Abstract
Objective
The postpartum period is a window of opportunity for diabetes prevention in women with recent gestational diabetes (GDM), but recruitment for clinical trials during this period of life is a major challenge.
Methods
We adapted a social-ecologic model to develop a multi-level recruitment strategy at the macro (high or institutional level), meso (mid or provider level), and micro (individual) levels. Our goal was to recruit 100 women with recent GDM into the Balance after Baby randomized controlled trial over a 17-month period. Participants were asked to attend three in-person study visits at 6 weeks, 6 months, and 12 months postpartum. They were randomized into a control arm or a web-based intervention arm at the end of the baseline visit at six weeks postpartum. At the end of the recruitment period, we compared population characteristics of our enrolled subjects to the entire population of women with GDM delivering at Brigham and Women's Hospital (BWH).
Results
We successfully recruited 107 of 156 (69%) women assessed for eligibility, with the majority (92) recruited during pregnancy at a mean 30 (SD± 5) weeks of gestation, and 15 recruited postpartum, at a mean 2 (SD±3) weeks postpartum. 78 subjects attended the initial baseline visit, and 75 subjects were randomized into the trial at a mean 7 (SD±2) weeks postpartum. The recruited subjects were similar in age and race/ethnicity to the total population of 538 GDM deliveries at BWH over the 17-month recruitment period.
Conclusions
Our multilevel approach allowed us to successfully meet our recruitment goal and recruit a representative sample of women with recent GDM. We believe that our most successful strategies included using a dedicated in-person recruiter, integrating recruitment into clinical flow, allowing for flexibility in recruitment, minimizing barriers to participation, and using an opt-out strategy with providers. Although the majority of women were recruited while pregnant, women recruited in the early postpartum period were more likely to present for the first study visit. Given the increased challenges of recruiting postpartum women with GDM into research studies, we believe our findings will be useful to other investigators seeking to study this population.
Keywords: women, postpartum, recruitment, gestational diabetes
Background
Gestational mellitus diabetes (GDM), glucose intolerance beginning or first detected after the first trimester of pregnancy [1], complicates 3-7% of pregnancies in the United States [1, 2] with prevalence on the rise as rates of overweight and obesity continue to increase [3, 4]. Women with GDM are at high risk for future type 2 diabetes (T2DM), with 30-70% of women developing T2DM within 5-10 years of an affected pregnancy [5]. Given this risk, the postpartum period provides a window of opportunity for diabetes prevention in women with recent GDM [6, 7]. However, few studies have enrolled women with recent GDM for interventions in the postpartum period [8]. This may be due, at least in part, to difficulties recruiting this cohort.
Failure to meet recruitment goals is a major reason that clinical trials are not completed on time [9, 10]. Potential subjects report that barriers to their participation in clinical trials include time and cost constraints [11-12] and general distrust of the medical field [13]. Investigators report that time constraints and lack of support staff interfere with their recruitment efforts [14]. Postpartum women face additional barriers to study participation, including time constraints related to childcare, challenges adapting to life with a newborn, lack of energy and sleep, and time constraints related to returning to work [11, 15-19]. Many studies report challenges recruiting and studying women during pregnancy and the postpartum period, including a lack of response from mailed invitations, difficulty using providers as recruiters in clinic, and poor retention. The published recruitment rates from other studies range from 13% to 81% [20-27], with the more successful studies employing in-person recruitment techniques, integrating recruitment into clinic flow, and allowing flexibility in the recruitment process. Studies requiring less participant burden were also more likely to have higher recruitment yield [24] than those studies requiring increased participant burden [28-29]. Few interventions have been studied among postpartum women with recent GDM. In two on-going trials of postpartum interventions for women with prior GDM, investigators reported challenges meeting recruitment goals [25-27]. Given the potential difficulties recruiting for a postpartum intervention trial among women with recent GDM, we developed a multilevel recruitment strategy for the Balance after Baby trial [30] by adapting a social-ecologic model to incorporate determinants of recruitment at the macro (high or institutional level), meso (mid or provider level), and micro (individual) levels [31, 32]. In this paper we describe our approach and which components appear to be most successful, as well as examine the representativeness of our recruited sample.
