Abstract
Glucagon-like peptide-1 (GLP-1) and GLP-1 receptors (GLP-1Rs) are responsible for glucose homeostasis, and have been shown to reduce inflammation in preclinical studies. The aim of the present study was to determine whether sitagliptin, an inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4), as a GLP-1 receptor agonist, exerts an anti-inflammatory effect on cardiomyoblasts during lipopolysaccharide (LPS) stimulation. Exposure to LPS increased the expression levels of tumor necrosis factor (TNF)-α, interleukin-6 (IL)-6 and IL-1β in H9c2 cells, and also resulted in elevations in cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression and nuclear factor-κB (NF-κB) nuclear translocation. Treatment with the DPP-4 inhibitor sitagliptin dose-dependently downregulated the mRNA levels of IL-6, COX-2 and iNOS in LPS-stimulated H9c2 cells. In addition, sitagliptin inhibited the increased protein expression of IL-6, TNF-α and IL-1β. NF-κB mRNA expression was reduced and its translocation to the nucleus was suppressed by treatment with sitagliptin. The present results demonstrated that sitagliptin exerts a beneficial effect on cardiomyoblasts exposed to LPS by inhibiting expression of inflammatory mediators and suppressing NF-κB activation. These findings indicate that the DPP-4 inhibitor sitagliptin may serve a function in cardiac remodeling attributed to sepsis-induced inflammation.
Keywords: lipopolysaccharides, sitagliptin, cardiomyopathy, sepsis
Introduction
Severe bacterial infection may contribute to systemic dysfunction, including heart failure, which is the predominant cause of morbidity and mortality in patients with sepsis. Lipopolysaccharide (LPS) is a major component of the bacterial outer membrane and has been shown to play an important role in the inflammatory response in the cardiovascular system (1). Under septic conditions, the LPS-induced activation of toll-like receptor 4 (TLR-4) results in the enhanced production of proinflammatory cytokines and results in myocardial dysfunction (2). Previous studies have shown that LPS has adverse effect on cardiomyoblasts, which induces the activation of TLR-4 and triggers nuclear factor-κB (NF-κB) signaling and results in increased the expression of proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) (3–5). During sepsis, the pathological production of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) may also have harmful effects on cardiomyocyte and impair cardiac contractile function (6,7). Interventions for sepsis involving anti-inflammatory agents have been demonstrated to reduce risk of cardiovascular complications (8,9).
Glucagon-like peptide-1 (GLP-1) and GLP-1 receptors (GLP-1Rs) serve crucial function in glucose-stimulated insulin release, leading to great interest in their use for glycemic control (10). Furthermore, recent studies have shown that GLP-1 reduces inflammation and oxidative stress in endothelial cells (11,12). The importance of GLP-1 in glucose homeostasis is emphasized by the inhibitors of dipeptidyl peptidase-4 (DPP-4); enzymes which have been found to exert positive effects on cardioprotection in previous studies (13,14). DPP-4 inhibitors may improve cardiac outcomes following myocardial infarction via cytoprotective pathways (15). Notably, DPP-4 inhibitors have been demonstrated to exert antiatherogenic effects on improving endothelial function through augmenting GLP-1 activity, and the anti-inflammatory effect of DPP-4 inhibitors has been suggested in preclinical and clinical studies of type 2 diabetes and coronary artery disease (16,17). However, the role of DPP-4 inhibitors in septic inflammation, which may lead to cardiovascular complications, remains unclear.
The aim of the present study was to clarify whether septic complications may be effectively reduced by DPP-4 therapeutic targeting of LPS-induced immune responses. It is conceivable that intervention in inflammatory process is beneficial for sepsis-associated cardiac dysfunctions. We investigated whether the DPP-4 inhibitor, sitagliptin, was able to exert inhibitory effects on an LPS-stimulated inflammatory response in cardiomyoblasts.
Materials and methods
Cell culture
H9c2 cells, a rat ventricular myoblast cell line, were obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). The cells were maintained in Gibco Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% Gibco fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) in humidified air (5% CO2) at 37°C. H9c2 cells were incubated overnight prior to treatment. Following stimulation with 10 µg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA) for 4 h, cells were treated with the DPP-4 inhibitor sitagliptin (0, 0.1, 0.5, 1, 2 and 4 µM; Sigma-Aldrich) and accompanied LPS at 37°C for 20 h.
