, ~5 cm) for 8 weeks, while the other 11 rats (T2DM group; DM) and the NC group received an equivalent administration of peanut oil (Shandong Luhua Group Co., Ltd., Shandong, China) daily for 8 weeks. During the experimental period, the NC group was fed the basic diet, while the DM and VD groups were fed the high-fat and high-sugar diet. At the end of the experiment, the NC and VD groups had retained 10 rats each, while 9 rats remained in the DM group. The study was approved by the ethics committee of Xinjiang Medical University.
Tissue sampling and preparation
Following the trial, the rats were sacrificed using 2% sodium pentobarbital injection (50 mg/kg; Merck Millipore, Darmstadt, Germany). The serum and liver specimens were harvested. Blood samples were collected from the abdominal aorta and were centrifuged at 990 × g for 20 min (5430R centrifuge; Eppendorf, Hamburg, Germany) to separate the plasma for use in assays. The serum levels of fasting plasma glucose (FPG) and fasting insulin (FINS) were detected using a modular chemical analyzer (7600; Hitachi, Tokyo, Japan) and an Architect i2000SR immunoassay analyzer (Abbott Laboratories, Lake Bluff, IL, USA), respectively. HOMA-IR was calculated using the following formula: (FPG × FINS)/22.5. Liver tissues were cut into small pieces, immersed into RNAlater Stabilization Solution (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and stored at −80°C for subsequent reverse transcription quantitative-polymerase chain reaction (RT-qPCR) and western blot analyses. Remaining liver tissues were fixed in 4% paraformaldehyde (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and stored at 4°C for hematoxylin and eosin (H&E; Shun Tian Biological, Ltd., Shanghai, China) staining and immunohistology.
Histopathological staining
Fresh liver tissues were washed with saline, and fixed in 4% paraformaldehyde. Following dehydration these tissues were embedded in paraffin, and then cut into 5-µm sections using a microtome (Leica Biosystems Nussloch GmbH, Nussloch, Germany). Liver tissues from all rats were subjected to routine histological examination. The sections were mounted on glass slides and depleted of paraffin using xylene (Weber Liyang Chemical Group). Then, sections were subjected to H&E staining. The sections were observed using a digital image-capture system (DM300B; Leica Microsystems).
Immunohistochemistry
Serial sections of 5 µm thickness were cut from paraffin-embedded tissue blocks and placed onto glass slides. After drying for 2 h, paraffin sections were deparaffinized and hydrated through a series of graded alcohol. Endogenous peroxidase activity was inactivated with 0.3% hydrogen peroxide. For antigen retrieval, the glass slides were immersed in citrate buffer (0.01 M) at 95°C for 20 min, and refrigerated at room temperature. Tissue slices were incubated with rabbit anti-rat antibodies against C-Jun (bs-0670R; 1:100; Beijing Biosynthesis Biotechnology, Co., Ltd., Beijing, China), JNK, TNF-α and IL-1β (BA1648, BA0131 and BA2782; 1:100; Wuhan Boster Biological Technology, Ltd., Wuhan, China) respectively at 4°C overnight. Then secondary antibody (5:100; ZLI-9017; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) was added to incubate the slices for 20 min at room temperature. After staining with 3,3′-diaminobenzidine chromogenic reagent (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) and counterstained with hematoxylin for 5–10 min. The slices were sealed and visualized by microscopy using a digital image-capture system (DM300B; Leica Microsystems). Brown staining was considered to indicate positive detection. A negative control was performed by omitting the primary antibody. The intensity of staining was evaluated semi-quantitatively in five random microscopic fields under high magnification (×400). The scoring criteria were based on the percentage of positive area and the staining intensity. The percentage of positive area was scored as follows: No positive staining was scored as 0 points; <25% as 1 points; 25–50% as 2 points; 51–75% as 3 points; and >75% as 4 points. The staining intensity was scored as 0–3 points, representing negative, weak, moderate and strong staining, respectively. Five fields of each section were evaluated and the average scores for each parameter were calculated, then added. Zero points can be defined as negative (−), 2–3 points were divided into weakly positive (+); 4–5 points were divided into medium positive (++); and 6–7 points were divided into strong positive (+++).
