Abstract
Dendritic cells (DCs) are important in the initiation of primary T‐cell responses, while transforming growth factor‐β (TGF‐β)‐activated kinase 1 (TAK1) is a critical regulator of DC survival and homeostasis. This study evaluated the T‐cell dependent antibody response (TDAR) to sheep red blood cells (SRBC) on a DC‐specific TAK1‐deficient mice model. The results showed that TAK1 deficiency in DCs significantly suppressed the humoral and cellular immune response in mice. DC‐specific TAK1 deletion impaired splenic T‐cell population and conventional DCs, abolished the cytokine production of splenic T cells and down‐regulated some functional gene expression in the spleen. Collectively, this study suggests that TAK1 plays an essential role in the development of the humoral immune response.
Keywords: dendritic cells, sheep red blood cells, T‐cell dependent antigen response, transforming growth factor‐β‐activated kinase 1
Abbreviations
- APC
antigen‐presenting cell
- DC
dendritic cell
- DTH
delayed type hypersensitivity
- IFN
interferon
- IL
interleukin
- MHC
major histocompatibility complex
- PFC
plaque‐forming cell
- SRBC
sheep red blood cells
- TAK1
transforming growth factor‐β activated kinase 1
- TDAR
T‐cell dependent antibody response
- Th cell
T‐helper cells
- WT
wild‐type
Dendritic cells (DCs), a morphologically distinct form of antigen‐presenting cell (APC), can capture, process, and present antigens in the form of peptides bound to major histocompatibility complex (MHC) molecules to activate T cells. It has been reported that in vivo antigen targeting to DCs induces strong T‐cell priming and long‐lived T cell help for antibody response 1, 2. DCs are famous for their role in activating and expanding T‐helper (Th) cells, which in turn induce B‐cell growth and immunoglobulin secretion 3. Immunoglobulin class‐switching also requires interaction between B cells and DCs 4. Therefore, DCs play a central role in initiating and modulating humoral immunity.
Transforming growth factor‐β (TGF‐β)‐activated kinase 1 (TAK1, encoded by Map3k7) belongs to the mitogen‐activated protein kinase kinase kinase (MAP3K) family 5. As a key regulator in inflammatory and immune signaling pathway, it can be activated by various stimuli 6, 7. TAK1 has an essential effect on promoting the survival, proliferation and function of adaptive immune cells T and B lymphocytes 6, 8, 9, 10, 11. Besides, it also controls the homeostasis of innate immune cells such as DCs 12, NK cells 13 and neutrophils 14, 15. Sato et al. 6 reported that antigen‐induced humoral immune responses to T‐cell dependent and independent antigens were impaired in B cell‐specific TAK1‐deficient mice. Wang et al. 12 showed that deficiency of TAK1 in DCs caused apoptosis and depletion of DC subsets in lymphoid and nonlymphoid tissues and suppressed T‐cell priming. But how DC‐specific TAK1 deficiency affects T‐cell dependent antibody response (TDAR) has not been reported.
The TDAR depends on the cooperation and interaction of numerous immune cell types, including APCs, such as DCs, Th cells and B cells 16. The TDAR is used as an immunotoxicity index, because it represents a comprehensive evaluation of immune function based on the assessment of various components of the immune system. Sheep red blood cell (SRBC) is a common T‐cell dependent antigen to evaluate immune status in mice. After immunized with SRBC, this SRBC‐specific IgM response in spleen can be measured by plaque‐forming cell (PFC) assay 17. This test is an IgM/complement dependent in vitro assay in mice, with SRBC as both the antigen and the target for complement mediated lysis. The SRBC PFC assay is considered the ‘gold standard’ for TDAR based on extensive intra‐ and inter‐laboratory validation in mice and the fact that it has been utilized for over 35 years 18. Although PFC assay is commonly used for assessing the potential immunotoxicity of xenobiotics 19, 20, 21, we used it in this study to evaluate the function of TAK1 in DCs on humoral immune response due to its integrated assessment capability and its sensitivity and stability.
The purpose of the study was to investigate the effect of DC‐specific TAK1 deficiency on adaptive immune response in SRBC‐immunized mice and to identify the impact of TAK1 in DCs on maintaining immune homeostasis and function. Here, we immunized the animals with SRBC and then performed functional assays including PFC assay, hemolysis test, and delayed type hypersensitivity (DTH) and quantified the antibody subsets in serum. Furthermore, splenic immune cell subpopulations, splenic T‐cell cytokine production and splenic functional gene expressions were also detected.