Methods
Description of trial
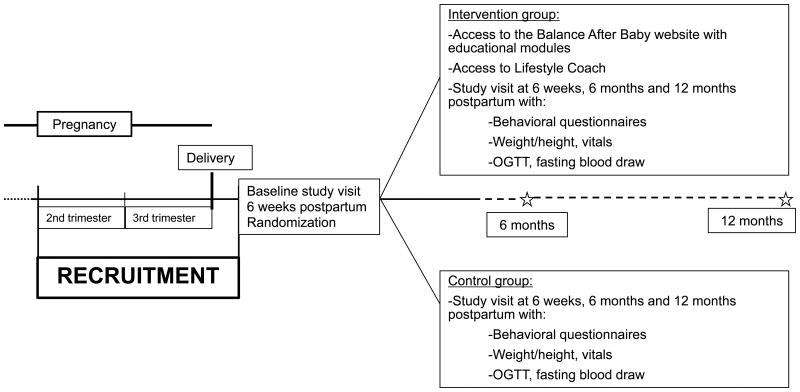
The Balance after Baby study was a year-long randomized controlled lifestyle intervention trial for postpartum women with recent GDM (Trial number: NCT01158131). The methods and results have been previously published [30]. Women received the tip sheet “It's Never Too Early to Prevent Diabetes” at the time of enrollment. The lifestyle program was web-based to address potential barriers to participation cited by women with prior GDM [18]. Subjects were asked to come fasting for three in-person study visits over the first postpartum year, with each visit lasting 3-4 hours. Each study visit included fasting blood measurements and an oral glucose tolerance test (OGTT). The subject burden included either two venipunctures for blood draws or placement of an indwelling catheter for the blood draws, as well as measurements of weight, height, and blood pressure, and completion of behavioral questionnaires (Figure 1). Potential benefits from study participation included increased surveillance for T2DM, and potential health benefits as a result of making lifestyle changes [33] if randomized to the Balance after Baby program.
Figure 1. Overview of the Balance after Baby randomized controlled trial.
Recruitment took place between May 2010 and September 2011. We recruited women who were pregnant or within 6 weeks of delivery. To be eligible, participants had to be diagnosed with GDM in the second or third trimester of their most recent pregnancy, be 18-45 years old, have a self-reported pre-pregnancy body mass index (BMI) 18-50 kg/m2, and not have type 1 or T2DM. Subjects had to be planning to deliver at BWH or have recently delivered there, and they could not be taking prescription medications known to affect weight, be planning to join a weight loss program, or have a history of gastric bypass. Because the intervention was only available in English, subjects had to be able to understand and read English at an 8th grade level. Women were scheduled for the baseline study visit at 6 weeks postpartum if they continued to meet postpartum eligibility criteria, including delivery of an infant at ≥32 weeks gestation, being at or above their pre-pregnancy weight at the time of delivery, and a postpartum BMI ≥24 (≥22 kg/m2 for Asian participants). The postpartum BMI requirements were the same as the eligibility requirements for the Diabetes Prevention Program, which use a lower BMI cutoff for Asians given their greater susceptibility for diabetes at a lower BMI [33]. If a woman randomized into the web-based intervention did not have a computer and/or access to the internet, we provided a laptop computer (Dell Mini) and/or internet service to ensure that everyone had the opportunity to participate. Women diagnosed with T2DM during the initial baseline study visit were not eligible for randomization. The Institutional Review Board at Brigham and Women's Hospital (BWH) approved the study and all subjects gave written informed consent.
Recruitment strategy
Our multilevel recruitment strategy was adapted from a social-ecologic model to address recruitment at different levels, including activities at the macro (high or institutional level), meso (mid or provider level), and micro (individual) levels [31, 32].
Macro-Level
Given that we were recruiting women to begin an intervention at six weeks postpartum, we focused recruitment on women planning to deliver or delivering at the BWH in Boston, Massachusetts, a large hospital with over 7,000 deliveries annually. The BWH serves a racially, ethnically and socio-economically diverse population, with approximately 16% of deliveries by African-American women, 14% by Hispanic women, and 3% by Asian women. About 5% of these pregnancies are complicated by GDM, with highest rates among Asian women (11%), compared to Hispanic (6%), African-American (5%), and White non-Hispanic (4%) women. We planned to recruit and consent 100 participants to allow for an estimated 25-30% to either not attend the randomization visit or not qualify for randomization, leaving 70-75 participants who could be randomized into the study.