RT-qPCR
Total RNA was extracted using an RNeasy Mini kit (Qiagen, Hilden, Germany). The concentrations of total RNA were measured by absorbance at 260/280 nm (MD1000; Beijing Thmorgan Biotechnology Co., Ltd., Beijing, China). Prior to reverse transcription, gDNA eraser (Qiagen) was used to remove genomic DNA. A QuantiTect Reverse Transcription Kit (Qiagen) was used to perform reverse transcription using 14 µl RNA and oligo(dT) primers, according to the manufacturer's instructions. The qPCR assays were performed using an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using QuantiFast SYBR green PCR Master Mix (Qiagen, Hilden, Germany) and 2 µl cDNA. The sequences of the primers (designed by Sangon Biological Engineering Co., Ltd., Shanghai, China) used were listed in Table I. A 20-µl reaction system was used containing 10 µl master mix (Qiagen), 2 µl cDNA, 7.6 µl H2O and 0.2 µl each of upstream and downstream primers. The cycling conditions were as follows: Melt at 95°C for 10 min; and 95°C for 10 sec, 60°C for 30 sec, for 40 cycles. Testing the SYBR green I fluorescent intensity in the reaction process was used to detect the increment of PCR products. A melting curve analysis was used to confirm the specificity of the PCR product, which was demonstrated as a single peak. The expression of β-actin served as the internal reference. Every sample was analyzed in triplicate. The relative expression levels of JNK, C-Jun, TNF-α and IL-1β were calculated using the 2−ΔΔCq method (5).
Table I.
Primers sequences for reverse transcription-quantitative polymerase chain reaction analysis.
| Primer | Sequence no. | Sequence (5′-3′) |
|---|---|---|
| JNK | NM-053829 | Forward: GATTTGGTGTAGCCCTTGGA |
| Reverse: GCTCACCCTTACCTGGAACA | ||
| C-Jun | NM-021835 | Forward: GCACATCACCACTACTCCGA |
| Reverse: GACACTGGGCAGCGTATT | ||
| TNF-α | NM-012675 | Forward: TACTGAACTTCGGGGTGATCG |
| Reverse: CCTTGTCCCTTGAAGAGAACC | ||
| IL-1β | NM-031512 | Forward: TCCAGTCAGGCTTCCTTGTG |
| Reverse: CGAGATGCTGCTGTGAGATT | ||
| β-actin | NM-031144 | Forward: AGTACCCCATTGAACACGGC |
| Reverse: TTTTCACGGTTGGCCTTAGG |
JNK, c-Jun N-terminal kinase; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β.
Western blot analysis
Total protein was extracted from liver tissues using radioimmunoprecipitation assay (RIPA) buffer (containing 0.1% PMSF), and the proteins were collected by centrifuging at 20,660 × g at 4°C for 10 min. As the protein phosphorylation was reversible and would be dephosphorylated by phosphatase, there was need to suppress or avoid cell phosphatase of endogenous and exogenous disturbances in the process of sample preparation and the immune detection, by using phosphatase inhibitor and protease inhibitor to draw reliable conclusions. Phosphatase inhibitor and protease inhibitor (Thermo Fisher Scientific, Inc.) were added to RIPA buffer (Thermo Fisher Scientific, Inc.) according to manufacturer's instructions. Protein concentrations were quantified using a bicinchoninic protein assay kit with bovine serum albumin as the standard (both purchased from Thermo Fisher Scientific, Inc.). Equal quantities of protein (50 µg) were separated by 10% SDS-PAGE (Beijing Solarbio Science & Technology, Co., Ltd.) and electrotransferred to polyvinylidene fluoride membranes (Roche AG, Basel, Switzerland). The membranes were blocked with 5% nonfat skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST; Beijing Solarbio Science & Technology Co., Ltd.) for 1 h at room temperature and then incubated overnight at 4°C with polyclonal rat anti-rabbit antibodies against JNK (cat. no. 9258S), phospho-JNK (cat. no. 4671S), C-Jun (cat. no. 9165S) and phospho-C-Jun (cat. no. 2361S) (all 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) then washed with TBST. Following this, membranes were incubated with secondary anti-rabbit antibody solution (1:1,000; cat. no. 1450236; Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at room temperature, and washed with TBST. Expression levels of the targeted proteins were detected using BCIP/NBT chromogenic substrate (Invitrogen; Thermo Fisher Scientific, Inc.). Protein concentrations were quantified using a bicinchoninic protein assay kit. The intensity of each band was captured digitally and measured using Quantity One software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation. SPSS software version 17.0 was used for statistical analysis (SPSS, Inc., Chicago, IL, USA). Significant differences were determined using one-way analysis of variance, and Least Significant Difference testing was performed for pair-wise comparison. The Wilcoxon Rank-Sum test was used for ranked data. P<0.01 was considered to indicate a statistically significant difference.
Results
Effects of vitamin D on biochemical parameters
The results of FPG, FINS and HOMA-IR are summarized in Table II. Compared with the NC group, the DM group exhibited increased levels of FPG, HOMA-IR and decreased levels of FINS (P<0.01). In the VD group, FPG and HOMA-IR were decreased, while FINS levels were increased compared with the DM group (P<0.01).
