Abstract
Due to type-specificity, commercially available human papillomavirus (HPV) vaccines are only effective against homologous HPV serotypes, providing limited protection. Recent studies have highlighted the role of HPV minor capsid protein (known as L2) in inducing cross-protection. The N-terminal peptides of L2 contain conserved cross-response epitopes that can induce neutralizing antibodies against heterogeneous HPVs. However, when compared with L1, these peptides have lower immunogenicity, which limits the application of these vaccines. The protein transduction domain (PTD), located in the Tat protein of human immunodeficiency virus, facilitates delivery of DNA, peptides, proteins and virus particles into cells by unknown mechanisms, and has been reported to enhance immunogenicity of several antigens. In the present study, two peptides derived from the N-terminal of HPV16L2 were chosen as model antigens and constructed a series of L2 peptide vaccines by either fusing or mixing with PTD. Subsequently their immunogenicity was evaluated. The results indicated that the L2 peptides fused with PTD show considerably enhanced humoral immunity. In particular, they increased the titer of cross-neutralizing antibodies, while L2 peptides that had only been mixed with PTD induced only small cross-protection responses. Overall, the data suggest that fusion of L2 peptides with PTD significantly enhances their cross-protection and may be a promising strategy for the development of broad-spectrum HPV prophylactic vaccines.
Keywords: protein transduction domain, human papillomavirus, L2, immunogenicity, cross-protection
Introduction
Tat-protein transduction domain (PTD) [the PTD domain of human immunodeficiency virus (HIV)-tat] is a peptide of 9–11 amino acids in length and is predominantly comprised of basic amino acids, such as arginine and/or lysine (1–3). Accumulating evidence indicates that PTD has the capability to carry proteins, peptides, nucleic acid and viral particles into cells. Although the detailed mechanisms for transduction remain to be elucidated, negatively charged heparan sulphate on the cell surface could have an important role in this process. Due to its unique characteristics, PTD has numerous applications, including delivery of functional proteins, viral particles and enhancing the DNA (RNA) transfection efficiency. A previous study has also suggested that PTD combined with certain antigens can enhance immunogenicity (4).
Currently, the cervical cancer prophylactic vaccines based on human papillomavirus (HPV) L1 virus-like particles (VLPs), including Gardasil (HPV6/11/16/18 quatrivalent vaccine developed by Merck, Kenilworth, NJ, USA) and Cervarix (HPV16/18 bivalent vaccine developed by GlaxoSmithKline, Brentford, UK) have been approved in a number of developed countries, and can induce satisfactory protective effects (5,6). However, this protection is type-specific and there is weak or no protection against other HPV types. The discovery of cross-neutralizing responses induced by L2 derived from rabbit, bovine and human papillomavirus underline the potential application as a promising broad-spectrum vaccine. Further studies have identified highly conserved common epitopes located within the first 200 amino acids of the N-terminal of L2 (7–9), which are essential for inducing cross-neutralizing antibodies. However, immunogenicity of polypeptide of L2 is much lower compared to L1 VLP, which has impeded its further application. Extensive efforts have been made to increase the immunogenicity of L2 peptides. Strategies include increasing the epitope number by constructing polypeptides with repeat cross-response epitopes from one serotype or tandem epitopes from several serotypes, and the utilization of Trx (recombinant tobacco mosaic virus) or bacteriophage VLP as vectors to display L2 epitopes (10–12). These strategies alone, or combined, improved the immunogenicity of L2 peptides to a certain extent.
In the present study, two HPV16L2 peptides, L2-N88 and L2-N200 (the first 88 or 200 amino acids of the N-terminal of L2, respectively) were chosen as model antigens and a series of L2 peptide vaccines were constructed by fusing or mixing with PTD, and evaluated their specific humoral responses and cross-protection by enzyme-linked immunosorbent assay (ELISA) and pseudovirion neutralization assay, respectively.