Materials and methods
Experimental animals
Floxed Map3k7 (Map3k7 fl/fl) 8 and CD11c‐Cre 22 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice are on C57BL/6 background. To delete TAK1 specifically in CD11c+DCs, we crossed mice bearing loxP‐flanked alleles of the Map3k7 gene with mice expressing Cre under the control of the CD11c promoter to generate Map3k7 fl/fl CD11c‐Cre mice (called ‘Map3k7 DC mice’ hereafter). Wild‐type (WT) controls were in the same genetic background and included Cre+ mice to account for Cre effects. Guinea pigs (250 g) were used for the preparation of complement for PFC assay and hemolysis test and they were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). All animals were healthy, housed in a barrier system (temperature: 20–26 °C; relative humidity: 40–70%) with a 12 h light/dark cycle. Water and standard diet were available ad libitum. The study was approved by the Animal Experimental Welfare & Ethical Inspection Committee of Peking University. Animal experiments and housing procedures were carried out in accordance with the laboratory animal administration rules of the Ministry of Science and Technology of the People's Republic of China.
Chemicals and antibodies
Sheep red blood cells (SRBC) were purchased from Vital River Laboratory Animal Technology Co. Ltd. PE‐Cy™5 hamster anti‐mouse CD3e, PE rat anti‐mouse CD8a, FITC hamster anti‐mouse CD11c and PE rat anti‐mouse I‐A/I‐E were purchased from BD Pharmingen (San Diego, CA, USA). Anti‐mouse CD4FITC and anti‐human/mouse CD45R (B220) PE were obtained from eBioscience (San Diego, CA, USA). Mouse IgG, IgA, and IgM ELISA kits were purchased from Freemore Biotechnology Co. Ltd (Beijing, China). Guinea pig complement, Hanks balanced salt buffer (HBSS), SA buffer solution and Drabkin's reagent were homemade.
Preparation of spleen cell suspensions
Each spleen isolated above was transferred to the 70 μm cell strainer in culture dishes containing 4 mL of cold HBSS and smashed the tissue with the plunger of a 1 mL syringe. Filtered the splenocyte suspensions through the two‐layer carbasus on top of the tubes and washed twice in HBSS and centrifuged for 10 min at 230 g at room temperature. Splenocytes were resuspended in 10 mL HBSS. Cell numbers were determined for each splenocyte suspension by counting in a hemocytometer.
Plaque‐forming cell assay
The Cunningham modification of Jerne and Nordin antibody plaque‐forming cell assay was used 23, 24, 25 to determine IgM production by spleen cells. Mice were immunized on Day 1 with 0.2 mL of 2% (v/v) SRBC suspension in sterile saline via intraperitoneal injection. On day 5, the mice were killed and spleen cell suspensions were prepared as stated in the part of “Preparation of spleen cell suspension”. About 20 μL of spleen cell suspension in HBSS, 50 μL of 10% (v/v) SRBC in SA buffer solution and 500 μL of agar solution (0.5 g/100 ml in HBSS, pH 7.2–7.4) were mixed in a glass tube, and then poured onto slides. The slides were inverted on a special frame after the mixtures were solidified, and incubated at 37 °C for 1.5 h, then diluted guinea pig complement (1 : 10 diluted with SA buffer solution) was added to the slot between the slides and the bottom of the frame. The slides were incubated at 37 °C for another 1.5 h, then plaque production was enumerated and the results were expressed as the number of PFC per 106 splenocytes.
Hemolysis test
Mice were immunized with SRBC as stated in the PFC assay section. The sera were obtained and assayed for HC50. One millilitre of SA buffer solution, 0.5 mL of 15% (v/v) SRBC, 1 mL of diluted guinea pig complement (1 : 10 diluted with SA buffer solution) and 3.3 μL of WT mice serum or 50 μL of Map3k7 DC mice serum were added into sample tubes and the blank control was the tube without serum. All tubes were kept in water bath for 15 min at 37 °C, and then the tubes were kept in ice bath to terminate the reaction. After centrifuged for 10 min at 890 g, 1 mL of supernatant was collected and added into 3 mL of Drabkin's reagent, at the same time, 0.25 mL of 15% (v/v) SRBC and 3.75 mL of Drabkin's reagent were mixed and placed for 10 min as positive control. The absorbance at 540 nm was detected using UV‐2100 spectrophotometer (UNICO Instrument Co. Ltd, Shanghai, China), the HC50 value was got by the equation: HC50 = (ODsample/ODpositive control) × dilution factor (300 for WT mice and 20 for Map3k7 DC mice).
Serum immunoglobulin isotypes quantification
The animals were immunized with SRBC as stated in the PFC assay section. Blood for immunoglobulin isotypes quantification was collected into tubes without anticoagulant and centrifuged to obtain serum. Total IgG, IgM, and IgA titers in the serum of each animal were determined by ELISA kits. The assay procedures were in accordance with the descriptions in the manufacturer's instructions. The absorbance (450 nm) of the contents of each well was determined using FLUOstar Omega multidetection micro‐plate reader (BMG Labtech, Ortenberg, Germany).
Delayed‐type hypersensitivity to SRBC
Mice were sensitized on Day 1 via intraperitoneal injection of 0.2 mL of 2% (v/v) SRBC in sterile saline. Five days later, the thickness of left rear footpad of each mouse was determined with a digital caliper (Mahr, Göttingen, Germany). Then, the left rear footpad was injected with 20 μL of 20% (v/v) SRBC by subcutaneous injection. After 24 h, the thickness of left rear footpad was measured again. Swelling was expressed by the difference between two measurements before and after injection of SRBC to left rear footpad.
