Abstract
Heterogeneous nuclear ribonucleoprotein K (hnRNP K) is a member of the hnRNP family, which exists in the nucleus and the cytoplasm simultaneously. It is a multifunctional protein that can participate in a variety of regulatory progressions of gene expression and signal transduction, such as chromatin remodeling, transcription, RNA alternative splicing and translation. hnRNP K not only directly binds to the kinases, but also recruits the associated factors regarding transcription, splicing and translation to control gene expression, and therefore, it serves as a docking platform for integrating transduction pathways to nucleic acid-directed processes. Numerous studies also show that abnormal expression of hnRNP K is closely associated with the tumor formation. This protein is overexpressed in numerous types of cancer and its aberrant cytoplasmic localization is also associated with a worse prognosis for patients. These results consistently indicate that hnRNP K has a key role in cancer progression. To understand the hnRNP K pathophysiological process in tumor disease, the previous research results regarding the association between hnRNP K and tumors were reviewed.
Keywords: heterogeneous nuclear ribonucleoprotein K, gene regulation, signal transduction, tumors
1. Introduction
RNA-binding proteins are the proteins that have similar characteristics and intracellular distribution, and are termed heterogeneous nuclear ribonucleoproteins (hnRNPs) (1). Their role is in sharp contrast with the roles of small nuclear ribonucleoproteins (snRNPs) and mRNA proteins (mRNPs). Thus far, ~20 types of hnRNPs have been identified, ranging from A1 to U. A large number of studies have shown that these proteins have a significant role in the progression of gene regulation, including DNA repairing, telomerase extending, signal transduction, and transcriptional and translational levels (2). Of which, hnRNP K is one type of DNA and RNA-binding protein involved in various regulatory progressions by means of protein-protein interaction (3).
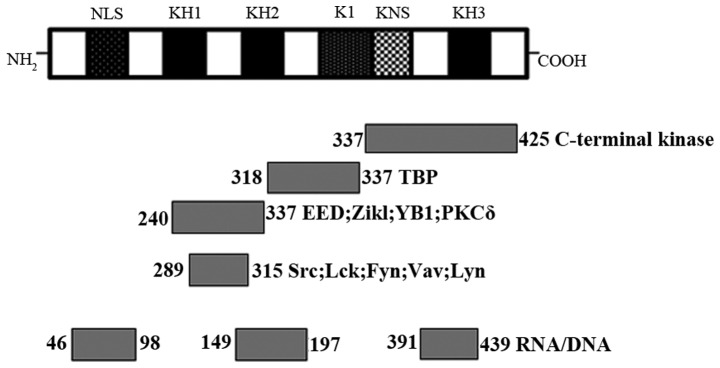
The relative molecular weight of hnRNP K is ~66 kDa, which is comprised of three DNA-RNA binding homology domains (KH1, KH2 and KH3), a K-protein-interactive region (KI) and a C-terminal protein kinase-binding domain (4). Each of these three KH domains contains 65–70 amino acids, two of them located at the N-terminus, and the remaining one at the C-terminus. KH domains have evolutionarily conserved features making the KH domain with the same number of amino acids or the same amino acid sequence exhibit similar functions in different tissues. The typical function of KH domains is to recognize and bind to RNA and single-stranded DNA. The KI domain lies between KH2 and KH3, which specifically exists in hnRNP K. This domain is responsible for regulating the interaction between hnRNP K and other proteins in the nucleus and cytoplasm. The KI region contains the proline-rich docking sites, such as RXXPXXP and PXXPXR, which interact particularly with SH3 domains of the Src-family signals. Furthermore, hnRNP K contains a nuclear-localization signal with the function of mediating its transport from the cytoplasm to the nucleus (5,6). Therefore, it acts as a nucleocytoplasmic shuttling protein to regulate gene expression by the nuclear pore complex with the help of a nuclear shuttling domain (7) (Fig. 1).
Figure 1.
Diagrammatic illustration of the K protein. The rectangles represent K homology domains (KH), K interactive region (KI), nuclear localization signal (NLS), nuclear shuttling domain (KNS) and domains that recruit protein and RNA partners. The numbers indicate the positions of amino acid (aa) residues. NLS in aa 1–40; KH 1 in aa 46–98; KH 2 in aa 149–197; KH 3 in aa 391–439; KI domain in aa 240–337; and KNS in aa 338–361.
