Abstract
Mature striatal medium size spiny neurons express the dopamine and cyclic AMP-regulated phosphoprotein, 32 kDa (DARPP-32), but little is known about the mechanisms regulating its levels, or the specification of fully differentiated neuronal subtypes. Cell extrinsic molecules that increase DARPP-32 mRNA and/or protein levels include retinoic acid (RA), brain-derived neurotrophic factor (BDNF), and estrogen (E2). We now demonstrate that RA regulates DARPP-32 mRNA and protein in primary striatal neuronal cultures. Furthermore, DARPP-32 induction by RA in vitro requires phosphatidylinositide 3-kinase (PI3K), but is independent of tropomyosin-related kinase b (TrkB), cyclin-dependent kinase 5 (cdk5), and protein kinase B (Akt). Using pharmacologic inhibitors of various isoforms of protein kinase C, we also demonstrate that DARPP-32 induction by RA in vitro is dependent on protein kinase C zeta (PKCζ). Thus, the signal transduction pathways mediated by RA are very different than those mediating DARPP-32 induction by brain derived neurotrophic factor (BDNF). These data support the presence of multiple signal transduction pathways mediating expression of DARPP-32 in vitro, including a novel, important pathway via which PI3K regulates the contribution of PKCζ.
Retinoic acid (RA) is a key regulator of development and function of the nigrostriatal dopaminergic system and of the medium size spiny neuron, the output neuron of the dorsal (caudate-putamen) and ventral (nucleus accumbens) striatum. Several groups have demonstrated regulation of the D2 receptor promoter and abnormalities of dopaminergic neurotransmission in retinoic acid receptor-null mice (Samad et al., 1997; Krezel et al., 1998; Valdenaire et al., 1998). High levels of multiple key enzymes in the retinoic acid-synthesizing and receptor pathway have been identified in the lateral ganglionic eminence and striatum (Zetterstrom et al., 1994; McCaffery and Drager, 1994; Toresson et al., 1999; Waclaw et al., 2004; Molotkova et al., 2007) and retinoic acid receptor beta (RARβ) appears to be most important for RA regulation of striatal-specific gene expression (Liao et al., 2005a,b; Wang and Liu, 2005). Importantly, RA does not lead to a general maturation of MSNs, but instead, induces expression of specific MSN markers, including dopamine- and cyclic AMP-regulated phosphoprotein Mr=32kDa (DARPP-32) (Toresson et al. 1999; Wang and Liu, 2005).
DARPP-32 is one of the best-characterized MSN-enriched molecules, and it is the most commonly used marker of this neuronal subtype in developmental and adult studies (e.g. Anderson et al., 1997; Toresson et al., 1999; Luthi-Carter et al., 2000). Other molecules known to regulate DARPP-32 levels include brain-derived neurotrophic factor (BDNF) (Nakao et al., 1995; Ivkovic et al., 1997; Ivkovic and Ehrlich, 1999) and estrogen (E2) (Stroppolo et al., 2004). Cell-specific MSN gene transcription is altered during the pathogenesis and treatment of many neuropsychiatric diseases, including Huntington's disease, Parkinson's disease, drug addiction, affective disorders, attention deficit hyperactivity disorder, and schizophrenia (e.g.Berke and Hyman, 2000; Gerfen, 2000; Luthi-Carter et al., 2000; Svenningsson et al., 2002). Despite the clinical significance of gene regulation in MSNs and the requirement for cell-specific transcription in development of the nervous system, few details are available regarding how the MSN phenotype is specified and maintained at the molecular level. Indeed, little is known about the molecular mechanisms underlying the specification of most central nervous system neuronal sub-types and their maintenance in the adult. Delineation of these pathways presents a major challenge to current molecular neurobiology.
Induction of DARPP-32 by BDNF is mediated via the tropomyosin-related receptor, trkB, and the phosphatidylinositide 3-kinase (PI3K) pathway (Stroppolo et al., 2001), the latter using either Akt and/or cdk5/p35 as effector molecules (Bogush et al., 2007). It has been hypothesized that trkB also mediates induction of DARPP-32 by RA, following its own induction (Wang and Liu, 2005), similar to what occurs in neuroblastoma and hippocampal neurons in vitro (Kaplan et al 1993; Takahashi et al. 1999). In fact, there is no direct evidence in MSNs to support this hypothesis, and there are multiple direct signal transduction pathways via which RA may induce gene expression in MSNs. We used cultured MSNs to investigate the signal transduction pathways mediating DARPP-32 induction by RA, and to determine whether trkB is necessary. We demonstrate that in vitro, trkB is not required for DARPP-32 induction by RA, but as with BDNF, PI3K activity is necessary. However, in the case of RA, PI3K appears to regulate DARPP-32 levels via protein kinase C zeta (PKCζ), a novel pathway, and the effect is independent of Akt and cdk5/p35.