Flow cytometric analysis
The animals were immunized with SRBC as stated in the PFC assay section and the spleen cell suspensions were prepared as stated in the part of “Preparation of spleen cell suspension”. The splenocytes were stained with combination of antibodies in 100 μL staining buffer (2% FBS in PBS) at 4 °C for 30 min in the dark. Excessive unreacted antibody was removed by washing the cells with staining buffer. Cells were resuspended at 106 cells per tube in 300 μL staining buffer and then analyzed on a FC500‐MPL flow cytometry (Beckman Coulter, Brea, CA, USA). A minimum of 20 000 events per sample was collected and analyzed for antigen expression.
Stimulation of cytokine production and intracellular cytokine staining
The animals were immunized with SRBC as stated in the PFC assay section and the spleen cell suspensions were prepared as stated in the part of “Preparation of spleen cell suspension”. The aliquots of 2 × 106 splenocytes were stimulated with cell stimulation cocktail (500×; eBioscience) for 4 h in the presence of Brefeldin A (1000×; eBiscience). The cells were stimulated in RPMI 1640 medium (Gibco, Shanghai, China) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 2 mm l‐glutamine and 50 μm 2‐mercaptoethanol and incubated under a humidified atmosphere of 5% CO2/95% air at 37 °C. Different cell surface markers and intracellular cytokines were stained with cocktails of fluorescence‐conjugated monoclonal antibodies according to the manufacturer's protocols (eBioscience).
Gene expression in spleen
The animals were immunized with SRBC as stated in the PFC assay section. Total RNA was extracted from 1 × 107 splenocytes using TRIzol reagent (Transgen Biotech, Beijing, China) according to the manufacturer's instruction. Total RNA (1 μg) was reverse transcribed with Oligo dT primer to cDNA using PrimeScript™ RT‐PCR kit (Takara, Dalian, China). All real‐time PCR primers were designed and synthesized by AuGCT DNA‐SYN Biotechnology (Beijing, China). Real‐time PCR primer sequences used are listed in Table 1. All real‐time PCR reactions were carried out using SYBR® Premix Ex Taq™ II (TliRNaseH Plus) (Takara) and the IQ5 iCycler (Bio‐Rad Laboratories, Hercules, CA, USA) according to manufacturer protocols. The relative gene expression was calculated using the delta‐delta Ct (threshold cycle) method based on normalization to the reference gene Gapdh (glyceraldehyde‐3‐phosphate dehydrogenase).
Table 1.
Primer sequences used for real‐time PCR
| Gene | Primer sequences (5′ to 3′) | Tm (°C) |
|---|---|---|
| Ifng | ||
| Sense | CTC AAG TGG CAT AGA TGT GG | 60.0 |
| Antisense | CAA TGA CGC TTA TGT TGT TG | 56.0 |
| Il2 | ||
| Sense | CTC TGC GGC ATG TTC TGG ATT | 60.3 |
| Antisense | CAG AAA GTC CAC CAC AGT TGC T | 60.3 |
| Il4 | ||
| Sense | GGT CTC AAC CCC CAG CTA GT | 64.0 |
| Antisense | CCC TTC TCC TGT GAC CTC GT | 64.0 |
| Il10 | ||
| Sense | TGGACAACATACTGCTAACC | 58.0 |
| Antisense | GGATCATTTCCGATAAGGCT | 58.0 |
| Tbet | ||
| Sense | CACTAAGCAAGGACGGCGAA | 62.0 |
| Antisense | CCACCAAGACCACATCCACA | 62.0 |
| Gata3 | ||
| Sense | AAGAAAGGCATGAAGGACGC | 60.0 |
| Antisense | GTGTGCCCATTTGGACATCA | 60.0 |
| Cxcr4 | ||
| Sense | ATCCCAGCCCTCCTCCTGACTA | 64.3 |
| Antisense | TCCACAGGCTATCGGGGTAAAG | 62.3 |
| Ccr7 | ||
| Sense | ATGTATTCTGTCATCTGCTTCGTGG | 61.3 |
| Antisense | GCCAGGTTGAGCAGGTAGGTATC | 64.3 |
| Mhc I | ||
| Sense | GCCCAAGAAGTGGATTACGGAG | 62.3 |
| Antisense | TCCAGAACCATTTGGCGACC | 62.0 |
| Mhc II | ||
| Sense | CTGTCTGGATGCTTCCTGAGTTT | 60.3 |
| Antisense | CAGCTATGTTTTGCAGTCCACC | 60.3 |
| Gapdh | ||
| Sense | GCT GAG TAT GTC GTG GAG T | 58.0 |
| Antisense | GTT CAC ACC CAT CAC AAA C | 56.0 |
Statistical analysis
Statistical analysis was performed using graphpad prism software (GraphPad, La Jolla, CA, USA). Data are expressed as mean ± standard deviation (SD). P values were calculated using Student's t‐test. Statistics with P value less than 0.05 were considered significant.