Table II.
Biochemical parameters.
| Group | FPG (mmol/l) | FINS (mIU/l) | HOMA-IR |
|---|---|---|---|
| NC (n=10) | 5.80±0.94 | 19.49±1.53 | 5.01±0.81 |
| DM (n=9) | 24.66±2.47a | 11.81±1.36a | 12.86±1.33a |
| VD (n=10) | 20.39±2.12a,b | 13.07±1.15a,b | 11.77±0.80a,b |
| F-value | 153.214 | 59.724 | 78.73 |
P<0.01 vs. NC group
P<0.01 vs. DM group. FPG, fasting plasma glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
Effects of vitamin D on liver histopathology
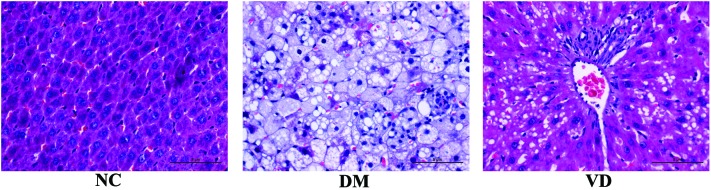
In the NC group, the structure of liver lobule was normal and clear, the hepatic cord was orderly with a large, round nucleus in the central of the hepatocyte and the cytoplasm is uniform. The size of liver appeared larger by naked eye in DM group compared with the NC group and was greasy to the touch. Various pathological changes appeared in the DM group, including narrow liver sinusoid, distortion of liver architecture, hepatocyte swelling, fatty degeneration, cytoplasm rarefaction, spotty necrosis scattering in the hepatic lobule as well as abundant inflammatory cell infiltration. However, the changes described above were markedly abated in the VD group (Fig. 1).
Figure 1.
Changes in liver histology (magnification, ×400; stain, hematoxylin and eosin). In the NC group, liver cell alignment is normal. In the DM group, liver cells appear swelled with fatty degeneration and inflammatory cell infiltration. In the VD group, fatty degeneration and inflammatory lesions are alleviated, in comparison with the DM group. NC, normal control group; DM, Type 2 diabetes mellitus group; VD, vitamin D-treated group.
Immunohistochemical analysis of the effects of vitamin D on liver and inflammatory cytokines
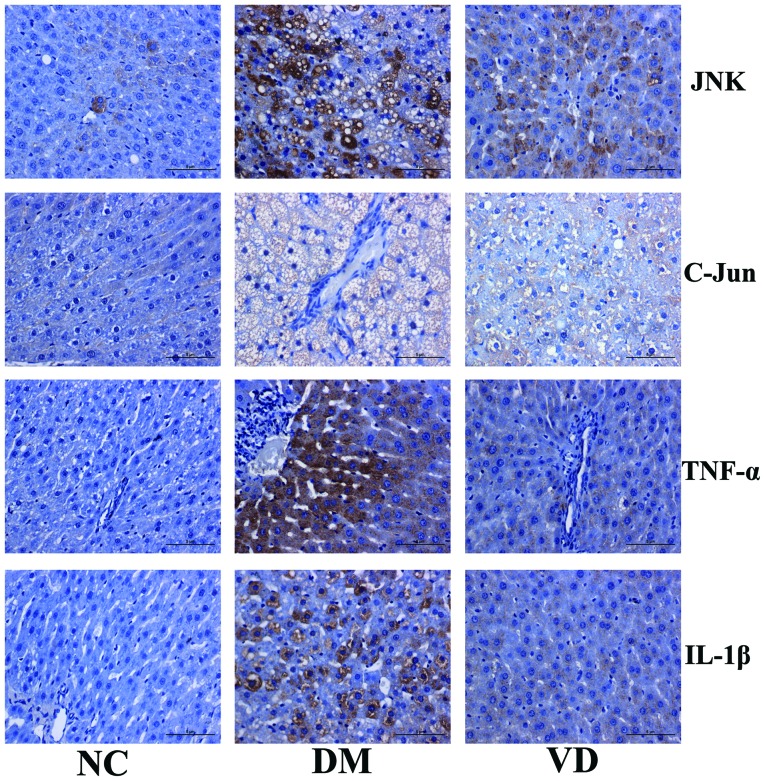
To investigate the molecular mechanisms underlying the therapeutic effects of vitamin D on liver tissues in T2DM model rats, immunohistochemical staining was performed to detect the expression of JNK, C-Jun, TNF-α and IL-1β in the liver tissues. The results showed that JNK, TNF-α and IL-1β were predominantly expressed in the cytoplasm, while C-Jun was primarily expressed in nucleus (Fig. 2). For JNK, C-Jun, TNF-α and IL-1β there was obvious brown staining and their protein expression levels were increased in the DM group compared with the NC group (P<0.01). Conversely, compared with the DM group, vitamin D administration significantly decreased the positive areas and staining intensity of each target protein, and hence their expression levels were significantly decreased in the VD group (P<0.01) (Tables III and IV).