EXPERIMENTAL PROCEDURES
Cell Culture
Timed-mated Swiss-Webster mouse dams were anesthetized with pentobarbital (Day of plug = E0-0.5) and the embryos were removed. The striatum (E15-17) was isolated and the meninges were removed. The tissue was minced with a scalpel blade and incubated in Ca2+/Mg2+-free Hanks’ balanced salt solution (CMF-HBSS) for 8 minutes at 37°C in a clinical rotator (40 rpm). The incubation mixture was replaced with 0.01% trypsin/CMF-HBSS, incubated for 8 minutes, and rinsed twice in Leibovitz's medium (L-15). It was then suspended in Dulbecco's minimum essential medium with 10% fetal calf serum, glucose (6 mg/ml), glutamine (1.4mM), and penicillin/streptomycin (100U/ml). Cells were triturated through a glass bore pipette and plated onto either Lab Tek 8-well slides (75,000 cells/well) for immunocytochemistry or 12-well plates (2 × 106 cells/well) for Western blotting, each previously coated with polymerized polyornithine (0.1 mg/ml in 15 mM borate buffer, pH 8.4) and air-dried. One hour later, the media was replaced with Neurobasal/B27 (GIBCO-Invitrogen, Carlsbad, CA), with glutamine and penicillin/streptomycin, as above. In the absence of anti-mitotic agents, these cultures contain 90-95% neurons, almost all of which are GABA-positive (Ivkovic and Ehrlich, 1999). An extra slide was plated each time and stained with enolase and gamma-aminobutyric acid (GABA) to confirm uniformity of the cultures between experiments. Treatments included roscovitine, K252a, bisindolylmaleimide-I, and LY 294,002 (Sigma-Aldrich, St. Louis, MO); calphostin C (Tocris Bioscience, Ellisville, MO); and myristolated PKCζ pseudosubstrate inhibitor (Calbiochem, San Diego, CA).
Cell Immunocytochemistry
Cultures were immersion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 and processed using the polyclonal anti-DARPP-32 antibody (Chemicon, Temecula, CA) (1:2000) and anti-rabbit secondary antibody (1:400) and the immunoperoxidase/ABC method (Vector Laboratories Elite Vectastain, Burlingame, CA). Neuronal purity was assessed by staining parallel cultures with neuron-specific enolase (Polyscience, 1:5000).
Western blot analysis
For analysis of phosphoproteins, and as a control, their respective non-phosphorylated isoforms, total cellular protein was prepared by lysis in boiling sample buffer [20% glycerol, 62.5 mM Tris-HCl, pH 6.9, 1% sodium dodecyl sulfate, 5% β-mercaptoethanol, and 0.025% bromophenol blue], followed by sonication, 15 min of centrifugation and recovery of the supernatants. An equal volume of lysate was loaded in each well on a gel, run on 10% SDS-polyacrylamide gels, and protein content verified by visualization of total protein by Ponceau Red after transfer to nitrocellulose and/or by demonstration of an equal amount of a “marker” protein, e.g. Akt or actin, in each lane. For analysis of holoproteins, non-phosphorylated, e.g. DARPP-32, cells and tissues were harvested in 137 mM NaCl, 20 mM Tris-HCl, pH 8.0, 1% v/v NP-40, and 10% v/v glycerol, supplemented with 1 X Mini-Complete protease inhibitors (Roche Diagnostics Corporation Indianapolis, IN, USA GmbH). Equal amounts of protein were loaded in each lane following measurement of protein concentrations with the BCA assay (Pierce, Rockford, IL, USA), and verified by visualization of total protein by Ponceau Red after transfer to nitrocellulose and/ or by demonstration of an equal amount of a marker protein. Blots were developed with NEN-DuPont chemiluminescence system (Boston, MA, USA). Densitometric values are obtained using BIOSOFT ScanAnalysis for Apple (Biosoft, Fersuson, MO, USA) and/or by direct UVP Imaging. Effects of treatments were calculated by ANOVA, with Bonferroni post-test. Results were considered significant if p < 0.05. Each lane contained either 25 or 50 μg from a single well from a 12-well plate, seeded with 2 × 106 neurons at the time of plating, and ultimately yielding 75-100 μg of total protein. Antibodies used included DARPP-32 (1:3000) and trkB (1:1000) (Chemicon, Temecula, CA); phosphor-ser473-Akt (1:2500), Akt (1:1000), PKCζ and phospho-PKCζ (1:1000) (Cell Signaling Technology, Beverly, MA); actin (1:3000, Sigma-Aldrich, St. Louis, MO).
Immunoprecipitation
For the analysis pf PKCζ, 200 μg ot total protein from primary neurons were immunoprecipitated o/night with a mouse antibody raised against PKC (Santa Cruz, Santa Cruz, CA), followed by 4 hrs incubation with protein G at 4°C. Samples were then boiled and loaded onto a 7.5% SDS-polyacrylamide gel. Western blot was performed using rabbit antibodies raised against PKCζ or phospho-PKCζ (Cell Signaling, Danvers, MA).