Results
The effect of TAK1 deficiency in DCs on adaptive immune response in SRBC‐immunized mice
To confirm the successful generation of Map3k7 DC mice, we checked the TAK1 knockout efficiency in the mice. The results showed that the mRNA expression level of TAK1 was significantly decreased in splenic DCs (CD11c+ cells) but not CD4+ T cells compared to WT mice (Fig. 1). The deficiency of TAK1 in DCs induced markedly reduction in the number of lysate plaques (PFCs) counted in 106 splenocytes and total serum hemolytic activity (HC50) (Fig. 2A,B). The deletion of TAK1 specifically in DCs elicited a suppressive effect in DTH response, as the increase in foot pad thickness was significantly reduced compared to WT controls (Fig. 2C). The serum IgA, IgM and IgG levels in Map3k7 DC mice were dramatically lower than those of WT mice (Fig. 2D).
Figure 1.

Deletion of TAK1 in DCs from Map3k7 DC mice. Analysis of TAK1 mRNA expression in splenic DCs (CD11c+) and CD4+ T cells of WT and Map3k7 DC mice. Data are representative of three independent experiments. The bars represented mean ± SD (n = 6). **P < 0.01 indicate significant changes compared to the WT group.
Figure 2.
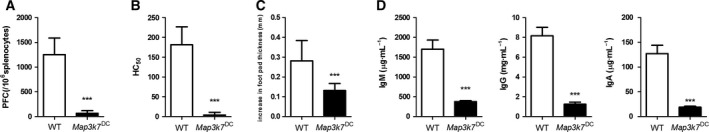
Deletion of TAK1 in DCs results in suppressed humoral and cellular immune response. WT and Map3k7 DC mice were immunized with SRBC as stated in the PFC assay section. (A) Counts of PFCs/106 splenocytes. (B) Serum hemolytic activity (HC 50). (C) The increase in foot pad increase. (D) Determination of serum IgM, IgG, and IgA. The bars represented mean ± SD (n = 9). ***P < 0.001 indicate significant changes compared to the WT group.
The effect of DC‐specific deletion of TAK1 on spleen cell subtyping in SRBC‐immunized mice
The total number of splenocytes of Map3k7 DC mice was significantly lower than that of WT controls (Fig. 3A). Map3k7 DC mice had significantly reduced percentages of conventional DCs (cDCs, MHC‐II+CD11c+) and T cells (CD3+), especially CD8+ T cells (cytotoxic T cells, Tc) (Fig. 3B,C). However, there was no dramatic change in B cells (B220+) proportion between these two groups (Fig. 3B,C). TAK1 deficiency in DCs caused significant decrease in the counts of T cells, B cells, CD4+ T cells (Th cells), CD8+ T cells, cDCs in spleen (Fig. 3D).
Figure 3.
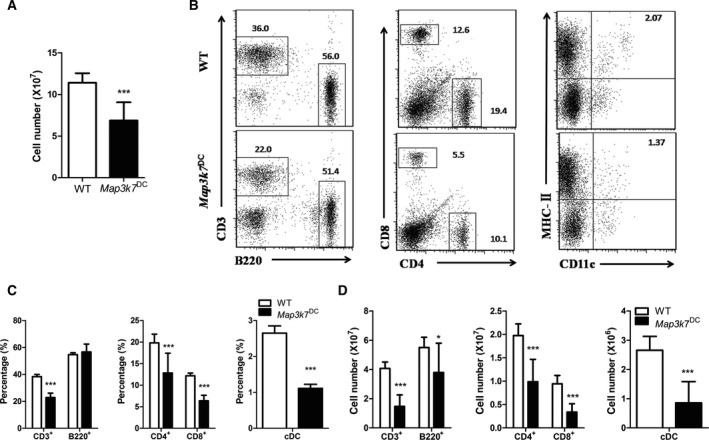
DC‐specific deficiency of TAK1 disrupts the spleen cellularity and cell subpopulation. T and Map3k7 DC mice were immunized with SRBC as stated in the PFC assay section and the splenocytes were subtyped by flow cytometer. (A) Total cell number of spleen. (B) Flow cytometry analysis of splenic B, T, Tc, Th cells, and cDC. (C) The percentage and (D) cell numbers of splenic cell subtypes. The bars represented mean ± SD (n = 9).*P < 0.05, ***P < 0.001 indicate significant changes compared to the WT group.
The effect of DC‐specific deficiency of TAK1 on splenocytes cytokine production in SRBC‐immunized mice
Using intracellular cytokine staining after stimulated with PMA and ionomycin to measure the expression of cytokines in splenic T cells, we found that splenic CD4+ and CD8+ T cells from Map3k7 DC mice secreted significantly less IFN‐γ and IL‐4 than that of WT controls (Fig. 4).
Figure 4.