According to previous results, there is a close association between tumors and hnRNP K; it often shows a high expression state in a variety of tumors, such as prostate cancer, colon cancer, nasopharyngeal cancer, oral squamous cell carcinoma, leukemia and breast cancer. hnRNP K is able to interact with multiple molecular partners and is involved in a number of gene regulation steps (7–9) (Table I). hnRNP K is specific for hnRNP family members, and compared with other hnRNP proteins, has different structural characteristics (KH domain and DNA binding sites), so that it can participate in numerous cellular processes in the nucleus and cytoplasm. Of note, in addition to having the same functions with other hnRNPs, such as mRNA splicing and the cytoplasmic transport of mRNA, it can regulate DNA transcription, RNA processing and RNA translation, particularly with regards to the process of oncogene expression (2). All these features make it exhibit multiple roles in the cell cycle, inhibition of apoptosis and tumor metastasis. The present review assessed certain studies from the perspective of the role and molecular mechanisms of hnRNP K in promoting tumors, providing a more in-depth and comprehensive understanding of the function of hnRNP K, and information for future investigations to further explore its role in the tumor progression.
Table I.
hnRNP K interacts with diverse groups of molecular partners to regulate gene expression and signal transduction.
| Process | Protein partner | Regulated gene |
|---|---|---|
| Transcription | General factors: TBP and HMGB1 Activators: Pura, Sox10 and C/EBPb Repressors: Zik1, Kid1 and MZF-1 | c-Myc, c-Src, thymidine kinase eIF4E, CHRNA4 and CD43 |
| Chromatin remodeling | Eed, DNA-methyltransferase, scaffold attachment factor B and MARs | AR |
| RNA processing | hnRNP E2, I, K, L and U 9G8, SRp20, YB-1 and Sam68 | β-tropomyosin, renin |
| Translation | EF-1α | c-Myc, 15-lipoxygenase, human papilloma virus type 16, eIF4E and p21 |
| Signal transduction | Src, Lyn, Fyn, Lck, Itk, PKCα, PKCδ, PKCε, ERK1/2, JNK, Vav and PRMT1 |
hnRNP K, heterogeneous nuclear ribonucleoprotein K.
2. hnRNP K as a transcription factor to promote tumors
hnRNP K can be a transcription factor to promote the expression of certain oncogenes (10,11), which combines the upstream pyrimidine-rich regions of promoters. In vivo it is able to interact directly with transcription machinery-related factors, such as the TATA box-binding protein (TBP), a subunit of the eukaryotic transcription factor TFIID, the RNA polymerase and others (12). These factors act synergistically to promote the transcription process by the way of protein-protein interaction.
There are CT repetitive sequences in the promoter region of c-myc, known as the CT element (13). It is comprised of four consecutive repeated CCCTCCCCA sequences and a fifth repeat sequence, which is separated by a 9-base pair long sequence located downstream of the first four sequences. Pioneer studies have shown that the N-terminus of hnRNP K contains 35-amino acid residues that are necessary for transactivating the CT element. When hnRNP K recognizes the CT element of the c-Myc promoter region in a specific-binding manner, it can recruit and interact with TBP and RNA polymerases to upregulate the expression of c-Myc. For example, it was found that c-myc and hnRNP K simultaneously increased in breast cancer (14). Following further exploration of hnRNP K, hnRNP K promoted transcription of c-myc in a CT element-dependent manner in these tumors, and subsequently c-Myc stimulated cell proliferation and inhibited apoptosis during the progression of malignant transformation.
Activation or overexpression of c-Src, a non-receptor tyrosine kinase of numerous signal pathways, has been associated with a host of malignant cancers (15,16). c-Src expression is regulated by the housekeeping-like SRC1A promoter in numerous tissues (17). There are three substantial polypurine/polypyrimidine (TC1, TC2 and TC3) tracts within this promoter that have a role in enhancing transcriptional activity. In addition, hnRNP K was shown to regulate the SRC1A promoter cooperatively with the transcription factor Sp1 (18,19). The study by Ritchie et al (20) proposed that hnRNP K recognizes and binds to TC1 and TC2 of the promoter region at first, which facilitates double strands to separate and become a single strand, leading to the affinity of hnRNP K with the increase in single-stranded DNA, followed by hnRNP K recruiting the basal transcriptional machinery, TBP and TFIID. The intact TC3 tract is capable of binding the single-stranded form with a high affinity to retain promoter activity. This series of processes promotes the transcription complex formation, so as to upregulate the expression of src.