Cell viability assay
Cell viability was determined using an MTT assay. Briefly, H9c2 cells were seeded at a density of 1×105 cells/well in 24-well plates 24 h prior to treatments. Following treatment, the culture medium was replaced with MTT solution (Sigma-Aldrich), consisting of 5 mg/ml stock solution in PBS, diluted with DMEM to a final concentration of 0.5 mg/ml. After 3 h incubation at 37°C the supernatant was aspirated, and the formazan produced was solubilized in dimethyl sulfoxide (Sigma-Aldrich). The resulting absorbance was measured at a wavelength of 540 nm with background subtraction at 650 nm using an EMax® Endpoint ELISA Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Following treatment, total RNA was extracted using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.). Genomic DNA was removed by adding nanopure water, RNA, DNase buffer and DNase (Qiagen, Hilden, Germany) to samples. The quantity of each RNA sample was determined using a Qubit fluorometer (Thermo Fisher Scientific, Inc.). Reverse transcription was performed in a 20-µl reaction system containing 200 ng total RNA using high capacity cDNA reverse transcription kits (cat. no. 4368814; Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative quantification of inflammation mediators and apoptosis indicators were assessed by qPCR, which was performed using SYBR Green mix Bio-Rad Laboratories, Inc., Hercules, CA, USA), using an ABI 7900HT Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences for each gene were supplied by Gene Probe Technologies, Inc. (Gaithersburg, MD, USA) and are presented in Table I. The reaction mixture for RT-qPCR was as follows: SYBR Green, 10 µl; forward primer, 0.4 µl; reverse primer, 0.4 µl; cDNA, 2 µl; and nuclease-free H2O, 7.2 µl. The reaction system for PCR used the following thermal cycle conditions: 35 Cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 45 sec. Negative controls containing all PCR components without template DNA were used to ensure that the reagent mix was free of contamination. Cycle threshold (Cq) values were determined by automated threshold analysis. The average Cq, ΔCq, ΔΔCq and RQ were calculated by detection software (7500 Fast System SDS Software; version 1.4; Applied Biosystems; Thermo Fisher Scientific, Inc.). The RQ (ΔΔCq value) was used to compare gene expression between samples. A housekeeping gene β-actin was used as an internal control. Primer sequences are indicated in Table I.
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer (5′-3′) | Reverse primer (3′-5′) |
|---|---|---|
| TNF-α | CACCGGCAAGGATTCCAA | CACTCAGGCATCGACATTCG |
| IL-6 | TCTCCAGCAACGAGGAGAAT' | TGTGATCTGAAACCTGCTGC |
| IL-1β | GACCTTCCAGGATGAGGACA | AGGCCACAGGTATTTTGTCG |
| iNOS | TGCTTACAGGTCTACGTTCAAGACAT | CGGCCACCAGCTTCTTCA |
| COX2 | GGATCCCCAAGGCACAAATAT | TCGCTTCTGATCTGTCTTGAAAAA |
| NF-κB | TGAGTCCCGCCCCTTCTAA | TGATGGTCCCCCCAGAGA |
| β-actin | ATGCCCCGAGGCTCTCTT | CAACGTCACACTTCATGATGGA |
TNF-α; IL, interleukin; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; NF-κB, nuclear factor-κB.
Enzyme-linked immunosorbent assay (ELISA)
Highly specific quantitative sandwich ELISA kits were used to measure the expression of the cytokines IL-6 (KHC0061), IL-1β (KHC0011)and TNF-α (KAC1751) (all purchased from Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. The optical density of each ELISA sample was determined at 450 nm, with the correction wavelength of 570 nm, using a microplate reader. Concentrations of cytokine are calculated on the basis of a standard curve.
Subcellular fractionation
Cells were washed with PBS and incubated with a lysis buffer (10 mM HEPES, pH7.6; containing 15 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1 mM dithiothreitol, 0.05% v/v Igepal CA-630 and 1 mM PMSF, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 10 µg/ml leupeptin and 10 µg/ml aprotinin; Sigma-Aldrich) for 10 min at 4°C. Resulting lysates were centrifuged at 2,500 × g for 10 min at 4°C. The supernatant was collected and centrifuged at 20,000 × g for 15 min at 4°C, comprising the cytosolic fraction. Pellets were washed with PBS and suspended in nuclear buffer 5 mM HEPES, pH7.6, 0.1% v/v Igepal CA-630, 1 M KCl, 0.1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 2 mM sodium fluoride, 10 µg/ml leupeptin and 10 µg/ml aprotinin; Sigma-Aldrich) followed by a centrifugation at 10,000 × g for 15 min at 4°C. The resulting supernatants were collected, comprising the nuclear fraction.