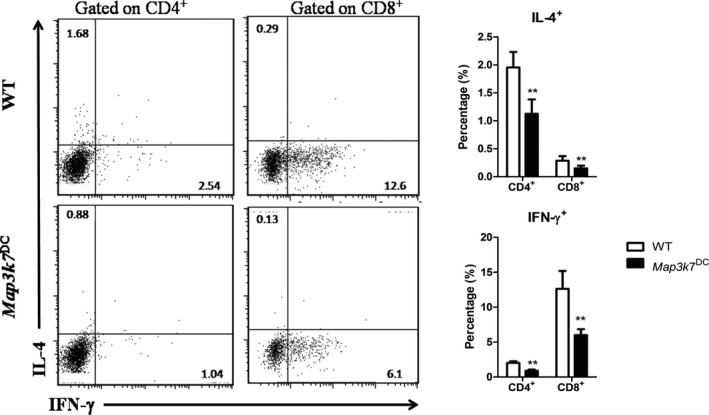
DC‐specific deletion of TAK1 impaired the cytokines production of T cells in spleen. WT and Map3k7 DC mice were immunized with SRBC as stated in the PFC assay section and the expression of IFN‐γ and IL‐4 in splenic CD4+ and CD8+ T cells was determined by intracellular cytokine staining after stimulation with PMA and ionomycin. (Left) Flow cytometry of splenic IFN‐γ+ CD4+, IFN‐γ+ CD8+, IL‐4+ CD4+ and IL‐4+ CD8+ populations. (Right) The proportion of these populations. The bars represented mean ± SD (n = 6). **P < 0.01 indicate significant changes compared to the WT group.
The effect of TAK1 deletion in DCs on splenocyte gene expression in SRBC‐immunized mice
Map3k7 DC mice showed markedly lower expression levels of several cytokines including IFN‐γ, IL‐2, IL‐4 and IL‐10 and reduced expression of transcription factors T‐bet and GATA‐3. In addition, expression of chemokine receptor CXCR4 was slightly down‐regulated without statistical significance, but the expression of CCR7 was significantly reduced in Map3k7 DC mice. We also detected the MHC molecules mRNA expression levels in the spleen, and both MHC‐I and MHC‐II molecules were dramatically down‐regulated in Map3k7 DC mice (Fig. 5).
Figure 5.
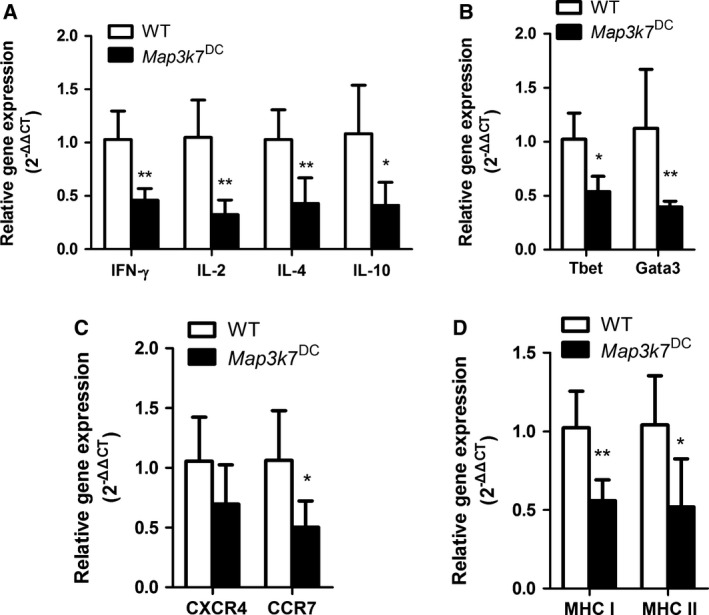
TAK1 deficiency in DCs down‐regulates the gene expression levels in spleen. WT and Map3k7 DC mice were immunized with SRBC as stated in the PFC assay section and total RNA of spleens was analyzed for the expression levels of the indicated genes by real‐time PCR. (A) Cytokines gene expression. (B) Transcription factors gene expression. (C) Chemokine receptors gene expression. (D)MHC molecules gene expression. The bars represented mean ± SD(n = 6). *P < 0.05, **P < 0.01 indicate significant changes compared to the WT group.
Discussion
This study was aimed to clarify how DC‐specific TAK1‐deficient mice responded to a T‐cell dependent antigen‐SRBC and to figure out its cellular and molecular mechanism. The results of this study showed that TAK1 deficiency in DCs suppressed SRBC‐induced PFC response and DTH response and decreased the levels of HC50 and immunoglobulin isotypes in serum. Besides, the balance of immune cell subpopulations in spleen and the cytokines produced by splenic T cells were disrupted, while mRNA expression levels of several cytokines, transcription factors, chemokine receptor, and MHC molecules were also down‐regulated in Map3k7 DC mice.