Viral transduction
Viral infections were performed after cells had attached for 24-48 hours. Vector was added in fresh medium, and the medium was changed again 18 hours later. BDNF (10 ng/ml) was added when appropriate after another 24-48 hours to allow time for expression of proteins from the virus. Cells were harvested or fixed from 24-72 hours later, i.e. 72-96 hours following addition of virus, depending on the experiment. The goal with each vector was to obtain maximal level of infection/expression without toxicity, as measured by the Molecular Probes Live/Dead assay (data not shown), used as per the manufacturer's instructions. The dominant negative AKT-CAAX (van Weeren et al. 1998; Bogush et al., 2007) and dnTrkB constructs (Elmariah et al., 2005) (generously provided by Dr. Rita Balice-Gordon, University of Pennsylvania) were transferred into adenoviral expression constructs and produced and amplified in HEK293A cells as per the manufacturer's instructions (ViraPower Adenoviral Expression System, Invitrogen, Carlsbad, CA). DARPP-32 levels were not significantly altered in the presence of control virus (Null virus), and expression of the protein from the virus was confirmed by Western blotting. Multiplicity of infection (MOI) ranged from 2-10:1.
Semiquantitative RT-PCR
Total RNA was isolated from 3×106 cells with the use of an RNeasy Minikit (QIAGEN, Valencia, CA) according to the manufacturer's protocol (RNeasy Mini Handbook). After the final step, mRNA samples were pretreated with DNase (RQ1 RNase-free DNase; Promega, Madison, WI) at 37 °C for 30 min with 1 unit of DNAse for 1μg of RNA in 10 μl of 1x buffer. For each cDNA synthesis reaction, 1μg of total RNA was reverse transcribed with Cells-to-cDNA™ II kit (Ambion, Austin, TX USA). The amount of synthesized cDNA, approximately 50 ng, was then used for simultaneous amplification of DARPP-32 and β-actin in a Gene Amp PCR System 9700 (Applied Biosystems) using Platinum PCR SuperMix (Invitrogen) (N = 3 separate platings). The primers were designed with Primer Express 3.0 Applied Biosystems software based on published cDNA sequences (GenBank accession nos. NM 144828 and NM 007393, respectively). Primer sequences were as follows: DARPP-32 upstream (5’-CACCACCCAAAGTCGAAGAGA-3’, corresponding to nucleotide residues 540–560); DARPP-32 downstream (5’-CGAAGCTCCCCTAACTCATCCT-3’, corresponding to nucleotide residues 676–655); β-actin upstream (5’-CGATGCCCTGAGGCTCTTT-3’, corresponding to nucleotide residues 846–864); β-actin downstream (5’-TGGATGCCACAGGATTCCA-3’, corresponding to nucleotide residues 904–886); The expected lengths of the RT-PCR products were 137 bp for DARPP-32, and 59 bp for β-actin. Primers for each gene were located in different exons to distinguish from amplification of contaminating genomic DNA. The thermocycle parameters were as follows: 3 minutes at 94°C, followed by 20 cycles of 30 seconds at 94°C, 15 seconds at 53°C, and 1 minute at 72°C, and final incubation for 7 minutes at 72°C. Control reactions were performed to verify that no amplification would occur without cDNA. The number of cycles was determined empirically by sampling product between 10 and 40 cycles to select the approximate midpoint of linear amplification for the PCR reactions (not shown). Eight microliters from each PCR amplification were loaded onto 4% agarose gels and the intensity of DNA bands was estimated using UVP BioImaging System (UVP, Inc, Upland, CA) and Lab Works Image Acquisition and Analysis Software V 4.00. The abundance of DARPP-32 PCR fragments was normalized by intensity of the β-actin PCR fragment. Statistical analysis was performed as for Western blotting.
RESULTS
Retinoic acid increases levels of DARPP-32 mRNA and protein in MSNs in vitro independent of trkB
We first sought to determine whether trans-retinoic-acid directly increases DARPP-32 mRNA and protein in our well-characterized culture system. Liu and colleagues (2005) used explants from the embryonic striatum, i.e. the lateral ganglionic eminence, and did not see an induction of DARPP-32 by RA until after 72 hours of treatment. In previous experiments, we used the commercial B27 additive which contains Vitamin A, an obvious substitute for retinoic acid. We therefore examined DARPP-32 levels in the presence of B27 without vitamin A in dissociated primary neuronal cultures. We found that after 24 hours of treatment with RA (10 μM), both DARPP-32 protein and mRNA levels were increased by approximately 500% (Figures 1A,B). In simultaneously plated cultures, brain-derived neurotrophic factor (BDNF) increased DARPP-32 protein by an average of 200% (Figure 1A) and of note, combined treatment with RA and BDNF did not induce DARPP-32 levels above those seen with either treatment alone (Figure 1A). As the cultures were plated in defined medium, we concluded that RA induction of DARPP-32 appeared to be independent of BDNF. We therefore sought to directly test, as hypothesized by others, whether RA induction of DARPP-32 was actually dependent on the receptor for BDNF, trkB, and possibly induction of BDNF. We found that inhibition of receptor tropomyosin-related kinase, including trkB, activity by k252A (Tapley et al., 1992), or more specifically, by transduction of cultures with a kinase-dead dominant negative form of trkB (Elmariah et al., 2005), had no effect on the ability of RA to induce DARPP-32 (Figures 1C,D). As a control, we demonstrated that induction of DARPP-32 by BDNF was attenuated in the presence of DN-TrkB (Figure 1D).