Western blot analysis
Following treatment, cells were lysed by incubation with radioimmunoprecipitation assay buffer (Sigma-Aldrich) for 5 min on ice. Samples were centrifuged at 4°C for 15 min at 16,000 × g and the supernatants were collected. The protein concentration was measured using a Qubit® Protein Assay kit (Thermo Fisher Scientific, Inc.). Next, ~20 µg protein per sample was loaded in 10–12% SDS-PAGE, then transferred to PVDF (EMD Millipore, Billerica, MA, USA) using 100 V for 1 h at 4°C. Blocking was performed with 5% bovine serum albumin in Tris-buffered saline (Sigma-Aldrich) with Tween 20 (TBST) and subsequently immunoblotted with the following primary antibodies overnight at 4°C: Rabbit polyclonal anti-human NF-κB (sc-372; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal anti-human Actin (A5441; Sigma-Aldrich) and rabbit polyclonal anti-human histone H3 (ab18521; Abcam, Cambridge, UK) and used at a dilution of 1:1,000. The blots were then washed with TBST and incubated with horseradish peroxidase-conjugated donkey anti-mouse (ab97030) and donkey anti-rabbit (ab97064) secondary antibodies at a 1:5,000 dilution for 30 min at room temperature. The blots were developed using ECL Western Blotting Detection Reagents (Amersham; GE Healthcare Life Sciences, Little Chalfont, UK).
Statistical analysis
Statistical analyses were performed using SPSS version 13.0 for Windows (SPSS, Inc., Chicago, IL, USA). The value of each treatment group was presented as a mean with the standard deviation of triplicates. Data were compared using one-way analysis of variance with post hoc Tukey-Kramer multiple comparisons test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of DPP-4 inhibitor on viability of H9c2 cells
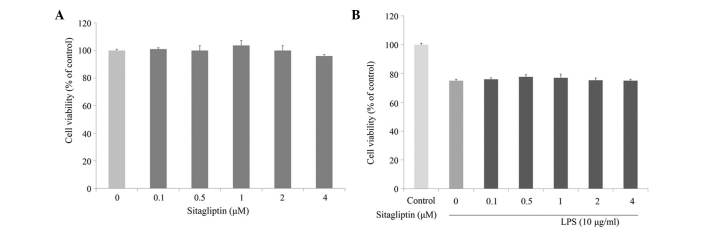
The cytotoxic effect of DPP-4 inhibitor on H9c2 cell viability was evaluated at various concentrations using MTT assay. As shown in Fig. 1A, incubation of H9c2 cells with a serial of concentration of DPP-4 inhibitor (0.1–4 µM) for 24 h slightly affected cell viability. Next, the cytotoxic effect of DPP-4 inhibitor on LPS-stimulated H9c2 cells was investigated. Cell viability of the H9c2 cells was slightly decreased in the presence of LPS; however, DPP-4 inhibitor exerted no effect on the viability of LPS-treated H9c2 cells (Fig. 1B).
Figure 1.
Effects of sitagliptin on the cell viability of (A) H9c2 cells and (B) LPS-treated H9c2 cells. Values are presented as the mean ± standard deviation. LPS, lipopolysaccharide.
Effect of LPS and the DPP-4 inhibitor sitagliptin on H9c2 cell morphology
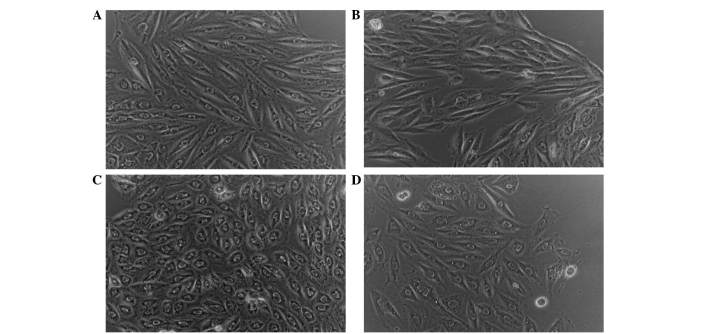
The effect of LPS and sitagliptin on H9c2 cell morphology were observed. Fig. 2A shows H9c2 cells without any treatment. When these cells were treated with sitagliptin alone, there was no apparent change in cellular shape as shown in Fig. 2B. However, following LPS stimulation the H9c2 cells exhibited cell rounding (Fig. 2C), which may indicate membrane blebbing due to morphological alterations. However, as a result of the administration of sitagliptin following LPS stimulation, H9c2 cells exhibited reduced phenotypic responses (Fig. 2D).