To recruit pregnant women with recently diagnosed GDM for the postpartum study, we targeted two Diabetes in Pregnancy clinics: one located in the main hospital at BWH and one in a satellite location. Women diagnosed with GDM at BWH community health centers and BWH-affiliated private groups are referred to these clinics. We also identified inpatient settings where we could encounter women with GDM, including the antenatal unit for pregnant women with complications requiring hospitalization (15 beds), and three floors (75 beds) where postpartum women are hospitalized after delivery. We did not recruit on the labor and delivery unit so as to not interfere with clinical care of women giving birth.
Meso-level
Prior to the scheduled start of recruitment, the principal investigator presented Obstetrics and Gynecology Grand Rounds describing the study rationale, protocol, and eligibility criteria. Approximately 50 obstetricians and midwives attended, in addition to residents in Obstetrics and Gynecology and nurses. A study team obstetrician gave separate presentations for midwives, obstetrical residents, inpatient nursing staff and the off-site Diabetes in Pregnancy program. To reach staff on the inpatient service, we provided informational handouts in the nursing break rooms on each floor. We also gave presentations to clinical staff at the two BWH Diabetes in Pregnancy clinics explaining the purpose of the study and discussing ways to integrate recruitment into clinical flow. Additionally, we sent an email to the 151 obstetricians and midwives who cared for women delivering at BWH describing the study and requesting permission to discuss the study with their patients. In this email, providers were told that if they did not want study staff to approach their patients they would need to opt-out by notifying the study team. This eliminated the need to follow-up with individual providers as would have been required for an opt-in strategy. During the recruitment period, we met with clinic staff at the BWH Diabetes in Pregnancy clinics every 6 months to update them about the study, to thank them for help with study recruitment, and to discuss any issues with clinic flow. Although they were not included in initial recruitment efforts, providers at an additional Diabetes in Pregnancy program at an affiliated institution chose to refer their patients for recruitment after hearing about the study.
We posted flyers and left brochures describing the study in waiting rooms, hallways, and exam rooms at the BWH Diabetes in Pregnancy clinics, as well as in the waiting rooms on each of the inpatient floors. During the 17-month recruitment period, a recruiter went twice per week to the BWH Diabetes in Pregnancy clinic sessions, requiring approximately 4-8 hours weekly, depending on the number of patients scheduled. A recruiter also visited the inpatient antepartum and postpartum units 2-3 times per week for about an hour each time, to identify eligible subjects and inform them about the study, as well as to remind previously enrolled women about the study and provide them with a small gift (picture frame). Due to staffing limitations, we did not recruit from these locations during evenings, weekends, or holidays, and we sent gifts by mail if women were not contacted in the hospital.
At the BWH Diabetes in Pregnancy clinics, we worked with providers to design the recruitment process to not interfere with clinic flow. When possible, clinic staff pre-identified potential participants and would also notify a recruiter when they encountered a patient interested in hearing about the study. Women were approached in the waiting room or in the exam room and asked if they were interested in learning about a postpartum study for women with prior GDM. We asked clinic staff to interrupt the recruitment process whenever the patient was needed for clinical care (i.e. to take her vital signs, see her provider, or get an ultrasound). Similarly, the clinic staff would notify the recruiter about breaks in clinical care that could allow time for recruitment. On the inpatient antepartum and postpartum units, nurses would notify the recruiter if a woman with GDM were present and available to be approached about the study.
Micro-level
At the subject level, we designed the recruitment process to ensure that we could maintain privacy and offer potential subjects multiple opportunities to give consent. Screening and consent took place privately, and if an interested patient became unavailable during the screening process due to clinical care, the recruiter would obtain contact information and attempt to complete screening within one week. In addition, subjects who were unsure whether or not they wanted to participate were provided the option to be contacted at a later time, so they could have more time to make a decision, and to discuss the study with their family if they wished. Since a recruiter was present at the BWH Diabetes in Pregnancy clinics every week, the majority of patients had more than one opportunity to learn about the study if they desired. On the inpatient units, the recruiter would approach a potential subject only if she were not otherwise occupied, including if sleeping, breastfeeding, or with visitors. If so occupied, the recruiter would leave information about the study and return at a later time. Through these approaches, women could be recruited into the study during the second or third trimester of their GDM-affected pregnancy, or during the early postpartum period following delivery.