Materials and methods
Expression and purification PTD-L2 polypeptides
L2 vaccines were constructed by fusing PTD with HPV16L2 peptides, L2-N88 and L2-N200, designated PTD-L2-N88 and PTD-L2-N200, respectively. First, the sequence-verified polymerase chain reaction amplification products encoding L2-N88, L2-N200, PTD-L2-N88 and PTD-L2-N200 were cloned into pET-22 (Novagen, Madison, WI, USA) plasmids using XhoI and NdeI restriction sites. The constructs were confirmed by sequencing, and were transformed into Escherichia coli Rosetta (DE3) cells. Transformed cells were grown at 37°C until they reached an optical density (OD) at 600 nm value of 0.8. Protein expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside for 4 h. Cell pellets were lysed and resuspended by binding buffer, 20 mM Tris-HCl, 500 mM NaCl, 20 mM imidazole and 8 M urea (pH 8.0), followed by centrifugation at 5,000 × g for 25 min at 4°C. The clear supernatant was applied to a HisTrap FF column (GE Healthcare, Beijing, China) according to the manufacturer's protocol. The peak fraction was collected and extensively dialyzed into phosphate-buffered saline (PBS) buffer (pH 7.4) for 12–14 h at 4°C. The dialyzed fractions were centrifuged for 10 min, and the clear supernatants collected. Protein concentration was determined by the bicinchoninic acid acid method (Bio-Rad, Hercules, CA, USA).
Immunization of mice
For immunization, purified L2-N88, PTD-L2-N88 and L2-N200, PTD-L2-N200 were diluted to proper concentration with PBS and sterilized using 0.22 µM filters. The mix-type L2 vaccines, termed PTD + L2-N88 and PTD + L2-N200 were prepared by mixing purified L2-N88 or L2-N200 (100 µg each) with PTD (Scilight Biotechnology LLC, Beijing, China) according to a molar ratio of 1:1. Female BALB/c mice (4–6-week old) were randomly divided into 8 groups, with 8 animals for each vaccination group, and 5 mice for the control groups. The mice were immunized subcutaneously 3 times. The priming injection at day 0 used vaccines formulated in complete Freund's adjuvant, and the subsequent 2 boost injections used vaccines prepared in incomplete Freund's adjuvant at days 14 and 28. Blood samples were collected 7 days after the last boost. All the animals were purchased from Vital River Laboratories (Beijing, China), and maintained under pathogen-free conditions at the animal facilities of Peking University First Hospital (Beijing, China). All the animal experimental procedures in this study were approved by the Animal Ethics Committee of Peking University First Hospital.
Detection of anti-L2 antibodies
Antibodies against HPV16L2 in immunized mice were measured from serum by ELISA. Microtiter plates were coated overnight at 4°C with 100 µl of coating buffer containing 1 µg of full-length HPV16L2 protein, washed twice using PBS with 0.2% Tween-20 (PBST), blocked with 100% fetal bovine serum at 37°C for 2 h, followed by washing twice again with PBST. Mouse serum (50 µl) was serially diluted in 2-fold steps starting at 1:100, subsequently added to the ELISA plate and incubated for 1 h at 37°C. Plates were washed and incubated for 1 h at 37°C with 50 µl horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (1:500 dilution) (CW0102; CWBio, Beijing, China). Subsequent to washing again with PBST, 100 ml of the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine was added to each well and the absorbance at 450 nm was measured after 10–20 min with an automated plate reader (Bio-Rad). An OD value 4× over that of the control sera was taken as a positive result.
Pseudovirion neutralization assay
HEK293FT cells were seeded 24 h prior to infection at a density of 1×104 cells/well in 96-well microtiter plates. The pseudovirions were diluted 1:2,000 in Dulbecco's modified Eagle's medium without phenol red and mixed with anti-sera at different dilutions. Following gentle agitation at 4°C for 1 h, the culture medium was replaced by 100 µl of the pseudovirion-sera mixture and cultured for a further 72 h. Finally, secreted embryonic alkaline phosphatase (SEAP) activity in cell culture supernatants was determined using a chemical chromatic assay. Briefly, 20 µl 0.05% CHAPS and 200 µl substrate solution (2 M diethanolamine with 1 mM MgCl2, 0.5 mM ZnCl2 and 3 mM pNPP) were added to 40 µl of culture supernatant and incubated for 2 h without light. The OD values at 405 nm were determined using a Bio-Rad Spectrophotometer. The neutralizing titer was expressed as the reciprocal of the maximum dilution of serum that caused ≥50% activity reduction of SEAP.