3. hnRNP K interaction with nuclear matrix proteins to promote tumors
Nuclear matrix (NM) is a fibrin protein-based grid system present in the eukaryotic nucleus, excluding the nuclear membrane, laminin, chromatin and nucleolus. This dynamic complex mainly contains a variety of proteins and a small amount of RNA and DNA. NM has an important role in gene regulation process, such as chromatin remodeling, DNA replication and transcription and RNA processing (21). hnRNP K activates at the chromatin level, exhibiting a transient recruitment to multiple sites within each of the inducible gene loci, including the promoter and transcribed regions (22). hnRNP K is abundant in the NM, which has a role in stabilizing the NM network. Furthermore, hnRNP K as one type of NM protein can bind to the NM attachment region (MAR) sequences, and is located in interchromatin granule clusters (23). MAR is a class of DNA sequence, which exists in eukaryotic cellular chromatins and specifically recognizes the NM (24,25). When MAR binds to NM, it creates a position segmentation effect and maintains each transcription unit relatively independent from each other to be free of interaction with the surrounding chromatins. As a consequence of the anchoring of MAR sequences to NM, chromatin fibers are organized into topologically isolated loops to regulate the progression of gene transcription and translation, and removal of gene silencing resulted from the position effect. It is the position of a gene within the loop that determines its activity (26). As hnRNP K is the constituent of NM, chromatin remodeling and the transcription process of gene expression will be affected accordingly if the NM internal structure is altered or the interaction between NM and MAR sequences is repressed, with the original normal regulatory process affected as well. In prostate cancer cells (27), phosphorylated AKT can promote the phosphorylation of hnRNP K. The effect of hnRNP K stabilizing AR will be weakened in succession, which is co-located with the AR in the NM at first. In turn, phosphorylated hnRNP K inhibits the expression of AR after it recognizes DNA-MAR sequences in the nucleus, which makes the androgen-sensitive prostate cancer cells convert to androgen-insensitive cancer cells and increases the risk of a poor prognosis in patients who have received androgen-deprivation therapy.
4. Involvement of hnRNP K in RNA alternative splicing (AS) to promote tumors
AS is an essential mechanism in post-transcriptional regulation, which is a crucial step of the gene expression process in eukaryotes (28). It is a major cause for protein diversity and has critical roles in differentiation, development and disease. Thus, a gene may encode a variety of proteins. Therefore, its regulation is associated with cancer. It has been confirmed that hnRNP K is involved in certain important splicing process by interacting with Sam68, TAF15, YB1, 9G8 and SRp20 (12). Of note, it participates in the expression of apoptosis-related genes by AS to promote the tumor formation. The mammalian B-cell lymphoma 2 (Bcl-2) family can be classified into the multi-motif Bcl-2 proteins that bear multiple BH motifs with pro-survival (Bcl-2, Bcl-xL, Bcl-w, myeloid leukemia-1, A1 and Bcl-B) and pro-apoptotic (Bcl-xS, Bcl-2-associated X protein and Bcl-2 homologous antagonist/killer) activity (29). hnRNP K can regulate the Bcl-2 AS process and inhibit Bcl-xS generation, which results in a reduction of apoptosis in tumor cells (30). In the event of AS, U1 snRNA identify the pre-mRNA 5′ splicing site in a nucleotide complementary manner while U2AF recognizes and combines the upstream pyrimidine-rich region of the 3′ splicing site and promotes U2 snRNP and the U4, U5 and U6 snRNP trimer to bind together to form a 60S spliceosome where a transesterification reaction occurs, leading to the generation of different isoforms at different sites (31,32). Furthermore, there is a B1 splicing-regulatory region existing in the 5′ splicing site of Bcl-xS. hnRNP K can bind to the pyrimidine-rich region of B1 to inhibit the production of the Bcl-xS isoform. Simultaneously, hnRNP K is able to interact with Sam68, which has a role in upregulating Bcl-xS expression to weaken its upregulation capacity. As a result, the apoptosis pathway is blocked, so that cancer cells survive to escape from the apoptotic signals. Due to this advantage condition, tumor cells can be maintained in a safe environment and proliferate rapidly.
5. Involvement of hnRNP K in RNA translation to promote tumors
The translation mechanisms of hnRNP K action are the most intensively studied. It has been confirmed that hnRNP K can affect the tumor growth and development at the translational level as well. Bomsztyk et al (7) found that hnRNP K have a direct interaction with the translation elongation factor 1α, confirming its role in translational regulation. Following this, it was also found that hnRNP K could bind to the polypyrimidine sequence of translation initiation factor eIF4E (4EBE) to upregulate oncoprotein expression and promote certain malignant phenotype formations (33). In addition, hnRNP K can interact with the CU-rich region of p21 mRNA 3′ untranslated region (UTR) to inhibit p21 translation and promote cell proliferation (34). When chronic myelogenous leukemia converts from the chronic phase to the acute phase (35), the expression product of B-cell surface receptor (BCR)/ABL, p210, can activates the tyrosine kinase activity of mitogen-activated protein kinase (MAPK)Erk1/2 in a dose-dependent manner in the bone marrow and lymphocytes cells with the BCR/ABL gene. Subsequently, activated MAPKErk1/2 induces hnRNP K expression and stability increased. Stable hnRNP K binds to the myc mRNA internal ribosome entry site to stimulate translational activation and expression upregulation (3). The increased myc protein will facilitate leukocyte cell proliferation, colony formation and stimulate the occurrence of leukemia.