During the recruitment process, we informed women about features of the study designed to decrease potential barriers to participation. Women could bring babies and/or other children with them to study visits if they needed to, and we provided financial compensation to allow subjects to pay for childcare and to cover transportation costs. We provided valet parking to facilitate getting out of a car with an infant and/or other children, and also we conducted study visits on weekends if necessary. We explained that women randomly assigned to the intervention arm would be using a web-based program that could be accessed any time of day or night, and could communicate with a lifestyle coach by phone or email.
Our goal was to consent 100 subjects over a 17-month period, including a ramping up month, where we intentionally recruited no more than one subject per week, and a ramping down month, where we only enrolled subjects who could potentially deliver by the end of the study. We set a goal of recruiting an average of 1-2 subjects per week during the core recruitment period. Recruiters entered data into a recruitment log during each session, detailing the women approached, whether or not they were interested in hearing about the study, and if they wanted to discuss the study again at a later date. The research group met weekly with the recruiters and discussed how many subjects were recruited that week and any issues that came up during the process. At each meeting we reviewed our progress, discussed whether or not we were on target with our goals, and identified strategies to improve our recruitment strategy when appropriate.
Statistical analysis
At the completion of the recruitment period, we compared our enrolled subjects with our recruitment goals to assess the efficacy of our recruitment strategy. To assess the representativeness of our study population, we compared our study population to all women with GDM who delivered at BWH during the recruitment period. We used the Partners Research Patient Data Registry (RPDR), a HIPAA compliant centralized clinical data registry of medical records, to identify women delivering with ICD-9 codes for GDM deliveries (648.80, 648.81 and 648.83). We then compared the age and race of our study population with all women with GDM who delivered over the study period using t-tests, chi-squared tests, and Fisher's exact tests.
Results
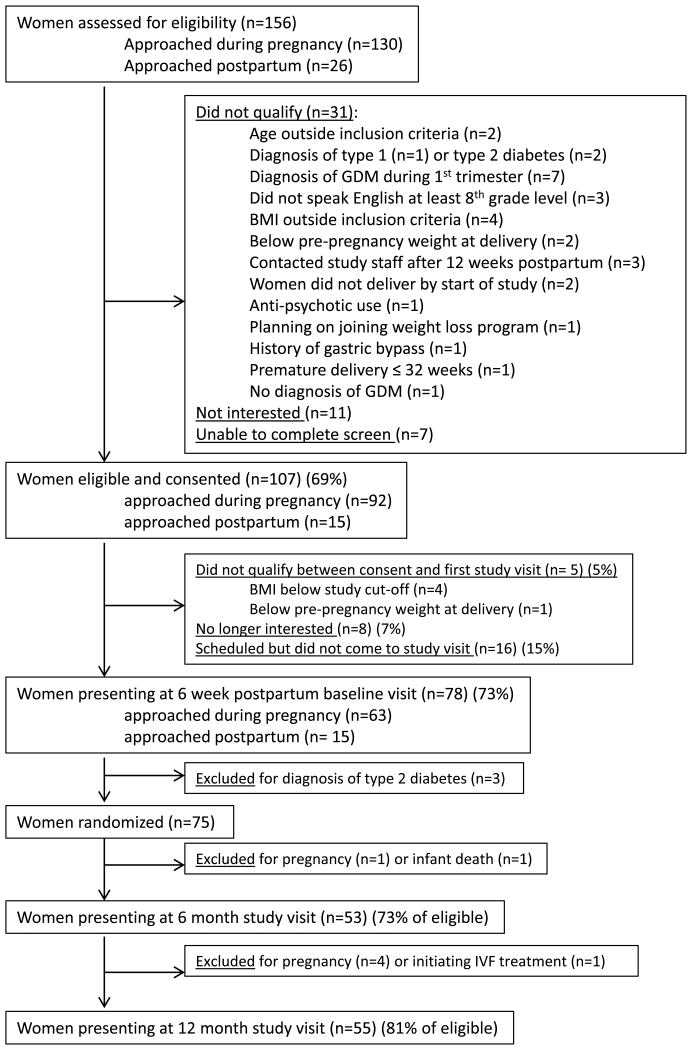
None of the BWH obstetric providers opted out of allowing us to approach their patients. We assessed 156 women for eligibility during the recruitment period. Of these, 107 were eligible after screening, still interested in participating, and signed the consent form (69% of the assessed women). Of the 107 consented subjects, most (78%) were recruited through the BWH Diabetes in Pregnancy clinics, while 16% were recruited on the inpatient units and 6% were referred by outside providers. (Table 1) Only one woman stated she learned about the study from a study flyer. The majority (92) were recruited during pregnancy at a mean 30 (SD±5) weeks of gestation, and 15 women were recruited postpartum, at a mean 2 (SD±3) weeks postpartum. Among the 107 consented subjects, 102 met the 6-week postpartum inclusion criteria and were still eligible to participate. Twenty-four