Statistical analysis
The neutralization titers were analyzed using parametric tests (one-way analysis of variance followed by Bonferroni comparisons). P<0.05 was considered to indicate a statistically significant difference. All statistical analysis was carried out with the GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Construction, expression and purification of L2 peptide vaccines
Previous studies reported that the antibodies induced by 1–88 and 1–200 peptides derived from the N-terminal region of HPV-16 L2 could neutralize HPV-16 pseudovirions and cross-neutralize heterologous pseudovirions. The peptide of 1–200 exhibited a higher immunogenicity compared to the 1–88 peptide. In order to study the effect of the transduction domain on the immunogenicity of L2 peptide, the L2 peptide vaccines were constructed with PTD mixed peptide (PTD: HPV16L2-88 and PTD: HPV16L2-200) and PTD fusion peptide (PTD-HPV16L2-88 and PTD-HPV16L2-200), respectively. The four proteins, HPV16L2-88, PTD-HPV16L2-88, HPV16L2-200 and PTD-HPV16L2-200, were expressed in the Escherichia coli expression system, and were purified under denaturing conditions using the HisTrap FF column (GE Healthcare). This was followed by dialysis for 12–14 h with PBS buffer (pH 7.4) at 4°C to harvest related purified L2 peptide vaccines. The purity of the four recombinant proteins obtained were 99.1, 98.9, 95.4 and 93.7%, respectively. The total protein concentrations were 1,626.1, 1,709.2, 1,660.6 and 1,570.3 µg/ml, respectively.
PTD-L2 peptide vaccines generate potent anti-HPV16 humoral immune responses
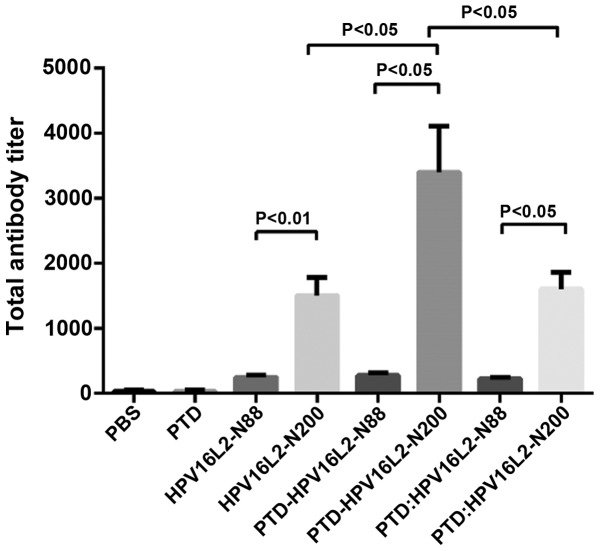
Inducing a high humoral immune response is the primary function of the preventive vaccine. In order to detect the effect of the humoral immune response induced by HPV16L2 peptide vaccines, the full-length HPV16L2 protein expressed by prokaryotic expression system was used as a coating antigen to detect the serum anti-L2 antibody (IgG) level by indirect ELISA results (Fig. 1) showed that each HPV16L2 vaccine could induce specific L2 antibodies with different titers and two results were summarized. First, total anti-L2 antibody induced by peptide vaccines of HPV16L2 N-88 (which included HPV16L2-N88, PTD-HPV16L2-N88 and PTD: HPV16L2-N88) was significantly lower than that of HPV16L2-N200 regardless of the involvement of PTD, and PTD participation mode was the mixture or fusion. The results are consistent with the view that the polypeptide size affects its immunogenicity. Second, in the HPV16L2-N88 groups, the use of PTD (fusion or mixture) did not result in a significant increase in the total antibodies produced by the vaccine, and the level of specific antibody titer was low in all groups. By contrast, in the HPV16L2-N200 groups, the antibody titer of the vaccine to PTD-HPV16L2-N200 (PTD fused with HPV16L2-N200) was twice that of HPV16L2-N200, which was 6–7 times the N88 groups. However, mixed use of PTD did not improve the total antibody titer.
Figure 1.
Antibody titer of mice vaccinated with HPVL2 relative proteins. Animals were immunized with HPV16L2-N88, HPV16L2-N200, PTD-HPV16L2-N88, PTD-HPV16L2-N200 and PTD: HPV16L2-N88 (mol ratio, 1:1) and PDT: HPV16L2-N200 (mol ratio, 1:1), the effective amount of protein was 100 µg/100 µl/animal. Protein was mixed with Freunds complete adjuvant, and the animals were immunized at weeks 0, 2 and 4. One week after the completion of the immunization, the serum was collected for indirect ELISA. No significant difference was identified between the total antibody levels in HPV16L2-N88-related groups. In the HPV16L2-N200-related groups, the total antibody titers in the PTD-HPV16L2-N200 groups were higher than those of the HPV16L2-N200 groups (P<0.05) and PTD: HPV16L2-N200 groups (P<0.05). No significant difference was identified between the HPV16L2-N200 and PTD: HPV16L2-N200 groups. In all conditions, the total antibodies in HPV16L2-N200-related groups were higher than those in the HPV16L2-N88 groups. HPV, human papillomavirus; PTD, protein transduction domain; ELISA, enzyme-linked immunosorbent assay.