Fig.1. Induction of DARPP-32 by RA does not require TrkB.
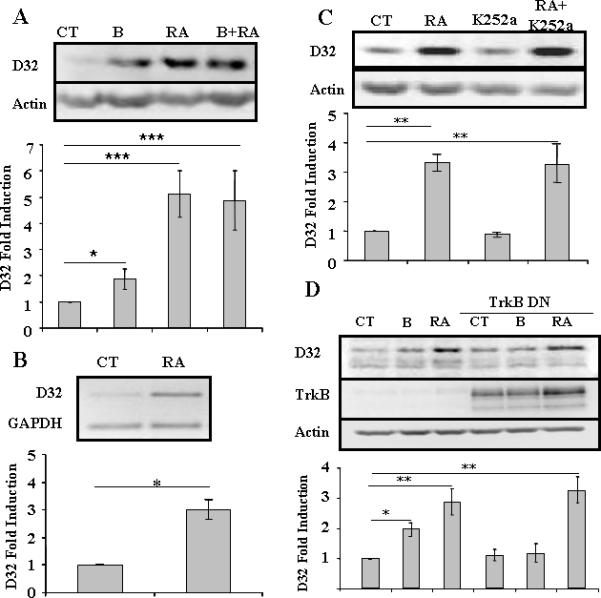
A) Primary striatal neurons were treated with BDNF (10 ng/ml) (B), retinoic acid (10 μM) (RA) or both for 24 hrs. Statistical analysis was performed using one-way ANOVA (p<0.0001) and significant differences were found between groups (*p<0.05 and ***p<0.001). (B) Primary striatal neurons were treated with RA (10 mM) for 24 hrs and levels of DARPP-32 and GAPDH mRNA were determined using semi-quantitative RT-PCR. Statistical analysis was performed using Student's T-test, * p< 0.01. (C) Primary striatal neurons were treated with RA (10 μM), K252a (100 nM) or both for 24 hrs. One-way ANOVA (p<0.001) and significant differences were found between groups (**p<0.01). (D) Primary striatal neurons were treated with RA (10 μM) in absence or presence of dominant negative TrkB (TrkB DN) adenovirus (m.o.i. = 2) for 24 hrs. Statistical analysis was performed using one-way ANOVA (p<0.0001) and significant differences were found between groups (*p<0.05 and **p<0.01) with the Bonferroni post-hoc test. (NOTE: TrkB was visible in wells without viral treatment upon over-exposure of the blot, data not shown).
Retinoic acid induction of DARPP-32 in MSNs in vitro is PI3K-dependent, but Akt- and cdk5/p35-independent
We previously demonstrated that induction of DARPP-32 by BDNF is PI3K-dependent, and downstream effectors include Akt1 and mTOR, and by a parallel pathway, cdk5/p35. Although ERK was phosphorylated following treatment with BDNF, inhibition of the MAP kinase pathway did not significantly reduce induction of DARPP-32 (Stroppolo et al., 2001; Bogush et al., 2007). Treatment of neurons with LY294002, an inhibitor of phosphatidylinositide 3-kinase (PI3K) totally abolished the RA-mediated induction of DARPP-32 protein (Figure 2A). We found that neither Akt nor ERK (Figure 2B) nor p70S6kinase (data not shown) were phosphorylated after 30 or 90 minutes of treatment with RA (10 μM or 1 mM). Moreover, transduction of neurons with a dominant negative Akt1 (Figure 2C) or treatment with roscovitine (Figure 2D), an inhibitor of cdk5, failed to prevent the RA-induced increase in DARPP-32 protein. We therefore concluded that induction of DARPP-32 by RA is PI3K-dependent, but Akt1-, ERK, and cdk5/p35 independent.
Fig.2. DARPP-32 induction by RA is PI3K dependent, but AKT- and cdk5-independent.
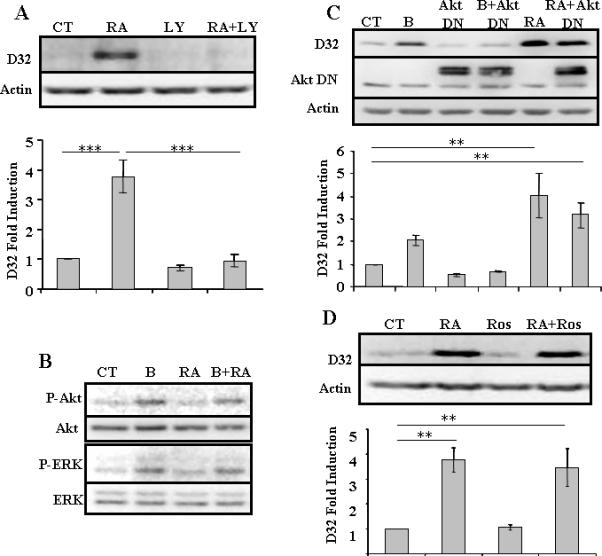
(A) Primary striatal neurons were treated with RA (10 μM), LY 294,002 (LY) (25 μM), or both, for 24 hrs. (B) Primary striatal neurons were treated with BDNF (B) (10 ng/ml), retinoic acid (RA) (10 μM), or both for 30 minutes. Similar results, i.e. absence of phosphorylation of Akt or ERK following treatment with RA, were seen in three different platings. (C) Primary neurons were treated with BDNF (B) (10 ng/ml) or retinoic acid (RA) (10 μM) for 24 hrs, in the absence or presence of Akt Dominant Negative (DN) adenovirus (m.o.i. = 10). Constant levels of endogenous Akt are also apparent. (D) Primary striatal neurons were treated with retinoic acid (RA) (10 μM), roscovitine (Ros) (10 μM) or both for 24 hrs. N=3 for all treatments and statistical analysis was performed using one-way ANOVA (p<0.001) and significant differences were found between groups (**p<0.01) (***p<0.001).