participants did not attend the baseline visit, of whom 8 (7%) were no longer interested and 16 (15%) did not show up for their visit. We attempted to contact these 16 but were unable to reach them. Among those attending the baseline visit, all of the 15 subjects who were recruited while postpartum presented for the baseline visit, while 63/87 (72%) of the eligible participants recruited during pregnancy attended. Seventy-eight subjects attended the initial baseline visit, and 75 subjects were randomized into the trial at a mean 7 (SD±2) weeks postpartum (Figure 2) (three patients met criteria for type 2 diabetes and were excluded). Among those who were eligible, 53/73 (73%) presented to the 6 month visit and 55/68 (81%) presented to the 12 month study visit. (Figure 2).
Table 1. Clinical characteristics of subjects recruited for the Balance after Baby study (n=107).
| Overall population (n=107) | Attended first study visit (n=78) | Did not attend first study visit (n=24) | Did not meet inclusion criteria at time of first visit (n= 5) | p-value (between groups) | |
|---|---|---|---|---|---|
|
| |||||
| Age, years (SD) | 33 (5) | 33 (5) | 32 (6) | 33 (4) | 0.62 |
|
| |||||
| Race (%) | 0.27 | ||||
|
| |||||
| White non-Hispanic | 38 (36%) | 32 (41%) | 6 (25%) | 1 (20%) | |
| Black/African-American | 28 (26%) | 21 (27%) | 6 (25%) | 1 (20%) | |
| Asian | 17 (16%) | 10 (13%) | 5 (21%) | 2 (40%) | |
| Hispanic | 24 (22%) | 15 (19%) | 7 (29%) | 1 (20%) | |
|
| |||||
| Pre-pregnancy BMI, kg/m2 (mean, SD) | 30 (6) | 30 (6%) | 30 (7) | 25 (7) | 0.1 |
|
| |||||
| 19-24.9 (%) | 25 (23%) | 16 (20%) | 5 (21%) | 4 (80%) | |
| 25-29.9 (%) | 36 (34%) | 28 (36%) | 8 (33%) | 0 (0) | |
| 30-34.9 (%) | 24 (22%) | 21 (27%) | 3 (13%) | 0 (0) | |
| >35 (%) | 22 (21%) | 13 (17%) | 8 (33%) | 1 (20%) | |
|
| |||||
| Location of recruitment (%) | 0.73 | ||||
|
| |||||
| DPC- satellite location | 35 (33%) | 26 (33%) | 7 (29%) | 2 (40%) | |
| DPC- main hospital | 48 (45%) | 33 (42%) | 12 (50%) | 3 (60%) | |
| Floors of the main hospital | 17 (16%) | 12 (15%) | 5 (21%) | 0 (0) | |
| Outside providers | 7 (7%) | 7 (9%) | 0 (0) | 0 (0) | |
DPC: Diabetes in Pregnancy Clinic
Figure 2. Flow of participants in the Balance after Baby randomized controlled trial.
The clinical characteristics of the 107 recruited subjects and the 78 subjects who attended the baseline visit are presented in Table 1. The mean age of recruited population was 33 (SD±5) years, with 36% describing themselves as White non-Hispanic, 26% as Black/African-American, 16% as Asian, and 22% as Hispanic. The median self-reported pre-pregnancy BMI was 30 (SD±6) kg/m2. Consented women who came to the randomization visit did not differ significantly from those who did not come to the randomization visit. Since women had to have a BMI ≥24 kg/m2 to be eligible at 6 weeks postpartum, women with lower pre-pregnancy BMIs were more likely to be ineligible at 6 weeks. We successfully recruited 1-2 women per week during the 17-month active recruitment period. In Table 2 we compare our study population with the 538 patients with GDM age 18-45 years delivering at the BWH during the recruitment period. There were no significant differences in age and race between recruited subjects and the total population of GDM deliveries.