6. hnRNP K interacts with signaling molecules to promote tumors
hnRNP K can cooperate with the Src tyrosine kinases family, tryptophan/threonine kinase PKCδ, Erk1/2, Vav and other molecules to regulate its interaction with the target proteins or gene sequences. As combination factors vary, the effect of the production of different signaling molecules is also significantly different (36). For example, Jeon et al (37) have demonstrated that hnRNP K can bind to the signal transducer protein Vav to become involved in the BCR signaling pathway. Interaction of the Vav proto-oncogene product with hnRNP K regulates and promotes the process of cell transformation by the SH3 domain (38,39). In hepatocellular carcinoma (40), it can increase the expression of the protein kinase inhibitor CFLP (cellular FLICE-like inhibitory protein) that prevents pro-caspase-8 activation and X-linked inhibitor of apoptosis protein and maintain them at a high level to inhibit the classic caspase apoptosis pathway activation. In breast cancer (41), the epidermal growth factor receptor family can increase the expression of hnRNP K following activation by exogenous growth signals, and subsequently, the upregulated hnRNP K binds to and activates the c-myc promoter region to improve the expression of c-myc to accelerate the tumor formation process. In prostate cancer, hnRNP K participates in the AKT/hnRNP K/AR/β-catenin signaling pathway (42), which has a crucial impact on converting prostate cancer into a hormone-insensitive neuroendocrine (NE) differentiation phenotype. The presence of this phenotype indicates a poor prognosis for patient. Phosphorylated AKT is present at prostate cancer cells in three pathways mainly; following promotion of GSK3β phosphorylation, the phosphorylated GSK3β will be transported from the cytoplasm into the nucleus; the second pathway promotes the intracytoplasm AR to be phosphorylated and subsequently degraded by the proteasome pathway; the last promotes hnRNP K phosphorylation and enters into the nucleus. GSK3β and phosphorylated hnRNP K of common positioning within the nucleus bind to the AR sequence and repress AR expression, while increasing the expression of NE differentiation phenotype markers, neuron-specific enolase (NSE), simultaneously, which causes hormone-sensitive prostate cancer to become hormone-insensitive and NSE-independent prostate cancer phenotype, ultimately resulting in ineffective androgen-withdrawal therapy. In the cytoplasm of tumor cells, the activation of Ras and MEK can also make hnRNP K stably exist in the cytoplasm (43). Stabilized hnRNP K is able to activate ERK to promote upregulation of matrix metalloproteinase 3 (MMP3) and MMP10. These factors have an important role in promoting tumor metastasis. A succession of studies are now providing a mechanistic basis, highlighting and reinforcing that specific MMPs are key in tumor invasion and metastasis through their catalytic and non-catalytic roles, including modulating tumor cell motility, promoting invadopodia formation, interactions of MMPs with pro-invasive pathways, sensing matrix stiffness and induction and maintenance of epithelial-mesenchymal transition (44). Therefore, hnRNP K simultaneously provided favorable conditions for tumor invasion and metastasis when upregulating MMPs expression.
7. hnRNP K interacts with non-coding RNAs (ncRNAs) to promote tumors
In the human genome, ~90% is transcribed into ncRNAs. ncRNAs are diverse RNA transcripts that are not transcribed into proteins but have been shown to regulate the transcription, stability or translation of protein-coding genes (45,46). According to their size, they can be divided into long ncRNAs and short ncRNAs (microRNAs). ncRNAs are associated with numerous diseases, including a variety of tumors (47). In addition, there is a close association between hnRNP K and ncRNAs, which may indicate that there is contact between hnRNPK and ncRNAs in tumors. Recently, Gumireddy et al (48) identified that a translational regulatory ncRNA (treRNA) was highly expressed in metastatic breast cancer and primary colon cancer through genome-wide computational analysis. It interacted with hnRNP K to promote tumor invasion and metastasis. treRNA can combine with hnRNP K, FXR1, puf60, SF3B3 and other factors to facilitate the formation of the treRNA-associated protein complex. This complex is able to directly or indirectly bind to the E-cadherin mRNA 3′UTR, and reduce translation efficiency of E-cadherin mRNA, and therefore E-cadherin expression decreased. Downregulated E-cadherin leads to a direct result of adhesion activity decrease between tumor cells. As a consequence, tumor cells shed from the primary tumor into the circulation system, and position in the new site. In addition, Qin et al (49) showed that hnRNP K is a target of miR-205. miR-205 has a complex regulatory role in tumor initiation and growth processes. It can inhibit or promote tumor formation depending on its binding targets and microenvironment. Furthermore, it was found that miR-205 can bind to the 3′UTR of hnRNP K to reduce hnRNP K expression (50). However, miR-205 is downregulated in prostate cancer, so its inhibition for hnRNP K is derepressed, which leads to promoting the state of tumors (51).