Retinoic acid induction of DARPP-32 in MSNs in vitro is at least partially dependent on protein kinase C (PKC)
Other downstream effectors of PI3K in neuronal and non-neuronal cells include various isoforms of protein kinase C (Kim et al., 2003; Shi et al., 2003; Montiel et al., 2006; Nakanishi et al., 2006). Expression of retinoic acid receptors and PKC isoforms has been positively correlated (Ghanooni et al., 2006), and RA-induced differentiation in multiple cell types, e.g. in promyelocytic leukemia cells and neuritogenesis in neurons, is mediated by PKC (Kambhampati et al., 2003; Miloso et al., 2004). PKC isoforms are comprised of three groups: 1) conventional (α, β, γ), 2) novel (δ, ∈, η, θ, μ), and 3) atypical (ζ, ι, κ). Calphostin C inhibits all isoforms, whereas bisindolylmaleimide I (also known as BIMI, GF 109203X or Go6850) (IC50 = 10-100 nM) and Go6976 inhibit conventional and novel isoforms.
We found that treatment of MSNs in vitro with calphostin C abolished approximately 50% of the induction of DARPP-32 protein (Figure 3A) and mRNA (Figure 3B) by RA. Failure of 100nM BIMI to inhibit any of the induction of DARPP-32 protein by RA indicated that the involved PKC isoform was likely in the atypical class (Figure 3C). Similar results were seen with Go6976 (data not shown). Cross-talk between PI3K and PKCζ has been identified (Martin et al., 2001; Canto et al., 2004), including directly in regulation of transcription by phosphorylation of the transcription factor SP1 (Zhang et al., 2006), We therefore treated the cells with a cell-permeable myristolated PKCζ pseudosubstrate inhibitor (EC50 = 10-20 μM), and treatment at 10 μM), which similarly to calphostinC, resulted in approximately a 50% decrease in the induction of DARPP-32 protein and mRNA by RA (Figures 3D and 3F). Higher concentrations of calphostin C and the myristolated PKCζ pseudosubstrate were toxic. Finally, we verified expression of PKCζ in MSNs in vitro, and phosphorylation following treatment by RA (Figure 3E).
Fig.3. Induction of DARPP-32 by RA is PKC-dependent.
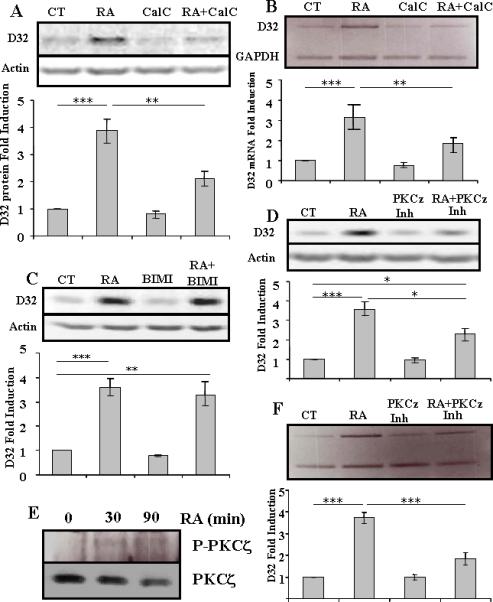
(A) Primary striatal neurons were treated with retinoic acid (RA) (10 μM), calphostin C (CalC) (250nM) or both for 24 hrs and DARPP-32 protein levels were measured. (**p<0.01 and ***p<0.001). (B) Primary striatal neurons were treated with retinoic acid (RA) (10 μM), calphostin C (CalC) (250nM) or both for 24 hrs and DARPP-32 mRNA levels were measured. (**p<0.01 and ***p<0.001). (C) Primary striatal neurons were treated with retinoic acid (RA) (10 μM), bisindolylmaleimide I (Bis) (100nM) or both for 24 hrs and DARPP-32 protein levels were measured (**p<0.01 and ***p<0.001). (D) Primary striatal neurons were treated with retinoic acid (RA), myristolated pseudosubstrate PKCζ Inhibitor (PKCζ Inh) or both for 24 hrs, and DARPP-32 protein levels were measured. (*p<0.05 and ***p<0.001). N =3 for all treatments, and statistical analysis was performed using one-way ANOVA and Bonferroni post-hoc test. (E) Primary striatal neurons were treated with retinoic acid (RA) (10 μM) for 30 or 90 minutes and proteins were immunoprecipitated with anti-PKCζ and blotted for PKCζ and phospho-PKCζ. Phospho-PKCζ is detectable only after treatment with retinoic acid, but levels of total PKCζ are not increased. Representative of 3 experiments. (F) Primary striatal neurons were treated with retinoic acid (RA), myristolated pseudosubstrate PKCζ Inhibitor (PKCζ Inh) or both for 8 hrs, and DARPP-32 mRNA, relative to actin, levels were measured. Representative of three experiments. Statistical analysis was performed using one-way ANOVA (p<0.0001) and significant differences were found between groups (***p<0.001)
DISCUSSION
In this study, we identify a novel pathway in medium size spiny neurons in vitro in which PKCζ serves as a downstream effector of retinoic acid in the induction of DARPP-32. Importantly, we also demonstrate that BDNF and trkB are not required for the direct action of RA on MSNs in vitro, and that the signal transduction pathways utilized by RA for the induction of DARPP-32 differ from those previously identified for BDNF except for the common involvement of PI3K. This study does not specifically address the source of retinoids in vivo, which is likely radial glia (Toresson et al., 1999). This would be consistent with the fact that the molecules induced by RA are by-and-large not detectable in vivo until the medium spiny neurons are post-migratory (Gustafson et al., 1992).