Figure 2.
Immunohistochemical analysis of the protein expression of target genes in the liver tissues of type 2 diabetes mellitus model rats (magnification, ×400; stain, hematoxylin and eosin). JNK, TNF-α and IL-1β are predominantly expressed in the cytoplasm, while C-Jun is primarily expressed in the nucleus. For JNK, C-Jun, TNF-α and IL-1β there is obvious brown staining; protein expression levels are increased in the DM group compared with the NC group. Conversely, compared with the DM group, VD administration decreased the positive areas and staining intensity of each target protein. JNK, c-Jun N-terminal kinase; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
Table III.
Immunohistochemical staining for JNK and C-Jun protein expression levels in the rat liver tissues.
| JNK | C-Jun | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | − | + | ++ | +++ | − | + | ++ | +++ |
| NC (n=10) | 8 | 2 | 0 | 0 | 6 | 4 | 0 | 0 |
| DM (n=9) | 0 | 1 | 2 | 6 | 0 | 0 | 1 | 8 |
| VD (n=10) | 1 | 4 | 3 | 2 | 0 | 4 | 3 | 3 |
JNK, X2=10.41, P<0.01; C-Jun, X2=16.74, P<0.01. JNK, C-Jun N−terminal kinase; negative; +, weakly positive; ++, medium positive; +++, strong positive; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
Table IV.
Immunohistochemical staining for TNF-α and IL-1β protein expression levels in the rat liver tissues.
| TNF-α | IL-1β | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | − | + | ++ | +++ | − | + | ++ | +++ |
| NC (n=10) | 7 | 3 | 0 | 0 | 8 | 2 | 0 | 0 |
| DM (n=9) | 0 | 0 | 3 | 6 | 0 | 1 | 1 | 7 |
| VD (n=10) | 1 | 4 | 4 | 1 | 2 | 3 | 4 | 1 |
TNF-α, X2=6.64, P<0.01; IL-1β, X2=11.30, P<0.01. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; −, negative; +, weakly positive; ++, medium positive; +++, strong positive; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
RT-qPCR analysis of the effects of vitamin D on the mRNA expression levels of inflammatory cytokines
RT-qPCR was used to evaluate the changes in the mRNA expression levels of the target genes in the liver tissues of the T2DM model rats. The mRNA expression levels of JNK, C-Jun, TNF-α and IL-1β in the NC group were relatively low, and were significantly elevated in the DM group (P<0.01). Compared with the DM group, vitamin D treatment appeared to significantly decrease the expression levels of the target mRNAs (P<0.01) (Table V).
Table V.
Reverse transcription-quantitative polymerase chain reaction analysis of mRNA expression levels.
| Group | JNK | C-Jun | TNF-α | IL-1β |
|---|---|---|---|---|
| NC (n=10) | 1.02±0.11 | 0.90±0.12 | 0.82±0.09 | 0.77±0.09 |
| DM (n=9) | 7.39±1.27a | 5.15±0.98a | 6.14±1.13a | 6.31±1.33a |
| VD (n=10) | 5.93±1.29a,b | 3.76±0.97a,b | 4.78±1.07a,b | 5.09±0.84a,b |
| F-value | 99.707 | 72.906 | 62.305 | 55.018 |
P<0.01 vs. NC group
P<0.01 vs. DM group, JNK, C-Jun N-terminal kinase; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
Western blot analysis of the effects of vitamin D on the protein expression of inflammatory cytokines
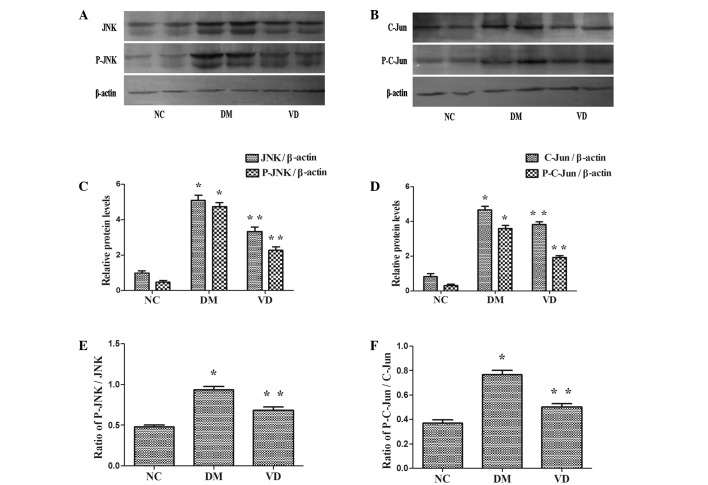
JNK is activated by phosphorylation and is able to phosphorylate/activate the transcription factor C-Jun. Consequently, the protein expression levels of JNK, phospho-JNK, C-Jun and phospho-C-Jun were assessed using a western blot assay. The protein expression levels of JNK, phospho-JNK, C-Jun and phospho-C-Jun were higher in the DM group compared with the NC group (P<0.01) (Fig. 3). Furthermore, they were reduced in the VD group compared with the DM group (P<0.01). Notably, the ratio of Phospho-JNK/JNK, Phospho-C-Jun/C-Jun was identical to above descriptions in each group (P<0.01).