8. Conclusion
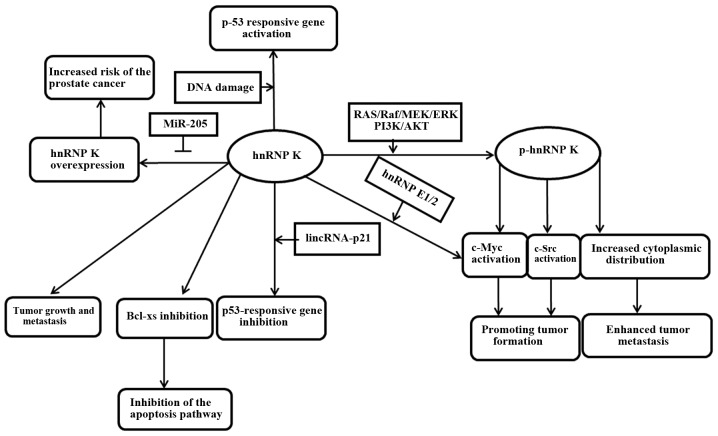
hnRNP K is an RNA/DNA-binding protein that is a target of multiple kinases or recruits factors involved in signal transduction and gene expression. Its abnormal expression can make the tumor formation risk increase significantly. In several tumors, the hnRNP K expression level progressively increases from normal to hyperplasia to carcinoma tissue, and it is often associated with tumor stage, indicating an asociation between hnRNP K expression and tumors progression (52,53). Inoue et al (54) have proved that hnRNP K has an important role in tumor invasion. They identified that hnRNP K is involved in tumor cell metastasis, and its cytoplasmic localization is essential for cell invasion and metastasis. Recently, it was also proved that if hnRNP K is overexpressed, cell malignancy and metastatic ability would be improved in vitro and in vivo. Furthermore, Hope and Murray (55) demonstrated in colon cancer that hnRNP K in addition to the high expression appeared with an abnormal cytoplasmic localization, and it correlated with lymph node metastasis, suggesting that it is a poor prognostic markers. Gao et al (43) have demonstrated that hnRNP K could induce the expression of certain genes involved in the cell extracellular matrix, cell motility and angiogenesis by cDNA microarray analysis and signaling pathway analysis. Therefore, regardless of the tumor type, the abnormal increase or cytoplasmic localization of hnRNP K may be regarded as a valid marker of poor prognosis (Table II). In summary, hnRNP K is involved in multiple cellular functions relevant to cancer development and progression (Fig. 2). The overexpressed hnRNP K in numerous tumors, as a multifunctional protein, may hopefully become a therapeutic target due to its role in promoting malignant transformation and tumor metastasis. If this hypothesis is true, reasonable drugs and therapies can be designed to intervene with tumor growth according to the regulatory characteristics of hnRNP K. Further investigations are required.
Table II.
Heterogeneous nuclear ribonucleoprotein K expression in individual types of cancer and its association with prognosis in different types of cancer.
| Type of cancer | Expression in tumor tissuea | Prognostic significance |
|---|---|---|
| Colorectal | Increased | Survival |
| Esophageal squamous cell | Increased | Poor prognosis |
| Hepatocellular | Increased | ND |
| Lung | Increased | ND |
| Melanoma | Increased | ND |
| Nasopharyngeal | Increased | Poor prognosis |
| Oral squamous cell | Increased | Poor prognosis |
| Prostate | Increased | Poor prognosis |
Expression was compared to normal or non-tumor tissue. ND, not determined.
Figure 2.
Signaling pathways of hnRNP K. The summary of the principal pathways regulated by hnRNP K, in which hnRNP K can drive cancer development and progression. hnRNP K, heterogeneous nuclear ribonucleoprotein K.
Acknowledgements
The present study was supported in part by grants from the National Natural Science Foundation of China (no. 81172322), Science and Technology Commission of Shanghai Municipality (no. 11ZR1421000) and Science and Technology Fund of Shanghai Jiao Tong University School of Medicine (no. YZ1027).