Table 2. Clinical characteristics of subjects recruited for the Balance after Baby study delivering at the BWH compared to the entire population of women age 18-45 with GDM who delivered at the BWH May 2010 - September 2011 (17 months).
| Balance after Baby population who gave birth at the BWH (n=100)ƚ | Total GDM deliveries at BWH during recruitment period (n= 538) | p-value | |
|---|---|---|---|
|
| |||
| Age (years), n (%) | 0.54 | ||
|
| |||
| 18-24 | 7 (7%) | 26 (5%) | |
| 25-29 | 16 (16%) | 84 (16%) | |
| 30-34 | 37 (37%) | 200 (37%) | |
| 35-39 | 28 (28%) | 165 (31%) | |
| 40-45 | 12 (12%) | 63 (12%) | |
|
| |||
| Race, n (%) | 0.16 | ||
|
| |||
| White non-Hispanic | 34 (34%) | 209 (39%) | |
| Black/African-American | 27 (27%) | 102 (19%) | |
| Asian | 15 (15%) | 116 (22%) | |
| Hispanic | 24 (24%) | 90 (17%) | |
| Mixed | 0 (0%) | 11 (2%) | |
| Unspecified-Declined to answer | 0 (0%) | 10 (2%) | |
Ƚ Seven patients from the Balance after Baby population did not give birth at the BWH and are not included
Discussion
We used a multi-level approach employing strategies at the macro (high or institutional level), meso (mid or provider level), and micro (individual) levels to recruit 107 of 156 women with recent GDM assessed for eligibility (69%) over 17-months, ultimately yielding 78 women presenting to the baseline visit (50%). Our recruited subjects were well representative of the overall population of women with GDM delivering at Brigham and Women's Hospital. We demonstrated good retention rates for women presenting to the 6 month and 12 month visits. We recruited the majority of women while they were pregnant and attending clinic visits at specialized Diabetes in Pregnancy clinics. We believe that our most effective recruitment strategies included: 1) employing an active approach by having a dedicated recruiter approach patients in clinic, 2) integrating recruitment efforts into clinic flow, 3) providing flexibility in recruitment 4) minimizing barriers to participation, 5) recruiting at a large hospital with a diverse population and focusing on Diabetes in Pregnancy clinics, and 6) not requiring providers to opt-in to allow us to recruit their patients.
We utilized an active, in-person recruitment strategy using dedicated recruiters, and had low yield from posted flyers and advertisements. In previous studies recruiting pregnant women who were also studied in the postpartum period, active recruitment methods including using dedicated recruiters or clinic staff were more effective than passive methods, such as using flyers, mailings or newspaper advertisements [19,24,34-35], and this has been noted in other populations as well [36-38]. Two studies using dedicated recruiters demonstrated recruitment rates of 16% and 52% [23, 24]. Another study, which is currently ongoing, was using clinic staff to recruit and they reported that they had to modify their protocol since recruitment was too slow. They added mailed invitations and increased the eligibility screening window [25]. In one study of pregnant and postpartum women, Kinnunen et al. trained clinic staff to recruit from maternity clinics and pediatric clinics during clinical care. The providers felt that the study protocol took too much time and the recruitment period had to be doubled to meet recruitment goals. However they did ultimately achieve a recruitment rate of 81% [21].