Figure 3.
Western blot analysis of the target protein levels in the liver tissues of T2DM model rats. (A and B) Expression of JNK and C-Jun and their phosphorylated forms in each group, with β-actin as the internal reference. (C and D) Protein expression levels of JNK, P-JNK, C-Jun and P-C-Jun were higher in the DM group compared with the NC group (P<0.01), and were reduced in the VM group compared with DM group (P<0.01). (E and F) Indicated that the ratio of P-JNK/JNK and P-C-Jun/C-Jun were identical to above descriptions in each group (P<0.01). *P<0.01 vs. NC group; **P<0.01 vs. DM group. JNK, C-Jun N-terminal kinase; P-, phosphorylated-; NC, normal control group; DM, type 2 diabetes mellitus group; VD, vitamin D-treated group.
Discussion
It has been demonstrated that inflammation and IR play an initial role in the onset of T2DM, and that the progression of its complications, suggesting that the regulation of inflammation and IR may inhibit the emergence and progression of this disease. Vitamin D is a well-known steroid hormone that has been identified as a regulator of inflammation and IR. Based on available clinical and epidemiological data, the positive effects of vitamin D appear to be primarily related to its action on inflammation and secondary to its action on insulin sensitivity and secretion.
In the present study, H&E staining and immunohistochemistry were performed to assess the direct effects of vitamin D on diabetes-induced liver complications. The results showed that the administration of vitamin D significantly alleviated certain pathological changes, particularly steatosis and inflammatory cell infiltration. Furthermore, immunohistochemistry and RT-qPCR analysis showed that the expression levels of JNK, C-Jun, TNF-α and IL-1β, which are implicated in inflammation and IR, were decreased following treatment with vitamin D. Based on these results, we speculate that vitamin D exerts therapeutic effects on diabetes-induced liver complications, possibly by downregulating the expression of JNK, C-Jun, TNF-α and IL-1β. This modulation may be in part due to the anti-inflammatory properties of vitamin D; however. the underlying mechanism remains to be elucidated.
A previous study suggested that the activation of inflammatory pathways interferes with normal metabolism and disrupts insulin signaling (6). The presence of chronic inflammatory diseases, such as rheumatoid arthritis or hepatitis, significantly increases the risk for the development of IR and/or T2DM, thus suggesting an association between chronic inflammatory state and impaired insulin activity (7,8). In addition, prior results have indicated that vitamin D improves insulin sensitivity by its anti-inflammatory activity. Incubation of isolated monocytes with vitamin D attenuated the expression levels of proinflammatory cytokines involved in IR, such as IL-1β, IL-6 and TNF-α in patients with T2DM (9). Similarly in the present study, in liver the tissues of T2DM model rats the levels of IL-1β and TNF-α were decreased following the administration of vitamin D, which was accompanied by an improvement in HOMA-IR. Thus, we speculate that vitamin D is able to interfere with the crosstalk between inflammation and IR.
Targher et al (10) demonstrated that the beneficial effects of vitamin D were mediated through the modulation of VDR, a receptor of vitamin D, thereby improving signal transduction in the treatment of diabetes-induced liver complications. VDR is constitutively expressed by immune cells, which suggests that vitamin D plays an important role in the modulation of the inflammatory response. The liver contains a large quantity of Kupffer cells, which may be activated and release a wide range of products implicated in liver injury, such as inflammatory cytokines including TNF-α and IL-1β. These factors may further exacerbate hepatic inflammation and IR, as found in the present study, disabling the inflammatory pathway within these cells prevented the generalization of inflammation and IR in liver (11). VDR is almost universally expressed in nucleated cells (12), in which it controls vital genes associated with bone metabolism, chronic diseases and inflammation. Therefore, in the present study we hypothesized that cells exhibiting VDR may be a target of vitamin D. It has been demonstrated in monocytes, dendritic cells and T cells that vitamin D is able to inhibit the expression of inflammation-related genes by interacting with the NF-κB and AP-1/C-Jun pathways (13). In the present study, vitamin D appeared to downregulate the expression of C-Jun and its upstream signaling molecule JNK in liver tissues.