Figure 2.
Effects of lipopolysaccharide (LPS) and sitagliptin on H9c2 cell morphology. (A) Control without any treatment. (B) H9c2 cell morphology after treated by sitagliptin alone. (C) H9c2 cell morphology after treatment with LPS stimulation alone. (D) Administration of sitagliptin on H9c2 cells following LPS stimulation.
Effect of DPP-4 inhibitor on the regulation of proinflammatory mediator expression in LPS-treated H9c2 cells
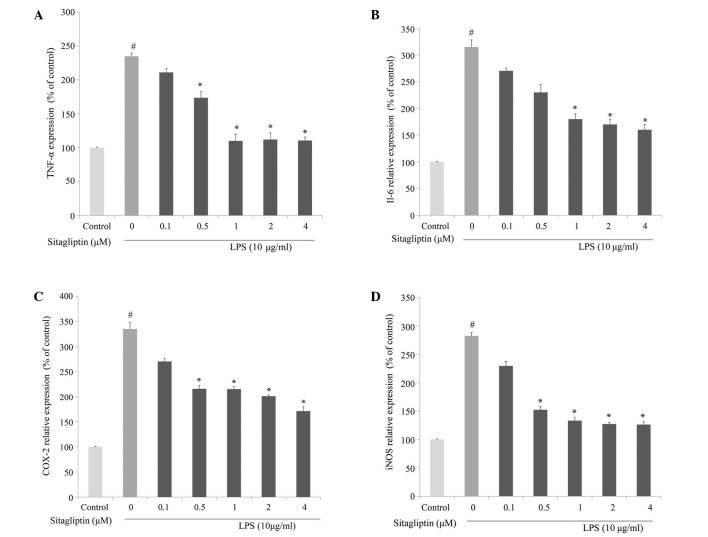
To investigate whether DPP-4 inhibitor alleviates inflammatory responses in cardiovascular tissue, the changes in the mRNA expression levels of inflammation-associated genes following DPP-4 inhibitor treatment in LPS-treated H9c2 cells were evaluated using qPCR analysis. The elevated mRNA expression of TNF-α was reduced following treatment with DPP-4 inhibitor (0.1–4 µM) (Fig. 3A). The mRNA expression of IL-6 in H9c2 cells was significantly increased in presence of LPS. The elevation of IL-6 in LPS-treated H9c2 cells was partially normalized as a result of exposure to DPP-4 inhibitor, and the alleviation was dose-dependent (Fig. 3B). It is known that LPS induces the activation of COX-2 transcription, leading to a release of prostaglandin E2 (18). The present data showed that LPS-treated H9c2 cells exhibited a significant increase in mRNA expression of COX-2. Treatment of LPS-stimulated H9c2 cells with DPP-4 inhibitor resulted in a suppression of the LPS-elevated expression of COX-2 (Fig. 3C). The mRNA expression levels of iNOS in H9c2 were significantly increased in response to exposure to LPS. The elevated expression of iNOS in H9c2 was significantly downregulated by DPP-4 inhibitor treatment at 0.5, 1, 2 and 4 µM (Fig. 3D). The amelioration of the LPS-induced upregulation of the expression of TNF-α, IL-6, COX-2 and iNOS by the DPP-4 inhibitor sitagliptin was dose-dependent.
Figure 3.
Effects of sitagliptin on the mRNA expression levels of (A) TNF-α, (B) IL-6, (C) COX-2 and (D) iNOS in LPS-treated H9c2 cells using quantitative polymerase chain reaction analysis. Values presented as the mean ± standard deviation. #P<0.01 vs. control group; *P<0.05 vs. LPS group. TNF-α, tumor necrosis factor-α, LPS, lipopolysaccharide; IL-6, interleukin-6; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase.