The use of different pathways by these two epigenetic factors may explain how DARPP-32 levels ultimately normalize in the BDNF-null mouse (Ivkovic et al.,1997), and for the apparently normal expression of DARPP-32 in RAR-receptor-null mice (Ehrlich, unpublished observations). PKC has also been implicated in the induction of MSN maturation by adenosine and its receptors (Canals et al., 2005), and may therefore represent another molecule on which multiple epigenetic maturation signals converge. Substantiating the potential relevance of the PKC pathway, previous studies have demonstrated the expression of all major isoforms of PKC in MSNs in vivo (Tanaka and Saito, 1992; Tanaka and Nishizuka, 1994; Battaini, 2001)
Inhibitors of PKCζ only abolished approximately 50% of the induction of DARPP-32 by RA, whereas inhibition of PI3K abolished 100% of the induction. It is possible that higher concentrations of the PKCζ inhibitors, had they been tolerated by the neurons, would have abolished 100% of the induction. It is also possible, of course, that other downstream effectors of PI3K are involved, and studies to identify these effectors are currently in progress.
Retinoic acid induces DARPP-32 mRNA and protein in the absence of BDNF and trkB, implying a direct effect on gene transcription. Importantly, therefore, there was a significant induction after 24 hours, contrary to what was previously reported in LGE explants, in which induction only occurred after 72 hours in vitro (Liao et al., 2005b). Prior experiments utilizing dissociated neurons from the LGE did not examine induction of DARPP-32 by RA until 5DIV (Toresson et al., 1999). It has previously been noted that there are at least three retinoic acid response elements (RARE) in the DARPP-32 gene (Liao and Liu, 2005), and some are included in the DARPP-32 genomic elements required for transgenic expression of cre-recombinase in the medium size spiny neurons (Bogush et al., 2005). It remains to be determined whether these genomic cis-elements are necessary or sufficient for DARPP-32 striatal gene expression.
The pathway of retinoic acid regulation of gene expression in the medium size spiny neuron is potentially relevant to several diseases and/or treatments which involve the striatum. Retinoic acid is of course a well-recognized morphogen, but evidence has mounted that it continues to play a significant role in brain function in the adult (reviewed in Lane and Bailey, 2005; Maden, 2007). Thus, dysfunction of RA pathways has been implicated in multiple neuropsychiatric diseases, including schizophrenia and depression. Genetic deletion of retinoic acid receptors leads to a movement disorder in mice (Krezel et al., 1998), and vitamin A deficiency leads to motor impairment in the adult rat (Carta et al., 2006). Retinoid signaling is also implicated in several neurodegenerative diseases (reviewed in Maden, 2007), including amyotrophic lateral sclerosis (Jokic et al., 2007) and Huntington's disease. Htt, the product of the gene mutated in Huntington's disease (HD), may function as a transcriptional coactivator of nuclear hormone receptors, including thyroid hormone and retinoic acid receptors. Expansion of the polyglutamine tract has been shown to enhance the ability of the nuclear corepressor transcription factor to repress thyroid hormone receptor-mediated transcription in the absence of ligand (Yohrling et al., 2003), and RARE-mediated transcription is dysregulated, perhaps due to sequestration of cofactors, in some cell models of HD (Sugars et al., 2004). Several of the genes that are down-regulated in human HD and in several mouse models of HD, are regulated by RA, including DARPP-32 (e.g. Bibb et al., 2000) and the D2 receptor (Valdenaire et al., 1998; Molotkova et al., 2007). It is conceivable therefore that retinoids could play a role in reversing transcriptional deficits in HD. In addition, retinoids have been implicated in the molecular alterations which follow chronic administration of neuroleptics (Ethier et al., 2004), and this pathway should therefore be further analyzed in regard to more specific therapeutic agents.
Acknowledgements
This work was supported by NIH R01NS045942 (to M.E.E.).