The adaptive immune response to the antigen includes both the cellular and humoral immunity and the mechanisms of which consist of antibody‐producing B cells, various kinds of Th cells, killer T cells and regulatory T cells. The humoral immune response induced by B cells is characterized by the specific antigen antibody reaction. The hemolysis test is an alternative to the PFC assay to measure the serum antibody level, which was first introduced in 1979 26, and it is now endorsed by Ministry of Health of China as a first‐line immune function test for evaluating the potential of health food to boost immunity 27. PFC assays is the most common method to evaluate the humoral immunity mediated by SRBC, and the amount of serum antibodies is also an accepted biomarker of the humoral immune response 28. DTH reaction is a kind of T‐cell mediated immune response to SRBC and is usually used to assess the cellular immunity in mice 29. In this study, compared with WT controls, PFC response was significantly suppressed and almost lost in Map3k7 DC mice (Fig. 2A). A dramatically reduced level of serum HC50 value and immunoglobulin isotypes—IgG, IgM, and IgA—were also found in DC‐specific TAK1‐deficient mice (Fig. 2B,D). This suggests an overall effect of TAK1 in DCs on antibody production, since variations attributed to the response to the immunogen and production of specific antibody should not explain a so dramatic reduction in the global concentration of immunoglobulins. In accordance with the phenotype of other mice harboring cDC depletion 30, the antigen‐specific antibodies generation after immunization was completely abolished. DTH reaction to SRBC in Map3k7 DC mice was abrogated (Fig. 2C). This result is in agreement with our previous study that Map3k7 DC mice failed to respond to 2, 4‐dinitrofluorobenzene (DNFB)‐induced contact hypersensitivity 31. These results strongly suggest that TAK1 deficiency in DCs has an immunosuppressive effect in mice on both humoral and cellular immune function.
To further clarify the mechanisms that the deletion of TAK1 in DCs caused immunosuppression, total, helper, and cytotoxic T‐cell percentages and counts, as well as total B‐cell and CD11c+ DCs percentages and counts in the spleens were analyzed via flow cytometry. It was clear that TAK1 deficiency in DCs decreased the percentage of total T cells, Th cells, Tc cells, and cDCs, but the percentage of B cells remained intact (Fig. 3C). Owing to the decreased total spleen cell number (Fig. 3A), the cell counts of all subsets of the immune cells above were markedly reduced in Map3k7 DC mice compared with those in WT controls (Fig. 3D). In nonimmunized Map3k7 DC mice, it also exhibited reduced populations of CD4+ and CD8+ T cells and increased apoptosis of cDCs in spleen 12. Collectively, TAK1 in DCs is essential for the homeostasis of both APCs and T‐cell populations, but has no impact on B cells. SRBCs are nonreplicating T‐cell dependent antigen that directly reach the spleen and induce the primary immune response there 32. DCs and T cells both play a key role in T‐dependent antibody formation. After T‐cell dependent antigen exposure, DCs uptake the antigen and subsequently process and present it to activate antigen‐specific Th cells. Then, Th cells activate B cells during cell–cell interaction and they also secrete several cytokines that induce B cells to proliferate and differentiate into plasma cells to produce antigen‐specific antibodies 33, 34, 35. Although Map3k7 DC mice have intact splenic B cells proportion, they are lack of splenic cDCs and T cells to initiate the SRBC‐induced humoral immune response. On the other hand, the number of B cells in the spleen of Map3k7 DC mice was much less than that in WT mice (Fig. 3D), which means that much less B cells could function in the immune responses. Besides, Map3k7 DC mice showed significantly increased percentages of macrophages and granulocytes in the spleen (Fig. 6). As DCs were much decreased in the spleen, macrophages, another kind of APCs, may be recruited to the spleen to function as the main source of APCs or to remove the cellular debris that accumulates after TAK1 was knockout in DCs. In nonimmunized Map3k7 DC mice, it also exhibited expansion of granulocytes resulting from myeloid proliferation as a consequence of loss of TAK1 in DCs 12. These might be possible explanations to why DC‐specific TAK1‐deficient mice are under immunosuppressed state.
Figure 6.

DC‐specific deficiency of TAK1 expanded macrophages and granulocytes. WT and Map3k7 DC mice were immunized with SRBC as stated in the PFC assay section and the splenocytes were subtyped by flow cytometer. (Left) Flow cytometry analysis of splenic macrophages and granulocytes. (Right) The percentage of splenic cell subtypes. The bars represented mean ± SD (n = 5). **P < 0.01 indicate significant changes compared to the WT group.