Effect of DPP-4 inhibitor on the protein expression of proinflammatory cytokines in LPS-treated H9c2 cells
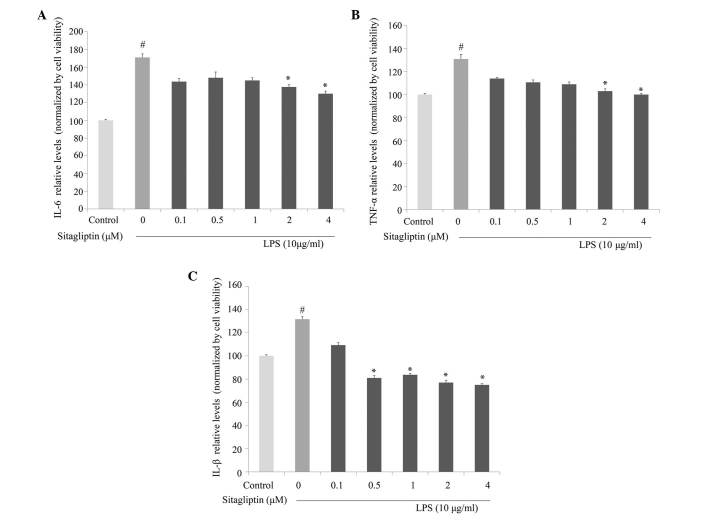
Next, the anti-inflammatory activity of DPP-4 inhibitor against the production of proinflammatory cytokines was investigated in LPS-treated H9c2 cells. IL-6 and TNF-α production in culture medium were evaluated using ELISA. As shown in Fig. 4A, IL-6 production was significantly decreased by DPP-4 inhibitor treatment at concentrations of 2 and 4 µM compared with cells treated with LPS alone. Exposure of H9c2 cells to LPS led to a significant increase in TNF-α secretion, which is consistent with the observed upregulation of expression of TNF-α at the mRNA level. Treatment with DPP-4 inhibitor led to partially normalized protein expression of TNF-α compared with the cells treated with LPS alone (Fig. 4B). Following stimulation, IL-1β is often upregulated in association with TNF-α and IL-6 under inflammation conditions. The LPS-elevated expression of IL-1β in the H9c2 cells was significantly inhibited by DPP-4 inhibitor treatment at 0.5, 1, 2 and 4 µM (Fig. 4C).
Figure 4.
Effects of sitagliptin on the protein expression of (A) IL-6, (B) TNF-α and (C) IL-1β in LPS-treated H9c2 cells. Values presented as the mean ± standard deviation. #P<0.01 vs. control group; *P<0.05 vs. LPS group. IL, interleukin; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
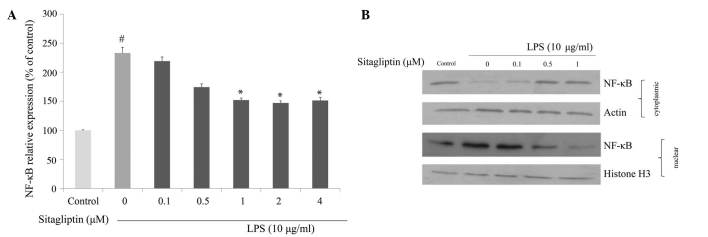
Effect of DPP-4 inhibitor on NF-κB regulation
It is evident that NF-κB plays a crucial role in regulating the expression of proinflammatory genes, including TNF-α and COX-2. We investigated whether DPP-4 inhibitor exerts anti-inflammatory effect via the inhibition of NF-κB in LPS-treated H9c2 cells. mRNA expression and nuclear translocation of NF-κB were examined using qPCR and western blot analyses, respectively. The mRNA expression of NF-κB in H9c2 cells was significantly upregulated in the presence of LPS. Exposure of LPS-treated H9c2 cells to DPP-4 inhibitor resulted in suppression of the LPS-elevated NF-κB expression (Fig. 5A). We also examined the influence of DPP-4 inhibitor on the translocation of NF-κB in response to LPS exposure. The data from western blot analysis indicated that LPS triggered nuclear translocation of NF-κB in H9c2 cells, and that degree of translocation of NF-κB was reduced by DPP-4 inhibitor treatment (Fig. 5B).
Figure 5.
Effects of sitagliptin on the expression of NF-κB. (A) mRNA levels of NF-κB in LPS-treated H9c2 cells. Values presented as the mean ± standard deviation. #P<0.01 vs. control group; *P<0.05 vs. LPS group. (B) Nuclear and cytoplasmic protein expression levels of NF-κB in LPS-treated H9c2 cells. Histone H3 as loading control for nuclear fraction and actin as loading control for cytoplasmic fraction. NF-κB, nuclear factor-κB; LPS, lipopolysaccharide.