References
- 1.Swanson MS, Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988;8:2237–2241. doi: 10.1128/MCB.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter B, MacKay C, Alnabulsi A, MacKay M, Telfer C, Melvin WT, Murray GI. The roles of heterogeneous nuclear ribonucleoproteins in tumour development and progression. Biochim Biophys Acta. 2006;1765:85–100. doi: 10.1016/j.bbcan.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Evans JR, Mitchell SA, Spriggs KA, Ostrowski J, Bomsztyk K, Ostarek D, Willis AE. Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo. Oncogene. 2003;22:8012–8020. doi: 10.1038/sj.onc.1206645. [DOI] [PubMed] [Google Scholar]
- 4.Dejgaard K, Leffers H. Characterisation of the nucleic-acid-binding activity of KH domains. Different properties of different domains. Eur J Biochem. 1996;241:425–431. doi: 10.1111/j.1432-1033.1996.00425.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol. 1994;127:1173–1184. doi: 10.1083/jcb.127.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain: A novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomsztyk K, Van Seuningen I, Suzuki H, Denisenko O, Ostrowski J. Diverse molecular interactions of the hnRNP K protein. FEBS Lett. 1997;403:113–115. doi: 10.1016/S0014-5793(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 8.Ostareck-Lederer A, Ostareck DH, Cans C, Neubauer G, Bomsztyk K, Superti-Furga G, Hentze MW. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol. 2002;22:4535–4543. doi: 10.1128/MCB.22.13.4535-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostareck-Lederer A, Ostareck DH, Hentze MW. Cytoplasmic regulatory functions of the KH-domain proteins hnRNPs K and E1/E2. Trends Biochem Sci. 1998;23:409–411. doi: 10.1016/S0968-0004(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 10.Choi HS, Hwang CK, Song KY, Law PY, Wei LN, Loh HH. Poly(C)-binding proteins as transcriptional regulators of gene expression. Biochem Biophys Res Commun. 2009;380:431–436. doi: 10.1016/j.bbrc.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michelotti EF, Michelotti GA, Aronsohn AI, Levens D. Heterogeneous nuclear ribonucleoprotein K is a transcription factor. Mol Cell Biol. 1996;16:2350–2360. doi: 10.1128/MCB.16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shnyreva M, Schullery DS, Suzuki H, Higaki Y, Bomsztyk K. Interaction of two multifunctional proteins. Heterogeneous nuclear ribonucleoprotein K and Y-box-binding protein. J Biol Chem. 2000;275:15498–15503. doi: 10.1074/jbc.275.20.15498. [DOI] [PubMed] [Google Scholar]
- 13.Takimoto M, Tomonaga T, Matunis M, Avigan M, Krutzsch H, Dreyfuss G, Levens D. Specific binding of heterogeneous ribonucleoprotein particle protein K to the human c-myc promoter, in vitro. J Biol Chem. 1993;268:18249–18258. [PubMed] [Google Scholar]
- 14.Samuel SK, Spencer VA, Bajno L, Sun JM, Holth LT, Oesterreich S, Davie JR. In situ cross-linking by cisplatin of nuclear matrix-bound transcription factors to nuclear DNA of human breast cancer cells. Cancer Res. 1998;58:3004–3008. [PubMed] [Google Scholar]
- 15.Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/S0065-230X(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C, Rübsamen H. Expression of pp60c-src protein kinase in adult and fetal human tissue: High activities in some sarcomas and mammary carcinomas. Cancer Res. 1983;43:1696–1702. [PubMed] [Google Scholar]
- 17.Bonham K, Ritchie SA, Dehm SM, Snyder K, Boyd FM. An alternative, human SRC promoter and its regulation by hepatic nuclear factor-1alpha. J Biol Chem. 2000;275:37604–37611. doi: 10.1074/jbc.M004882200. [DOI] [PubMed] [Google Scholar]
- 18.Du Q, Melnikova IN, Gardner PD. Differential effects of heterogeneous nuclear ribonucleoprotein K on Sp1- and Sp3-mediated transcriptional activation of a neuronal nicotinic acetylcholine receptor promoter. J Biol Chem. 1998;273:19877–19883. doi: 10.1074/jbc.273.31.19877. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie S, Boyd FM, Wong J, Bonham K. Transcription of the human c-Src promoter is dependent on Sp1, a novel pyrimidine binding factor SPy, and can be inhibited by triplex-forming oligonucleotides. J Biol Chem. 2000;275:847–854. doi: 10.1074/jbc.275.2.847. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie SA, Pasha MK, Batten DJ, Sharma RK, Olson DJ, Ross AR, Bonham K. Identification of the SRC pyrimidine-binding protein (SPy) as hnRNP K: Implications in the regulation of SRC1A transcription. Nucleic Acids Res. 2003;31:1502–1513. doi: 10.1093/nar/gkg246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barboro P, D'Arrigo C, Diaspro A, Mormino M, Alberti I, Parodi S, Patrone E, Balbi C. Unraveling the organization of the internal nuclear matrix: RNA-dependent anchoring of NuMA to a lamin scaffold. Exp Cell Res. 2002;279:202–218. doi: 10.1006/excr.2002.5605. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski J, Kawata Y, Schullery DS, Denisenko ON, Bomsztyk K. Transient recruitment of the hnRNP K protein to inducibly transcribed gene loci. Nucleic Acids Res. 2003;31:3954–3962. doi: 10.1093/nar/gkg452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitoh N, Spahr CS, Patterson SD, Bubulya P, Neuwald AF, Spector DL. Proteomic analysis of interchromatin granule clusters. Mol Biol Cell. 2004;15:3876–3890. doi: 10.1091/mbc.E04-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barboro P, D'Arrigo C, Repaci E, Bagnasco L, Orecchia P, Carnemolla B, Patrone E, Balbi C. Proteomic analysis of the nuclear matrix in the early stages of rat liver carcinogenesis: Identification of differentially expressed and MAR-binding proteins. Exp Cell Res. 2009;315:226–239. doi: 10.1016/j.yexcr.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Barboro P, Repaci E, D'Arrigo C, Balbi C. The role of nuclear matrix proteins binding to matrix attachment regions (Mars) in prostate cancer cell differentiation. PLoS One. 2012;7:e40617. doi: 10.1371/journal.pone.0040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marenduzzo D, Faro-Trindade I, Cook PR. What are the molecular ties that maintain genomic loops? Trends Genet. 2007;23:126–133. doi: 10.1016/j.tig.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Barboro P, Borzì L, Repaci E, Ferrari N, Balbi C. Androgen receptor activity is affected by both nuclear matrix localization and the phosphorylation status of the heterogeneous nuclear ribonucleoprotein K in anti-androgen-treated LNCaP cells. PLoS One. 2013;8:e79212. doi: 10.1371/journal.pone.0079212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Dai X, Wu J. Alternative splicing: An important mechanism in stem cell biology. World J Stem Cells. 2015;7:1–10. doi: 10.4252/wjsc.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanave C, Santamaria M, Saccone C. Comparative genomics: The evolutionary history of the Bcl-2 family. Gene. 2004;333:71–79. doi: 10.1016/j.gene.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Revil T, Pelletier J, Toutant J, Cloutier A, Chabot B. Heterogeneous nuclear ribonucleoprotein K represses the production of pro-apoptotic Bcl-xS splice isoform. J Biol Chem. 2009;284:21458–21467. doi: 10.1074/jbc.M109.019711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahl MC, Will CL, Lührmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 33.Lynch M, Chen L, Ravitz MJ, Mehtani S, Korenblat K, Pazin MJ, Schmidt EV. hnRNP K binds a core polypyrimidine element in the eukaryotic translation initiation factor 4E (eIF4E) promoter, and its regulation of eIF4E contributes to neoplastic transformation. Mol Cell Biol. 2005;25:6436–6453. doi: 10.1128/MCB.25.15.6436-6453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yano M, Okano HJ, Okano H. Involvement of Hu and heterogeneous nuclear ribonucleoprotein K in neuronal differentiation through p21 mRNA post-transcriptional regulation. J Biol Chem. 2005;280:12690–12699. doi: 10.1074/jbc.M411119200. [DOI] [PubMed] [Google Scholar]
- 35.Notari M, Neviani P, Santhanam R, Blaser BW, Chang JS, Galietta A, Willis AE, Roy DC, Caligiuri MA, Marcucci G, et al. A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation. Blood. 2006;107:2507–2516. doi: 10.1182/blood-2005-09-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrowski J, Schullery DS, Denisenko ON, Higaki Y, Watts J, Aebersold R, Stempka L, Gschwendt M, Bomsztyk K. Role of tyrosine phosphorylation in the regulation of the interaction of heterogenous nuclear ribonucleoprotein K protein with its protein and RNA partners. J Biol Chem. 2000;275:3619–3628. doi: 10.1074/jbc.275.5.3619. [DOI] [PubMed] [Google Scholar]