A summary of the existing data shows that for the HPV N-end peptide vaccines, the involvement of PTD in the induction of HPV16L2-specific antibodies did not significantly increase the level of total antibody. Although, the combination of PTD and N200 doubled the antibody titer, which is still not a satisfactory consequences.
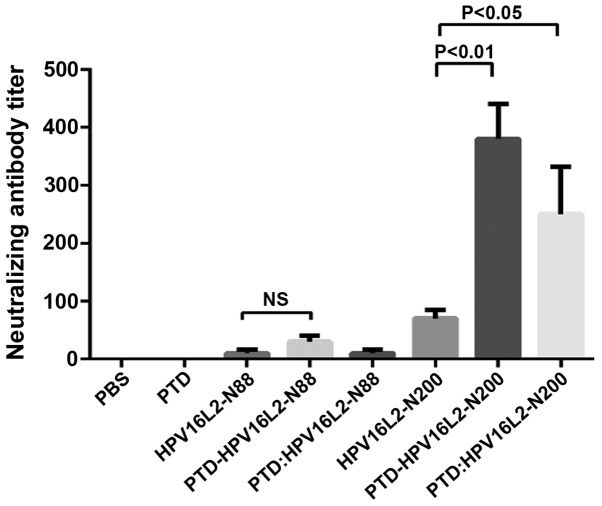
Serum-neutralizing antibodies can neutralize the related viral particles to prevent them from infecting host cells, which is key in protective efficacy of the vaccine-induced immunity. To determine whether the anti-L2 serum antibodies stimulated by L2 peptide vaccines with mixed or fusion form of PTD own the ability to neutralize the virus particles, the pseudovirion neutralization experiments were carried out to detect the serum levels of anti-HPV16 neutralizing antibody titers in each mouse group (Fig. 2). The result which was consistent with the total antibody test was that the neutralizing antibody titer of all the HPV16L2-N88 relative groups were still extremely low. It was demonstrated that PTD was not effective in improving the humoral immune response to the HPV-N88 vaccine. However, in the HPV16L2-N200 groups, the specific-neutralizing antibody titer induced by PTD-HPV16L2-N200 was 5 times that of the PTD group, and the PTD mixed involvement also increased the neutralizing antibody by ~2-fold. The neutralizing antibody titer of PTD-HPV16L-N200 was significantly higher than that of PTD: HPV16L-N200 (P<0.05).
Figure 2.
Pseudovirus neutralization assay of HPV16. Neutralizing antibody has a major role in protecting against the invasion of viruses. Subsequent to the animals being immunized according to the immunization schedule, the serum was collected for pseudovirus neutralization experiments to detect the neutralizing antibody titers. PTD-fused expression protein increased the neutralizing antibody titers by stimulating the body, although no significant difference was identified for the results in the HPV16L2-N88 group. However, for HPV16L2-N200 group, the PTD fusion or mixed significantly increased the neutralizing antibody titers. Wherein, the neutralizing antibody titers in the PTD-HPV16L2-200 group were significantly increased. HPV, human papillomavirus; PTD, protein transduction domain.
Humoral immune response results demonstrated that no change of humoral immune response occurred in the short peptide vaccine HPV16L2-N88 with or without PTD. However, the humoral immune response of the longer peptide vaccine (HPV16L2-N200) fused with PTD was improved significantly. This suggested that the fusion form appeared to have a stronger immune-enhancing effect.