Discussion
The expression of inflammatory markers is increased in sepsis patients with cardiovascular dysfunction. Prior studies have shown elevations in inflammatory marker expression in infectious individuals who undergo systemic bacterial infection (19,20). In patients with endoxemia and sepsis, circulating LPS induces the elevation of proinflammatory cytokines, which may contribute to myocardial depression (21). It was shown that LPS participates in the inflammatory response by resulting in enhanced expression of proinflammatory cytokines such as TNF-α and causes dysfunction in the cardiovascular system (22).
Under septic conditions, excessive LPS enhances the expression of proinflammatory cytokines and chemokine cascades via the activation of TLR-4. Previous studies have shown that cardiomyoblasts express TLR-4, through which LPS exerts a adverse effect mediated by NF-κB signaling, resulting in decreased cardiomyocyte contractility (23,24). It has been reported that the regulation of NF-κB was involved in the amelioration of inflammatory responses by DPP-4 inhibitor (25). Several studies also demonstrated that DPP-4 inhibitor has beneficial effects on cardiovascular system and gain the ability to improve renal microvasculature (26,27). However, the role of DPP-4 inhibition in ameliorating cardiovascular complication and potential anti-inflammatory properties during sepsis has not been largely investigated. Therefore, there is a requirement to determine whether DPP-4 inhibitor exerts direct anti-inflammatory effect on cardiomyoblast during sepsis-induced inflammation.
The aim of the present study was investigate the effects of sitagliptin on LPS-induced inflammation in cardiomyoblasts. The results indicate that sitagliptin inhibited the increased mRNA expression of inflammatory genes in LPS-stimulated cardiomyoblasts, including TNF-α, IL-6, COX-2 and iNOS. Furthermore, the activated expression of NF-κB was downregulated in the presence of sitagliptin. Additionally, treatment of LPS-stimulated H9c2 cells with sitagliptin resulted in the inhibition of the elevated protein expression of TNF-α, IL-6 and IL-1β. A previous study has reported that the increased expression of proinflammatory cytokines is associated with mortality and may indicate the severity of sepsis (20). In addition, IL-6 was modulated by IL-1β and TNF-α in cardiomyocytes due to circulating LPS, via NF-κB signaling pathway (28). The present results suggest that LPS significantly induced the mRNA expression of IL-6 and TNF-α, in addition to stimulating NF-κB activation in H9c2 cells. These inflammatory cytokines were reduced in the presence of sitagliptin, suggesting that sitagliptin has inhibitory effects on inflammation in response to proinflammatory agents such as LPS, which can lead to cardiovascular depression during sepsis. The present results are consistent with previous studies which have shown that sitagliptin can lead to reduced levels of proinflammatory markers, and may potentially contribute to the inhibition of cardiovascular complications (29,30).
In addition to proinflammatory cytokines, it has been demonstrated that sepsis results in significant expression of COX-2 in cardiomyocytes, with subsequent release of prostaglandin during myocardial inflammation (31). The present data revealed that the expression of COX-2 was upregulated by LPS in cardiomyoblasts, and that this upregulation could be suppressed by sitagliptin. This result suggested that treatment with sitagliptin inhibits LPS-induced overexpression of COX-2, leading to a reduction of prostaglandin release. It is evident that endotoxemia is associated with increased production of NO, mediated by iNOS (32). The increased NO production may reduce the endothelium-dependent vasodilatory response, due to the downregulation of endothelial NOS (eNOS) (33). The present results show that the mRNA expression of iNOS is increased in LPS-treated H9c2 cells, and the elevated expression of iNOS is inhibited in the presence of sitagliptin.
Prior studies have shown that the increased expression of TNF-α in cardiomyocytes during sepsis via the NF-κB signaling pathway (34). Over production of TNF-α and LPS stimulation may induce the activity of NF-κB and exert harmful effects on myocardial cells (35). The current results revealed that sitagliptin is able to reduce LPS-induced TNF-α production and inhibit the nuclear translocation of NF-κB. This study demonstrates that the DPP-4 inhibitor sitagliptin reduces the LPS-stimulated expression of inflammatory cytokines due, and these results indicate that DPP-4 has the potential to serve as a target for reverse cardiac remodeling due to endotoxemia and sepsis.