JNKs were initially described as a family of serine/threonine protein kinases, and may be activated by diverse stimuli including cytokines (such as TNF-α and IL-1β), reactive oxygen species, endoplasmic reticulum stress, free fatty acid, hyperglycemia and advanced glycation end products, all of which are known to be increased under diabetic conditions (14). JNK serves a crucial function in chronic inflammatory diseases involving the expression of specific proteases and cytokines (15). JNK activity and/or expression levels are elevated in various tissues in patients with T2DM, and have been shown to promote the progression of inflammation and IR in T2DM and its complications, which is in accordance with the present results. The specific function of JNK in the dysregulation of hepatic insulin signaling has also been demonstrated. Previous studies have indicated that hepatic inhibition of JNK using a dominant-negative protein or knockdown of JNK in diabetic mice significantly lowered blood glucose levels and improved insulin sensitivity. Silencing of hepatic JNK by small interfering RNA increased the expression of glycolysis enzymes, reduced the expression of gluconeogenic enzymes and the attenuated development of IR in a nutritionally-induced diabetes mouse model (16). These results directly demonstrate the therapeutic potential of JNK inhibitors in diabetes. On the basis of the present results, vitamin D may function as an inhibitor of JNK. Activated JNK is able to directly target insulin receptor substrate 1 (IRS)-1 for serine phosphorylation, which inhibits the insulin signaling cascade (17). In addition, Sharfi and Eldar-Finkelman (18) showed that the expression level of JNK is markedly elevated in the diabetic liver and that JNK/GSK-3-mediated phosphorylation of IRS-2 inhibited insulin signaling in liver cells. Thus, JNK has been viewed as a potential target for the prevention and treatment of T2DM (19).
At least 50 proteins have been identified as JNK substrates. Among these substrates, C-Jun is a representative target, as the functions of JNK depend largely on phosphorylating C-Jun (20). Phosphorylated JNK translocates into the nucleus and regulates downstream transcriptional programs via transcription factor C-Jun, and consequently promotes the activity of the activator protein-1 (AP-1). This promotion may result in the increased expression of proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α. These cytokines target cell membrane receptors, thus exacerbating the inflammatory response and inducing IR (21). In addition to being leaner and more insulin responsive, high-fat diet-fed JNK−/− mice exhibited reduced expression of inflammatory cytokines such as IL-6, TNF-α and MCP-1 (22).
Among the best-studied pathways leading to JNK activation is TNF-α signaling via TNF-α receptor 1 (TNFR1) (23). Mice that lack TNFR1 and rats injected with an antibody against TNF-α exhibit reduced liver activation of JNK and C-Jun/AP-1 (24). Notably, JNK expression has been implicated in hepatocyte injury mediated by TNF-α (25). In hepatocytes, TNF-α rapidly activates JNK, leading to the activation of C-Jun/AP-1 and consequently the production of proinflammatory cytokines, particularly TNF-α and IL-1β, which in return activate JNK as has been noted. In conclusion, the interaction between JNK and TNF-α forms a vicious circle and results in an increased quantity of proinflammatory cytokines, which exacerbated inflammation and IR in liver tissues in the present study. The administration of vitamin D has been shown to inhibit the inflammatory response of diabetic mice and reduce fasting glucose and serum TNF-α levels (26). Vitamin D administration also inhibited the expression of TNF-α in monocytes extracted from diabetic patients (9). Furthermore, TNF-α is hypothesized to play a major role in the pathophysiology of IR in rodents (27) via the phosphorylation of IRS-1 protein on serine residues. Recombinant TNF-α decreases insulin sensitivity, while TNF-α or TNF-α receptor null mice exhibit an increased sensitivity in response to this hormone, suggesting a direct role for TNF-α in the development of IR.