HPV16L2 peptide vaccines induce cross-neutralizing activity
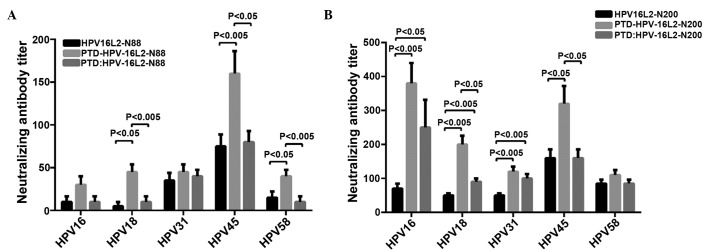
The N-terminal of HPV16 L2 contains linear cross-neutralizing epitopes, which cannot only neutralize the homotype of HPV virus particles, but also has a cross-neutralizing activity for allotype of HPV particles. To determine whether PTD can enhance the ability of L2 peptide vaccines in inducing cross-neutralizing antibodies, HPV18, HPV31, HPV45 and HPV58 pseudovariola were diluted to a suitable titer for the detection of serum levels of cross-protective neutralizing antibodies for corresponding HPV type in each group. The results showed that immunizing with HPV16L2-N88 alone cannot produce effective cross-neutralizing antibodies in the animals. Whereas HPV16L2-N200 alone resulted in the generation of cross-protective neutralizing antibodies for all types of pseudovirus (HPV18, HPV31, HPV45 and HPV58) in immunized mice of all the experimental groups. HPV16L2-N88-related vaccines induced cross-protective antibody, as shown in Fig. 3A, while Fig. 3B showed the result of HPV16L2-N200.
Figure 3.
(A) Pseudovirion cross-neutralization antibody assay for the HPV16L2-N88 groups (including HPV16L2-N88, PTD-HPV16L2-N88 and PTD: HPV16L2-N88). The animal serum was serially diluted, mixed and incubated with pseudovirion particles to infect cells, and fusion type of PTD significantly increased the titers of cross-neutralizing antibody. However, mixed PTD peptides were less effective, which was similar to the groups without PTD. (B) Pseudovirion cross-neutralization antibody assay for the HPV16L2-N200 group (including HPV16L2-N200, PTD-HPV16L2-N200 and PTD: HPV16L2-N200). The overall titers of the cross-neutralization antibody were higher than those of the HPV16L2-N88 groups. PTD-fused expression significantly improved the titers of the cross-protective antibody in each group. Several mixed types had specific effects. HPV, human papillomavirus; PTD, protein transduction domain.
HPV16L2-N88 alone resulted in an extremely low titer of cross-protective neutralizing antibodies in immunized animals, and the PTD fusion vaccine significantly increased the antibody titers in HPV18, 45 and 58 pseudovirions (P<0.05, P<0.005 and P<0.05, respectively). The PTD peptide mixed with HPV16L2-N88 also increased the antibody titers of partial types. The cross-protective neutralizing antibody induced by HPV16L2-N200 alone was higher than that of HPV16L2-N88, and that was also significantly increased by fusion of HPV16L2-N200 and PTD (Fig. 3B). When animals were immunized with the mixture of PTD and HPV16L2-N88 or HPV16L2-N200 (molar ratio of 1:1), the ability to produce cross-protective neutralizing antibodies was improved, but the effect was significantly worse than that of fusion forms. In conclusion, the pseudovirus neutralization experiments showed that the cross-neutralizing antibody levels in the HPVL2-N200 group (HPVL2-N200, PTD-HPVL2-N200 and PTD + HPVL2-N200) were significantly higher than those in the L2-N88 group (HPVL2-N88, PTD-HPVL2-N88 and PTD + HPVL2-N88). Whether fused or mixed with PTD, each L2-N200 peptide vaccine had a higher ability to induce cross-neutralizing antibody to different degrees, and the fusion of PTD had a better immune-enhancing effect compared to the mixed from PTD.
Discussion
High-risk HPV is an important virological cause of cervical cancer. In recent years, epidemiological studies have shown that HPV is strongly associated with head and neck cancer, and esophageal cancer. The present preventive HPV vaccine in the market is the L1 VLP-based subunit vaccine, and it can induce the body to generate a high titer of serum-neutralizing antibodies. However, as the serum-neutralizing antibodies are type-specific, there is little cross efficacy between different types of neutralizing antibody (13). Increasing the number of vaccines to extend the scope of protection will not only increase vaccine production costs further, but also increase the risk of side effects from the vaccine. Studies have found that L2 had cross-neutralization epitopes, and can induce a broad spectrum of cross-neutralizing antibodies. Further studies showed that the N-terminal of HPVL2 also had linear cross-neutralizing epitopes. The present studies on the L2 protein for HPV16 showed that the main neutralizing antibody epitopes present in the 11–200 region of the N-terminal can generate a cross-protective effect on 16, 18, 31, 45, 52 and 58 pseudovirion particles (14). However, the limitation of the HPVL2 peptide vaccine applications is low immunogenicity, and low titers of induced protection neutralizing antibody. Improving the immunogenicity has attracted increasing attention in research.