Antigen‐primed CD4+ T cells can be divided into at least two subsets based on the different cytokine they produced and these subsets are known as Th1 and Th2. Th1 cells secrete IFN‐γ and IL‐2, which stimulates the maturation of DCs and improve the cellular immunity, and its transcription is depending on T‐bet 16. Th2 cells secrete IL‐4, IL‐5, IL‐6, and IL‐10 and its transcription factor is GATA3, which regulate the humoral immune response by inducing the proliferation and differentiation of B cells 36. The intracellular cytokine staining results exhibited that TAK1 deficiency in DCs abolished the Th1 and Th2 cytokines secretion by T cells in the spleen (Fig. 4). Along with this result, the real‐time PCR results showed that DC‐specific TAK1 deficiency significantly down‐regulated the mRNA expression levels of Th1 and Th2 cytokines (Fig. 5A). Besides, the expression of transcription factors T‐bet and GATA‐3, the master regulators controlling Th1 and Th2 cells differentiation, were also down‐regulated in the spleen of Map3k7 DC mice (Fig. 5B), which indicated that deletion of TAK1 in DCs might inhibit the cytokines secretion by interfering with specific molecular programs to suppress Th‐cell differentiation. In nonimmunized Map3k7 DC mice, the splenic T cells were impaired to produce IFN‐γ and IL‐2 upon acute polyclonal stimulation, indicative of altered function of T cells owing to TAK1 deletion in DCs 12. In agreement with the phenotype of other mice harboring myeloid proliferation syndrome 37, the ability of T cells to produce IFN‐γ was reduced. The impaired cytokine secretion function of T cells in spleen of Map3k7 DC mice might attribute to the myeloid proliferation phenotype. And this further resulted in suppressing the SRBC‐induced humoral immune response.
MHC molecules play a crucial role in the response of T cells to antigens, which known as antigen processing and presentation 16. Our results showed that Map3k7 DC mice significantly down‐regulated mRNA expression levels of MHC I/II molecules (Fig. 5D), indicative the diminished ability of APCs to activate T cells after immunized with SRBC. Chemokines and their receptors constitute a molecular system to regulate immune cell migration for effective and robust immunity, while CCR7 and CXCR4 are two common chemokine receptors 16. In this study, Map3k7 DC mice showed significantly down‐regulated mRNA expression levels of CCR7 after immunized with SRBC (Fig. 5C). It is evident that CCR7 axis in the T‐ and B‐cell interactions is required to initiate effective high affinity humoral immune responses 38. The data revealed that the deletion of TAK1 in DCs disrupted normal function of CCR7 to induce the humoral immune response after immunized with SRBC. Taken together, TAK1 specific deficiency in DCs might damage the key molecules in the initiation of SRBC‐induced humoral immune responses.
In conclusion, our study demonstrated that TAK1 is critical for the development of humoral immune responses. Mechanistically, TAK1 is essential for survival of DCs, as well as the normal function and homeostasis of T‐cell populations. Therefore, DC‐specific targeting of TAK1 may be a feasible immunotherapy strategy.
Author contribution
YP and ZL conceived and designed the study and conducted the experiments. XW and WH contributed to the interpretation of results and the discussion. YP wrote the manuscript. All authors have read and approved the final version submitted for publication.
Acknowledgement
This study was supported by grant from the National Sciences Foundations of the People's Republic of China [Grant No. 81273112].
References
- 1. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC and Steinman RM (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC‐205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8 + T cell tolerance. J Exp Med 196, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boscardin SB (2006) Antigen targeting to dendritic cells elicits long‐lived T cell help for antibody responses. J Exp Med 203, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banchereau J and Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 4. Fayette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Banchereau J, Caux C and Briere F (1997) Human dendritic cells skew isotype switching of CD40‐activated naive B cells towards IgA1 and IgA2. J Exp Med 185, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang G, Shi LZ and Chi H (2009) Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine 48, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato S, Sanjo H, Takeda K, Ninomiya‐Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O and Akira S (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6, 1087–1095. [DOI] [PubMed] [Google Scholar]
- 7. Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M and Tanaka N (2005) TAK1, but not TAB 1 or TAB 2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19, 2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wan YY, Chi H, Xie M, Schneider MD and Flavell RA (2006) The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol 7, 851–858. [DOI] [PubMed] [Google Scholar]
- 9. Sato S, Sanjo H, Tsujimura T, Ninomiya‐Tsuji J, Yamamoto M, Kawai T, Takeuchi O and Akira S (2006) TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol 18, 1405–1411. [DOI] [PubMed] [Google Scholar]