In chronic liver disease, the overproduction of IL-1β and TNF-α is observed (28). Negrin et al (29) reported that the development of hepatic steatosis required IL-1β signaling, which upregulated fatty acid synthase to promote hepatic lipogenesis. Furthermore, the administration of IL-1β receptor antagonist to obese mice markedly reduced steatosis in hepatic tissues. IL-1β binding to IL-1β receptor I (IL-1RI) activated a number of inflammatory pathways, including JNK, which induced IR by attenuating IRS-1 activation while activating C-Jun/AP-1 and consequently inducing the transcription of inflammatory genes including itself (24), similarly to TNF-α, ultimately resulting in extensive inflammation reactions in the liver. IL-1RI−/− were protected against high-fat diet-induced IR. Recent clinical studies aimed at attenuating IL-1β activity in subjects with T2DM have yielded promising results; demonstrating improvements in hyperglycaemia, insulin secretion and sensitivity, β-cell function and reduced levels of systemic inflammation (30). Moore et al (31) suggested that the anti-inflammatory effect of vitamin D3 derived from its ability to downregulate the IL-1β-induced activation of JNK in microglial cells.
It is currently unclear whether JNK is the cause or effect of other cytokines, particularly TNF-α and IL-1β. The targeting of individual inflammatory cytokines may not be highly effective, whereas the targeting of inflammatory kinases JNK generated a robust anti-diabetic action as this factor integrated signals from multiple inflammatory mediators. Therefore, targeting JNK could potentially disable the inflammatory response and improve insulin action (32). For example, TNF-α is a key downstream effectors of JNK-mediated IR in 3T3-L1 adipocytes (33). In the present study, the protein expression levels of JNK and C-Jun, in addition to their active forms phospho-JNK and phospho-C-Jun, were evaluated using western blot assays. The results showed that compared with the DM group, vitamin D was able to downregulate the protein expression of JNK and C-Jun, their active forms and the ratios of phospho-JNK/JNK and phospho-C-Jun/C-Jun. These results suggested that vitamin D was able to regulate the phosphorylation of JNK and C-Jun. Notably, increased dietary vitamin D was beneficial in preventing inflammation-associated colon cancer via the suppression of inflammatory responses, particularly JNK (34). Supplementation with vitamin D improved IR in muscle cells by reducing the phosphorylation of JNK (35). These results suggest that vitamin D has a crucial impact on inflammation and IR induced by JNK. Thus, JNK signaling pathway may be a crucial target of vitamin D with regarding to anti-inflammation and improves IR in diabetes-induced liver complications. Hence, the inhibition of the JNK pathway prevents its activation by various stimuli, particularly TNF-α and IL-1β, and contributes to the reduced production of proinflammatory cytokines in the liver. In conclusion, vitamin D may play a role in attenuating the positive feedback loop between JNK signaling pathway and TNF-α and IL-1β, thus ameliorating the diabetes-induce liver complications in T2DM model rats.
Lipid infusion or high fat feeding promotes IR in rodents and humans (33). IR occurs in the liver and other tissues, resulting in metabolism disturbance which may result in the accumulation of fat in hepatocytes. Notably, the Third National Health and Nutrition Examination Survey identified an inverse association between vitamin D status and IR/diabetes (36). Significant improvements were observed in insulin sensitivity and IR in a randomized, controlled, double-blind intervention, which involved the administration of 100 µg vitamin D (4,000 IU) for 6 months to South Asian women who were insulin resistant (HOMA-IR, >1.93; serum 25(OH)D, <50 nmol/l) (37), which was in agreement with the present findings. In the present study, the HOMA-IR in the DM group was significant higher compared with the NC group following vitamin D treatment, thus showing a tendency toward improvement. Although the underlying mechanisms remain unclear, it may partly due to the anti-inflammatory properties of vitamin D. The JNK-C-Jun axes is a major inflammatory pathway involved in the disruption of insulin signaling, and modulating its action with anti-inflammatory agent is believed to improve insulin sensitivity and glucose homeostasis (38). In the present study, vitamin D may have served as the anti-inflammatory agent in regulating JNK/C-Jun, TNF-α and IL-1β in insulin signaling.
It has been reported that the combination of lipotoxicity and glucotoxicity induced by a high-fat diet and STZ injection leads to islet β-cell failure and apoptosis, and ultimately to hypoinsulinemia and hyperglycemia (39). This is similar to the present method of T2DM model establishment, and closely mimics the natural history and metabolic characteristics of late stage T2DM. T2DM is characterized by an increase in IR in conjunction with the inability of islet β-cells to secrete sufficient insulin to compensate. IR and hypoinsulinemia may result in a hyperglycemic state that is a major risk factor for the development of diabetes-induced liver complications. Hyperglycemia promotes the accumulation of fat in the liver, while releases oxidizing and inflammatory mediators and causing local and systemic damage. In the present study, the administration of vitamin D reduced the FPG and increased the FINS. These changes are in accordance with a prior study (40) which showed that vitamin D is able to act on β-cells through the vitamin D response elements present in insulin gene promoter, thereby increasing the levels of FINS in plasma which may result in decreased FBG levels in vitamin D-treated group. In addition, the immunomodulatory properties of vitamin D may decrease the release of inflammatory cytokines such as TNF-α and IL-1β, while improving IR-induced by inflammation, thereby restoring residual β-cells and decreasing FPG induced by IR.