The Tat-PTD, a basic amino acid-rich polypeptide fragment of 9–11 amino acids in length, can cross the cell membrane in a receptor-independent mechanism (15). This polypeptide could cross the lipid bilayer of cells either alone or as a fused form of certain polypeptides or nucleotides (16,17) and deliver the carried Tat/PTD-fused proteins/peptides to the relevant cells (18). However so far, the mechanism of how Tat-PTD mediates the associated substances entering the cell membrane and having a relevant role remains to be elucidated. In certain studies it has been shown that the HIV-1 tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. PTD can enhance the transduction potential of heterologous peptides and proteins in vitro and in vivo (1,3), improve immunogenicity of certain antigens and enhance the immune response results. Following the addition of PTD, the fusion type of PTD-L2-N200 can significantly improve the titers of anti-HPV16L2 total antibodies, as well as those of neutralizing antibody for HPV16 and other high-risk HPV types (HPV16, 18, 31, 45 and 58). By contrast, neither its fusion nor the mixed form of PTD in the L2-N88 group was able to improve the level of total antibodies and cross-neutralizing antibodies clearly. This suggested that the mechanism for immunogenicity of the L2 polypeptide enhanced by PTD is different from other carrier protein and antigen hapten. In the present study, the neutralizing antibody titer/total antibody titer was used to assess the quality of antibodies. Fused PTD significantly increased the quality of L2-N200 group-induced antibodies. However, for the full-length HPV16L2, the ratio was significantly lower than that of the PTD fusion polypeptide vaccine (data not shown), although it can induce higher titers of total antibody and neutralizing antibodies. We speculate that the PTD peptide can penetrate the lipid bilayer of the cell membrane, and PTD can cause certain channels to open so that certain sections of the peptide vaccine can enter the reaction site or get closer to the effect site. When the PTD and peptide vaccine were fused together, the peptide vaccine was incorporated into the membrane structure, and all the components of the vaccine can be used in the reaction of PTD, which make PTD-HPV16L2-N200 more effective in comparison to the mixed.
References
- 1.Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 2.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 3.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: Enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–477. [PubMed] [Google Scholar]
- 4.Chen X, Lai J, Pan Q, Tang Z, Yu Y, Zang G. The delivery of HBcAg via Tat-PTD enhances specific immune response and inhibits Hepatitis B virus replication in transgenic mice. Vaccine. 2010;28:3913–3919. doi: 10.1016/j.vaccine.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 5.Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, Wu TC. Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs. 2010;24:109–129. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal L, Wilby OK, Willoughby CR, Veenstra S, Deschamps M. Evaluation of the intramuscular administration of Cervarix™ vaccine on fertility, pre- and post-natal development in rats. Reprod Toxicol. 2011;31:111–120. doi: 10.1016/j.reprotox.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73:6188–6190. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawana K, Matsumoto K, Yoshikawa H, Taketani Y, Kawana T, Yoshiike K, Kanda T. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology. 1998;245:353–359. doi: 10.1006/viro.1998.9168. [DOI] [PubMed] [Google Scholar]
- 9.Hitzeroth II, Passmore JA, Shephard E, Stewart D, Müller M, Williamson AL, Rybicki EP, Kast WM. Immunogenicity of an HPV-16 L2 DNA vaccine. Vaccine. 2009;27:6432–6434. doi: 10.1016/j.vaccine.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Müller M, Ottonello S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine. 2009;27:1949–1956. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 11.Kondo K, Ishii Y, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology. 2007;358:266–272. doi: 10.1016/j.virol.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, McCormick AA, McCulloch M, Pogue GP, Smith ML, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006;24:5516–5525. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 13.Huh WK, Roden RB. The future of vaccines for cervical cancer. Gynecol Oncol. 2008;109(Suppl 2):S48–S56. doi: 10.1016/j.ygyno.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007;81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 16.Gupta B, Torchilin VP. Transactivating transcriptional activator-mediated drug delivery. Expert Opin Drug Deliv. 2006;3:177–190. doi: 10.1517/17425247.3.2.177. [DOI] [PubMed] [Google Scholar]
- 17.Dietz GP, Bähr M. Delivery of bioactive molecules into the cell: The Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]