- 37.Jeon HK, Ahn JH, Choe J, Park JH, Lee TH. Anti-IgM induces up-regulation and tyrosine-phosphorylation of heterogeneous nuclear ribonucleoprotein K proteins (hnRNP K) in a Ramos B cell line. Immunol Lett. 2005;98:303–310. doi: 10.1016/j.imlet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Bustelo XR, Suen KL, Michael WM, Dreyfuss G, Barbacid M. Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol Cell Biol. 1995;15:1324–1332. doi: 10.1128/MCB.15.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groysman M, Nagano M, Shaanan B, Katzav S. Mutagenic analysis of Vav reveals that an intact SH3 domain is required for transformation. Oncogene. 1998;17:1597–1606. doi: 10.1038/sj.onc.1202074. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Z, Ko HL, Goh EH, Wang B, Ren EC. hnRNP K suppresses apoptosis independent of p53 status by maintaining high levels of endogenous caspase inhibitors. Carcinogenesis. 2013;34:1458–1467. doi: 10.1093/carcin/bgt085. [DOI] [PubMed] [Google Scholar]
- 41.Mandal M, Vadlamudi R, Nguyen D, Wang RA, Costa L, Bagheri-Yarmand R, Mendelsohn J, Kumar R. Growth factors regulate heterogeneous nuclear ribonucleoprotein K expression and function. J Biol Chem. 2001;276:9699–9704. doi: 10.1074/jbc.M008514200. [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay NK, Kim J, Cinar B, Ramachandran A, Hager MH, Di Vizio D, Adam RM, Rubin MA, Raychaudhuri P, De Benedetti A, et al. Heterogeneous nuclear ribonucleoprotein K is a novel regulator of androgen receptor translation. Cancer Res. 2009;69:2210–2218. doi: 10.1158/0008-5472.CAN-08-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao R, Yu Y, Inoue A, Widodo N, Kaul SC, Wadhwa R. Heterogeneous nuclear ribonucleoprotein K (hnRNP-K) promotes tumor metastasis by induction of genes involved in extracellular matrix, cell movement, and angiogenesis. J Biol Chem. 2013;288:15046–15056. doi: 10.1074/jbc.M113.466136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 45.Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5:e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 47.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gumireddy K, Li A, Yan J, Setoyama T, Johannes GJ, Orom UA, Tchou J, Liu Q, Zhang L, Speicher DW, et al. Identification of a long non-coding RNA-associated RNP complex regulating metastasis at the translational step. EMBO J. 2013;32:2672–2684. doi: 10.1038/emboj.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin AY, Zhang XW, Liu L, Yu JP, Li H, Wang SZ, Ren XB, Cao S. miR-205 in cancer: An angel or a devil? Eur J Cell Biol. 2013;92:54–60. doi: 10.1016/j.ejcb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Szczyrba J, Nolte E, Hart M, Döll C, Wach S, Taubert H, Keck B, Kremmer E, Stöhr R, et al. Identification of ZNF217, hnRNP-K, VEGF-A and IPO7 as targets for microRNAs that are downregulated in prostate carcinoma. Int J Cancer. 2013;132:775–784. doi: 10.1002/ijc.27731. [DOI] [PubMed] [Google Scholar]
- 51.Szczyrba J, Löprich E, Wach S, Jung V, Unteregger G, et al. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol Cancer Res. 2010;8:529–538. doi: 10.1158/1541-7786.MCR-09-0443. [DOI] [PubMed] [Google Scholar]
- 52.Carpenter B, McKay M, Dundas SR, Lawrie LC, Telfer C, Murray GI. Heterogeneous nuclear ribonucleoprotein K is over expressed, aberrantly localised and is associated with poor prognosis in colorectal cancer. Br J Cancer. 2006;95:921–927. doi: 10.1038/sj.bjc.6603349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roychoudhury P, Chaudhuri K. Evidence for heterogeneous nuclear ribonucleoprotein K overexpression in oral squamous cell carcinoma. Br J Cancer. 2007;97:574–575. doi: 10.1038/sj.bjc.6603911. author reply 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue A, Sawata SY, Taira K, Wadhwa R. Loss-of-function screening by randomized intracellular antibodies: Identification of hnRNP-K as a potential target for metastasis. Proc Natl Acad Sci USA. 2007;104:8983–8988. doi: 10.1073/pnas.0607595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hope NR, Murray GI. The expression profile of RNA-binding proteins in primary and metastatic colorectal cancer: Relationship of heterogeneous nuclear ribonucleoproteins with prognosis. Hum Pathol. 2011;42:393–402. doi: 10.1016/j.humpath.2010.08.006. [DOI] [PubMed] [Google Scholar]