In summary, the present results indicate the protective effects of vitamin D against diabetes-induced liver complications, which may occur by modulating the inflammatory response, attenuating the crosstalk between inflammation and IR and ameliorating hyperglycemic state. The results suggest an association between vitamin D, inflammation and T2DM.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81160116).
References
- 1.Guadarrama-López AL, Valdés-Ramos R, Martínez-Carrillo BE. Type 2 diabetes, PUFAs, and vitamin D: Their relation to inflammation. J Immunol Res. 2014;2014:860703. doi: 10.1155/2014/860703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores M. A role of vitamin D in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus? Nutr Res Rev. 2005;18:175–182. doi: 10.1079/NRR2005104. [DOI] [PubMed] [Google Scholar]
- 3.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 4.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 5.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 6.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Ann Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, McCarey DW, Capell H, McInnes IB. ‘Explaining how high-grade’ systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 8.Knobler H, Zhornicky T, Sandler A, Haran N, Ashur Y, Schattner A. Tumor necrosis factor-alpha-induced insulin resistance may mediate the hepatitis C virus-diabetes association. Am J Gastroenterol. 2003;98:2751–2756. doi: 10.1016/j.amjgastroenterol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–170. [PubMed] [Google Scholar]
- 12.Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–5799. doi: 10.1128/MCB.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/S0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 15.Karin M, Gallagher E. From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 16.Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Trevillyan JM. c-Jun N-terminal kinase pathways in diabetes. Int J Biochem Cell Biol. 2008;40:2702–2706. doi: 10.1016/j.biocel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Sharfi H, Eldar-Finkelman H. Sequential phosphorylation of insulin receptor substrate-2 by glycogen synthase kinase-3 and c-Jun NH2-terminal kinase plays a role in hepatic insulin signaling. Am J Physiol Endocrinol Metab. 2008;294:E307–E315. doi: 10.1152/ajpendo.00534.2007. [DOI] [PubMed] [Google Scholar]
- 19.Kaneto H. The JNK pathway as a therapeutic target for diabetes. Expert Opin Ther Targets. 2005;9:581–592. doi: 10.1517/14728222.9.3.581. [DOI] [PubMed] [Google Scholar]
- 20.Tarantino G, Caputi A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J Gastroenterol. 2011;17:3785–3794. doi: 10.3748/wjg.v17.i33.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chagas CE, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 24.Seki E, Brenner DA, Karin M. A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNF alpha-and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Xie H, Fu M, Li W, Guo B, Ding Y, Wang Q. 25-hydroxyvitamin D3 ameliorates periodontitis by modulating the expression of inflammation-associated factors in diabetic mice. Steroids. 2013;78:115–120. doi: 10.1016/j.steroids.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Bieghs V, Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013;34:446–452. doi: 10.1016/j.it.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Negrin KA, Roth Flach RJ, Di Stefano MT, Matevossian A, Friedline RH, Jung D, Kim JK, Czech MP. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PloS One. 2014;9:e107265. doi: 10.1371/journal.pone.0107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 31.Moore M, Piazza A, Nolan Y, Lynch MA. Treatment with dexamethasone and vitamin D3 attenuates neuroinflammatory age-related changes in rat hippocampus. Synapse. 2007;61:851–861. doi: 10.1002/syn.20433. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 34.Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74:4398–4408. doi: 10.1158/0008-5472.CAN-13-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou QG, Hou FF, Guo ZJ, Liang M, Wang GB, Zhang X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab Res Rev. 2008;24:459–464. doi: 10.1002/dmrr.873. [DOI] [PubMed] [Google Scholar]
- 36.Scragg R, Sowers M, Bell C. Third National Health and Nutrition Examination Survey: Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 37.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient-a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 39.Nugent DA, Smith DM, Jones HB. A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol Pathol. 2008;36:529–551. doi: 10.1177/0192623308318209. [DOI] [PubMed] [Google Scholar]
- 40.Meerza D, Naseem I, Ahmed J. Effect of 1,25(OH)2 vitamin D3 on glucose homeostasis and DNA damage in type 2 diabetic mice. J Diabetes Complications. 2012;26:363–368. doi: 10.1016/j.jdiacomp.2012.05.013. [DOI] [PubMed] [Google Scholar]