- 10. Liu HH, Xie M, Schneider MD and Chen ZJ (2006) Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA 103, 11677–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schuman J, Chen Y, Podd A, Yu M, Liu HH, Wen R, Chen ZJ and Wang D (2009) A critical role of TAK1 in B‐cell receptor‐mediated nuclear factor kappaB activation. Blood 113, 4566–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Huang G, Vogel P, Neale G, Reizis B and Chi H (2012) Transforming growth factor beta‐activated kinase 1 (TAK1)‐dependent checkpoint in the survival of dendritic cells promotes immune homeostasis and function. Proc Natl Acad Sci USA 109, E343–E352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajasekaran K, Chu H, Kumar P, Xiao Y, Tinguely M, Samarakoon A, Kim TW, Li X, Thakar MS and Zhang J (2011) Transforming growth factor‐beta‐activated kinase 1 regulates natural killer cell‐mediated cytotoxicity and cytokine production. J Biol Chem 286, 31213–31224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D and Su B (2012) TAK1 negatively regulates NF‐kappaB and p38 MAP kinase activation in Gr‐1 + CD11b+ neutrophils. Immunity 36, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamothe B, Lai Y, Hur L, Orozco NM, Wang J, Campos AD, Xie M, Schneider MD, Lockworth CR and Jakacky J (2012) Deletion of TAK1 in the myeloid lineage results in the spontaneous development of myelomonocytic leukemia in mice. PLoS One 7, e51228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coico R, Sunshine G and Benjamini E (2003) Immunology‐A Short Course, 5th edn Wiley‐Liss, Hoboken, NJ. [Google Scholar]
- 17. White KL, Musgrove DL and Brown RD (2010) The sheep erythrocyte T‐dependent antibody response (TDAR). Methods Mol Biol 598, 173–184. [DOI] [PubMed] [Google Scholar]
- 18. Herzyk DJ and Holsapple M (2007) Immunotoxicity evaluation by immune function tests: focus on the T‐dependent antibody response (TDAR) [Overview of a Workshop Session at the 45th Annual Meeting of the Society of Toxicology (SOT) March 5‐9, 2006 San Diego, CA]. J Immunotoxicol 4, 143–147. [DOI] [PubMed] [Google Scholar]
- 19. US EPA (1998) Health Effects Test Guidelines, OPPTS 870.7800. Immunotoxicity pp. 1–11. http://www.epa.gov/opptsfrs/publications/OPPTS_Harmonized/870_Health_EffectTest_Guidelines/Series/870-7800.pdf/
- 20. Luster MI, Munson AE, Thomas PT, Holsapple MP, Fenters JD, White KJ, Lauer LD, Germolec DR, Rosenthal GJ and Dean JH (1988) Development of a testing battery to assess chemical‐induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam Appl Toxicol 10, 2–19. [DOI] [PubMed] [Google Scholar]
- 21. Luster MI, Portier C, Pait DG, White KJ, Gennings C, Munson AE and Rosenthal GJ (1992) Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol 18, 200–210. [DOI] [PubMed] [Google Scholar]
- 22. Caton ML, Smith‐Raska MR and Reizis B (2007) Notch‐RBP‐J signaling controls the homeostasis of CD8‐ dendritic cells in the spleen. J Exp Med 204, 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunningham AJ (1965) A method of increased sensitivity for detecting single antibody‐forming cells. Nature 207, 1106–1107. [DOI] [PubMed] [Google Scholar]
- 24. Cunningham AJ and Szenberg A (1968) Further improvements in the plaque technique for detecting single antibody‐forming cells. Immunology 14, 599–600. [PMC free article] [PubMed] [Google Scholar]
- 25. Jerne NK and Nordin AA (1963) Plaque formation in agar by single antibody‐producing cells. Science 140, 405. [PubMed] [Google Scholar]
- 26. Xueying X, Yuan L and Jin X (1979) An alternative humoral immunity assessment method‐ hemolysis test. Acta Pharm Sin 14, 443–446. [Google Scholar]
- 27. Ministry of Health of China (2003) Technical Standards for Testing and Assessment of Health Food.
- 28. Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R and De Rosa A (2011) Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccines 10, 1053–1061. [DOI] [PubMed] [Google Scholar]
- 29. Doherty NS (1981) Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents Actions 11, 237–242. [DOI] [PubMed] [Google Scholar]
- 30. Fahlen‐Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strombeck A, Blomquist M, MacPherson GG, Holmgren J and Yrlid U (2009) CD11c(high)dendritic cells are essential for activation of CD4 + T cells and generation of specific antibodies following mucosal immunization. J Immunol 183, 5032–5041. [DOI] [PubMed] [Google Scholar]
- 31. Zhao YG, Wang Y, Hao W and Wan YY (2011) An essential role for TAK1 in the contact hypersensitivity response. Cell Mol Immunol 8, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van den Eertwegh AJ, Boersma WJ and Claassen E (1992) Immunological functions and in vivo cell‐cell interactions of T cells in the spleen. Crit Rev Immunol 11, 337–380. [PubMed] [Google Scholar]
- 33. Inaba K and Steinman RM (1985) Antibody responses to T‐dependent antigens: contributions of dendritic cells and helper T lymphocytes. Adv Exp Med Biol 186, 369–376. [DOI] [PubMed] [Google Scholar]
- 34. Lanzavecchia A (1985) Antigen‐specific interaction between T and B cells. Nature 314, 537–539. [DOI] [PubMed] [Google Scholar]
- 35. Niiro H and Clark EA (2002) Regulation of B‐cell fate by antigen‐receptor signals. Nat Rev Immunol 2, 945–956. [DOI] [PubMed] [Google Scholar]
- 36. Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, Cherwinski HM, Savelkoul HF, Finkelman FD and Bond MW (1988) The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev 102, 5–28. [DOI] [PubMed] [Google Scholar]
- 37. Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L and Waring JF (1996) Immunodeficiency and chronic myelogenous leukemia‐like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317. [DOI] [PubMed] [Google Scholar]
- 38. Comerford I, Harata‐Lee Y, Bunting MD, Gregor C, Kara EE and McColl SR (2013) A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 24, 269–283. [DOI] [PubMed] [Google Scholar]