Abbreviations
- 5 DIV
days in vitro
- BDNF
brain-derived neurotrophic factor
- cdk5
cyclin-dependent kinase
- CMF-HBSS
Ca2+/Mg2+-free Hanks’ balanced salt solution
- DARPP-32
dopamine and cyclic AMP-regulated phosphoprotein, 32 kDa
- E2
estrogen
- ERK
extracellular signal-regulated kinase
- GABA
gamma-aminobutyric acid
- HD
Huntington disease
- MOI
multiplicity of infection
- MSN
medium size spiny neuron
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositide 3-kinase
- RA
retinoic acid
- trkB
tropomyosin-related kinase B
REFERENCES
- Anderson SA, Qiu M, Bulfone A, et al. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Battaini F. Protein kinase isoforms as therapeutic targets in nervous system disease states. Pharmacol. Res. 2001;44:353–361. doi: 10.1006/phrs.2001.0893. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, et al. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc. Natl Acad Sci. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. Bibb et al., 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogush AI, McCarthy LE, Tian C, et al. DARPP-32 genomic fragments drive Cre expression in postnatal striatum. Genesis. 2005;42:37–46. doi: 10.1002/gene.20118. [DOI] [PubMed] [Google Scholar]
- Bogush AI, Pedrini S, Pelta-Heller J, et al. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem. 2007;282:7352–7359. doi: 10.1074/jbc.M606508200. [DOI] [PubMed] [Google Scholar]
- Canals M, Angulo E, Casado V, et al. Molecular mechanisms involved in the adenosine A and A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J Neurochem. 2005;92:337–348. doi: 10.1111/j.1471-4159.2004.02856.x. [DOI] [PubMed] [Google Scholar]
- Canto C, Suarez E, Lizcano JM, et al. Neuregulin signaling on glucose tansport in muscle cells. J Biol. Chem. 2004;279:12260–12268. doi: 10.1074/jbc.M308554200. [DOI] [PubMed] [Google Scholar]
- Carta M, Stncampiano R, Tronci E, et al. Vitamin A deficiency induces motor impairments and striatal cholinergic dysfunction in rats. Neuroscience. 2006;139:1163–1172. doi: 10.1016/j.neuroscience.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Oh EJ, Hughes EG, et al. Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J Neurosci. 2005;25:3638–3650. doi: 10.1523/JNEUROSCI.3980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier I, Beaudry G, St.Hiliare M, et al. The transcription factor NGFI-B (Nur77) and retinoids play a critical role in acute neuroleptic-induced extrapyramidal effect and striatal neuropeptide gene expression. Neuropsychopharmacology. 2004;29:335–346. doi: 10.1038/sj.npp.1300318. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Ghanooni R, Decaestecker C, Simon P, et al. Characterization of patterns of expression of protein kinase C-alpha, -delta, -eta, -gamma and -zeta and their correlations to p53, galectin-3, the retinoic acid receptor-beta and the macrophage migration inhibitory factor (MIF) in human cholesteatomas. Hear Res. 2006;214:7–16. doi: 10.1016/j.heares.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Ehrlich ME, Trivedi P, et al. Developmental regulation of phosphoprotein gene expression in the caudate-putamen of rat: an in situ hybridization study. Neuroscience. 1992;51:65–75. doi: 10.1016/0306-4522(92)90471-d. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Polonskaia O, Farinas I, et al. Brain-derived neurotrophic factor regulates maturation of the DARPP-32 phenotype in striatal medium spiny neurons: studies in vivo and in vitro. Neuroscience. 1997;79:509–516. doi: 10.1016/s0306-4522(96)00684-7. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich ME. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J. Neurosci. 1999;18:5409–5419. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokic N, Ling YY, Ward RE, et al. Retinoid receptors in chronic degeneration of the spinal cord: observation in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2007;103:1821–1833. doi: 10.1111/j.1471-4159.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Li Y, Verma A, et al. Activation of protein kinase C delta by all-trans-retinoic acid. J Biol. Chem. 2003;278:32544–32551. doi: 10.1074/jbc.M301523200. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Matsumoto K, Lucarelli E, et al. Induction of TkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron. 1993;11:321–331. doi: 10.1016/0896-6273(93)90187-v. [DOI] [PubMed] [Google Scholar]
- Kim YB, Kotani K, Ciaraldi TP, et al. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- Krezel W, Ghyselinck N, Samad TA, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Liao WL, Tsai HC, Wu CY, et al. Differential expression of RARbeta isoforms in the mouse striatum during development: a gradient of RARbeta2 expression along the rostrocaudal axis. Dev. Dyn. 2005a;233:584–594. doi: 10.1002/dvdy.20344. [DOI] [PubMed] [Google Scholar]
- Liao WL, Wang HF, Tsai HC, et al. Retinoid signaling competence and RARbeta-mediated gene regulation in the developing mammalian telencephalon. Dev. Dyn. 2005b;232:887–900. doi: 10.1002/dvdy.20281. [DOI] [PubMed] [Google Scholar]