In conclusion, the present results suggest that the DPP-4 inhibitor sitagliptin reduced the LPS-induced inflammatory response, which was mediated by the NF-κB pathway signaling pathway. Treatment with sitagliptin decreased the protein expression levels of the inflammatory cytokines TNF-α, IL-6 and IL-1β, and the mRNA expression of COX-2 and iNOS in cardiomyoblasts. These findings suggest that DPP-4 inhibitors may be beneficial to the suppression of septic inflammation, which may lead to further cardiovascular complications. Further efforts to determine the most effective application of DPP-4 inhibitor to attenuate LPS-induced inflammatory response are required, and this strategy may provide novel therapies for treating septic patients and reducing subsequent cardiomyopathy.
Acknowledgements
The present study was supported by research grants (grant no. 101XDAA00012) from Taipei City Hospital.
References
- 1.Jirik FR, Podor TJ, Hirano T, Kishimoto T, Loskutoff DJ, Carson DA, Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989;142:144–147. [PubMed] [Google Scholar]
- 2.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E. Cardiomyocyte toll-like receptor 4 is involvedin heart dysfunction following septic shock or myocardial ischemia. J Mol Cell Cardiol. 2010;48:1236–1244. doi: 10.1016/j.yjmcc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Du J, An J, Wei N, Guan T, Pritchard KA, Jr, Shi Y. Increased resistance to LPS-induced myocardial dysfunction in the Brown Norway rats versus Dahl S rats: Roles of inflammatory cytokines and nuclear factor kappaB pathway. Shock. 2010;33:332–336. doi: 10.1097/SHK.0b013e3181b7819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuefermann P, Nemoto S, Baumgarten G, Misra A, Sivasubramanian N, Carabello BA, Vallejo JG. Cardiac inflammation and innate immunity in septic shock: Is there a role for toll-like receptors? Chest. 2002;121:1329–1336. doi: 10.1378/chest.121.4.1329. [DOI] [PubMed] [Google Scholar]
- 6.Panaro MA, Acquafredda A, Cavallo P, Cianciulli A, Saponaro C, Mitolo V. Inflammatory responses in embryonal cardiomyocytesexposed to LPS challenge: An in vitro model of deciphering the effects of LPS on the heart. Curr Pharm Des. 2010;16:754–765. doi: 10.2174/138161210790883516. [DOI] [PubMed] [Google Scholar]
- 7.Grandel U, Hopf M, Buerke M, Hattar K, Heep M, Fink L, Bohle RM, Morath S, Hartung T, Pullamsetti S, et al. Mechanisms of cardiac depression caused by lipoteichoic acids from staphylococcus aureus in isolated rat hearts. Circulation. 2005;112:691–698. doi: 10.1161/CIRCULATIONAHA.104.503938. [DOI] [PubMed] [Google Scholar]
- 8.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009;48:170–176. doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Rosenstiel N, von Rosenstiel I, Adam D. Management of sepsis and septic shock in infants and children. Paediatr Drugs. 2001;3:9–27. doi: 10.2165/00128072-200103010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Cariou B. Pleiotropic effects of insulin and GLP-1 receptor agonists: Potential benefits of the association. Diabetes Metab. 2015;41:6S28–6S35. doi: 10.1016/S1262-3636(16)30006-4. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Esposito K, Giugliano D, Genovese S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase T, Node K. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Lee TI, Kao YH, Chen YC, Huang JH, Hsu MI, Chen YJ. The dipeptidyl peptidase-4 inhibitor-sitagliptin modulates calcium dysregulation, inflammation and PPARs in hypertensive cardiomyocytes. Int J Cardiol. 2013;168:5390–5395. doi: 10.1016/j.ijcard.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 14.Chinda K, Palee S, Surinkaew S, Phornphutkul M, Chattipakorn S, Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol. 2013;167:451–457. doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang XM, Yang YJ, Wu YJ. The emerging role of dipeptidyl peptidase-4 inhibitors in cardiovascular protection: Current position and perspectives. Cardiovasc Drugs Ther. 2013;27:297–307. doi: 10.1007/s10557-013-6459-8. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, et al. Dipeptidyl peptidase-4inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–1344. doi: 10.1253/circj.CJ-12-1168. [DOI] [PubMed] [Google Scholar]
- 18.Panaro MA, Pricci M, Meziani F, Ragot T, Andriantsitohaina R, Mitolo V, Tesse A. Cyclooxygenase-2-derived prostacyclin protective role on endotoxin-induced mouse cardiomyocyte mortality. Cardiovasc Toxicol. 2011;11:347–356. doi: 10.1007/s12012-011-9127-x. [DOI] [PubMed] [Google Scholar]
- 19.Lobo SM, Lobo FR. Markers and mediators of inflammatory response in infection and sepsis. Rev Bras Ter Intensiva. 2007;19:210–215. doi: 10.1590/S0103-507X2007000200012. [DOI] [PubMed] [Google Scholar]
- 20.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L633–L640. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 21.Tavener SA, Kubes P. Cellular and molecular mechanisms underlying LPS-associated myocyte impairment. Am J Physiol Heart Circ Physiol. 2006;290:H800–H806. doi: 10.1152/ajpheart.00701.2005. [DOI] [PubMed] [Google Scholar]
- 22.Jatta K, Wågsäter D, Norgren L, Stenberg B, Sirsjö A. Lipopolysaccharide-induced cytokine and chemokine expression in human carotid lesions. J Vasc Res. 2005;42:266–271. doi: 10.1159/000085721. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: Role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol. 2002;282:H2316–H2323. doi: 10.1152/ajpheart.00763.2001. [DOI] [PubMed] [Google Scholar]
- 24.Panaro MA, Gagliardi N, Saponaro C, Calvello R, Mitolo V, Cianciulli A. Toll-like receptor 4 mediates LPS-induced release of nitric oxide and tumor necrosis factor-alpha by embryonal cardiomyocytes: Biological significance and clinical implications in human pathology. Curr Pharm Des. 2010;16:766–774. doi: 10.2174/138161210790883624. [DOI] [PubMed] [Google Scholar]
- 25.Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, Hirota D, Ono T, Usui HK, Makino H. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443:828–833. doi: 10.1016/j.bbrc.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 26.DeNicola M, Du J, Wang Z, Yano N, Zhang L, Wang Y, Qin G, Zhuang S, Zhao TC. Stimulation of glucagon-like peptide-1 receptor through exendin-4 preserves myocardial performance and prevents cardiac remodeling in infarcted myocardium. Am J Physiol Endocrinol Metab. 2014;307:E630–E643. doi: 10.1152/ajpendo.00109.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang WJ, Chang CH, Sun MF, Hsu SF, Weng CS. DPP-4 inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. PLoS One. 2014;9:e93447. doi: 10.1371/journal.pone.0093447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vona-Davis L, Zhu X, Yu AK, McFadden DW. Modulation of interleukin-6 in cardiac myoblasts during endotoxemia. J Surg Res. 2003;112:91–96. doi: 10.1016/S0022-4804(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 29.Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, Dandona P. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F, Xue Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK-and MAPK-dependent mechanisms. Cardiovasc Diabetol. 2014;13:32. doi: 10.1186/1475-2840-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazier WJ, Xue J, Luce WA, Liu Y. MAPK signaling drives inflammation in LPS-stimulated cardiomyocytes: The route of crosstalk to G-protein-coupled receptors. PLoS One. 2012;7:e50071. doi: 10.1371/journal.pone.0050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgarten G, Knuefermann P, Schuhmacher G, Vervölgyi V, von Rappard J, Dreiner U, Fink K, Djoufack C, Hoeft A, Grohé C, et al. Toll-like receptor 4, nitric oxide, and myocardial depression in endotoxemia. Shock. 2006;25:43–49. doi: 10.1097/01.shk.0000196498.57306.a6. [DOI] [PubMed] [Google Scholar]
- 33.Lee CC, Lin NT, Hsu YH, Chen HI. Inducible nitric oxide synthase inhibition potentiates multiple organ dysfunction induced by endotoxin in consciousrats. J Cardiovasc Pharmacol. 2005;45:396–403. doi: 10.1097/01.fjc.0000157438.72483.ae. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Shan L, Schiller PW, Mai A, Peng T. Histone deacetylase-3 activation promotes tumor necrosis factor-alpha (TNF-alpha) expression in cardiomyocytes during lipopolysaccharide stimulation. J Biol Chem. 2010;285:9429–9436. doi: 10.1074/jbc.M109.071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright G, Singh IS, Hasday JD, Farrance IK, Hall G, Cross AS, Rogers TB. Endotoxin stress-response in cardiomyocytes: NF-kappaB activation and tumor necrosis factor-alpha expression. Am J Physiol Heart Circ Physiol. 2002;282:H872–H879. doi: 10.1152/ajpheart.00256.2001. [DOI] [PubMed] [Google Scholar]