- Liao W-L, Liu F-C. RARbetA isoform-specific regulation of DARPP-32 gene expression: an ectopic expression study in the developing rat telencephalon. Eur. J Neurosci. 2005;21:3262–3268. doi: 10.1111/j.1460-9568.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Strand A, Peters NL, et al. Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum. Mol. Genet. 2000;22:1259–1271. doi: 10.1093/hmg/9.9.1259. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc. Natl. Acad. Sci. USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development,regeneration and maintenance of the nervous system. Nature Rev. Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Martin AG, San-Antonio B, Fresno M. Regulation of nuclear factor kappa B transactivation. Implication of phosphatidylinositol3-kinase and protein kinase C zeta in c-Rel activation by tumor necrosis factor alpha. J Biol. Chem. 2001;276:15840–15849. doi: 10.1074/jbc.M011313200. [DOI] [PubMed] [Google Scholar]
- Miloso M, Villa D, Crimi M, et al. Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J Neurosci. Res. 2004;75:241–252. doi: 10.1002/jnr.10848. [DOI] [PubMed] [Google Scholar]
- Molotkova N, Molotkov S, Duester G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventicular zone. Dev. Biol. 2007;303:601–610. doi: 10.1016/j.ydbio.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel M, de la Blanca EP, Jimenez E. P2Y receptors activate MAPK/ERK through a pathway involving PI3K/PDK1/PKC-zeta in human vein endothelial cells. Cell Physiol. Biochem. 2006;18:123–134. doi: 10.1159/000095180. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Aono S, Hirano K, et al. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J Biol. Chem. 2006;281:24970–24978. doi: 10.1074/jbc.M601498200. [DOI] [PubMed] [Google Scholar]
- Nakao N, Brundin P, Funa K, et al. Trophic and protective actions of brain-derived neurotrophic factor on striatal DARPP-32-containing neurons in vitro. Brain Res. Dev. Brain Res. 1995;90:92–101. doi: 10.1016/0165-3806(96)83489-4. [DOI] [PubMed] [Google Scholar]
- Samad TA, Krezel W, Chambon P, et al. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family. Proc. Natl. Acad. Sci. USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Barnes S, Coca-Prados M, et al. Protein tyrosine kinase and protein phosphatase signaling pathways regulate volume-sensitive chloride currents in a nonpigmented ciliary epithelial cell line. Invest. Ophthalmol. Vis. Sci. 2003;43:1525–1532. [PubMed] [Google Scholar]
- Stroppolo A, Tian C, Guinea B, et al. 17beta-Estradiol promotes striatal medium size spiny neuronal maturation in vitro. Neuroendocrinology. 2004;79:259–267. doi: 10.1159/000079320. [DOI] [PubMed] [Google Scholar]
- Stroppolo A, Guinea B, Tian C, et al. Role of phosphatidylinositide 3-kinase in brain-derived neurotrophic factor-induced DARPP-32 expression in medium size spiny neurons in vitro. J Neurochem. 2001;79:1027–1032. doi: 10.1046/j.1471-4159.2001.00651.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, et al. DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci U S A. 2002;99:3188–3193. doi: 10.1073/pnas.052712699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Palmer T, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 1999;38:65–81. [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu. Rev. Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Saito N. Localization of subspecies of protein kinase C in the mammalian central nervous system. Neurochem. Int. 1992;21:499–512. doi: 10.1016/0197-0186(92)90081-2. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Babacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes an neurotrohin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Toresson H, Mata de Urquize A, Fagerstrom C, et al. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal differentiation. Development. 1999;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Maus-Moatti M, Vincent J-D, et al. Retinoic acid regulates the developmental expression dopamine D2 receptor in rat striatal primary cultues. J. Neurochem. 1998 doi: 10.1046/j.1471-4159.1998.71030929.x. [DOI] [PubMed] [Google Scholar]
- van Weeren PC, de Bruyn KM, de Vries-Smits AM, et al. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Campbell The homeobox gene Gsh2 is required fo retinoid production in the embryonic mouse telencephalon. Development. 2004;131:4013–4020. doi: 10.1242/dev.01272. [DOI] [PubMed] [Google Scholar]
- Wang H-F, Liu FC. Regulation of multiple dopamine signal transduction molecules by retinoids in the developing striatum. Neuroscience. 2005;134:97–105. doi: 10.1016/j.neuroscience.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Yohrling GJ, Farrell LA, Hollenberg AN, et al. Mutant huntingtin increases nuclear corepressor function and enhances ligand-dependent nuclear hormone receptor activation. Mol. Cell. Neurosci. 2003;23:28–38. doi: 10.1016/s1044-7431(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Simon A, Giacobini NM, et al. Localization of cellular retinoid-binding proteins suggests specific roles for retinoids in the adult central nervous system. Neuroscience. 1994;62:899–918. doi: 10.1016/0306-4522(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lia M, Dufau MI. Phosphatidylinositol 3-kinase/protein kinase Cz-induced phosphorylation of Sp1 and p107 repressor release have a critical role in histone deacetylase inhibitor-mediated depression of transcription of the luteinizing hormone receptor gene. Mol. Cell. Biol. 2006;26:6748–6761. doi: 10.1128/MCB.00560-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