We focused on integrating recruitment with clinical care, building rapport with patients, providing flexibility in the recruitment process, and minimizing barriers to participation. Integrating recruitment into clinic flow allowed us to minimize time outside of clinical care required for subjects for screening and consent as well as to decrease interference with clinical care. This is similar to the strategy employed to recruit overweight and obese pregnant women in the Maternal Adiposity, Metabolism and Stress (MAMAS) Study, a behavioral intervention to reduce excessive gestational weight gain, where establishing a good relationship with the clinic staff to improve recruitment and retention was one of strategies [35]. They screened 135 subjects, identified 68 eligible subjects, and enrolled 47 (35%). In our study, having a dedicated recruiter in clinic allowed recruiters to gain familiarity with the clinic population and clinic staff, as well as to build rapport with patients over time. Similarly, other investigators have emphasized the importance of building rapport with patients [23, 34, 39]. In the Proyecto Salud study, recruiters met with clinic staff regularly to elicit their cooperation and to show appreciation [24], a strategy we also employed our study. Having a dedicated recruiter on site also ensured that the women would not need to spend time traveling, pay for childcare, or miss work to engage in recruitment. Similar to the Proyecto Salud study, where phone numbers were obtained in order to pursue the screening process at a later time, we designed the recruitment process to be flexible, allowing for clinical interruptions and for the screening and consent process to take place over several weeks if necessary. By offering an intervention that participants could access from home and providing financial compensation to help cover cost of childcare or transportation when assessments were scheduled, we tried to address barriers described in other studies by women with previous GDM [20].
Recruiting at BWH provided access to a large representative population of women with GDM. We recruited the majority of women while pregnant at the Diabetes in Pregnancy clinics. Although the yield from the inpatient floors was much lower, this is likely due, at least in part, to the fact that many women may have already been approached in the outpatient clinic setting. Although we recruited the majority of women during pregnancy, women recruited postpartum were more likely than those who were recruited during pregnancy to present for the first study visit. This may be because there was less time between recruitment and the first study visit, or because postpartum women were less likely to change their mind about participating since they already had some idea about the reality of life with a newborn.
We used an opt-out strategy with BWH obstetric providers to request permission to approach patients. Other studies employing this technique have noted that opt-out strategies shortened recruitment time and increased the number of participants recruited [40]. In addition, given that our study had minimal risks and may have had potential benefits for patients, including increased surveillance for T2DM, clinicians may have felt positively about their patients' participation.
Limitations
We cannot truly compare the rates of women recruited during pregnancy with those recruited postpartum since many women may have been approached first while pregnant. Although we suspect that using an opt-out strategy facilitated recruitment, we cannot be sure since no providers opted out. However we believe that an opt-in strategy would have required more time and resources, and likely would have slowed recruitment.
Conclusions
Using a multi-level recruitment approach, we recruited 107 patients, representative of the overall population of women with GDM delivering at the BWH, for a web-based lifestyle intervention for women with recent GDM, which allowed us to randomize 75 subjects. Employing a multi-level strategy at macro, meso and micro/individual levels allowed us to meet our recruitment goals, despite multiple barriers to participation in postpartum women that have been previously described. Key components of our multi-level recruitment strategy included using a dedicated in-person recruiter, integrating recruitment into clinical flow, allowing for flexibility in recruitment, minimizing barriers to participation, and using an opt-out strategy with providers. Decreasing the number of women lost-to-follow-up between pregnancy and the baseline postpartum study visit could potentially improve overall recruitment rates. Future studies may want to consider increased contact with participants during the period between recruitment and the first study visit.
Acknowledgments
The Balance after Baby study was supported by the Centers for Disease Control (CDC MM-1094-09/09), and work by the investigators was supported by an Institutional National Research Service Award #T32AT000051 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health, NIH BIRCWH K12 HD057022, Ruth L. Kirschstein National Research Service Award #T32HL007609 at the National Institutes of Health and a K24 from the National Heart Lung and Blood Institute at the National Institutes of Health (9K24HL096141). No relationships disclosed. The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Jacinda M. Nicklas, Email: Jacinda.Nicklas@ucdenver.edu, Division of General Medicine, University of Colorado School of Medicine, Anschutz Health and Wellness Center, Mailstop C263, 12348 E. Montview Blvd., Aurora, CO 80045, 303-724-9028.
Geraldine Skurnik, Division of Diabetes, Hypertension and Endocrinology, Brigham and Women's Hospital, Harvard Medical School.
Chloe A. Zera, Division of Maternal-Fetal Medicine, Brigham and Women's Hospital, Harvard Medical School
Liberty G. Reforma, Division of Diabetes, Hypertension and Endocrinology, Brigham and Women's Hospital
Sue E. Levkoff, College of Social Work, University of South Carolina
Ellen W. Seely, Division of Diabetes, Hypertension and Endocrinology, Brigham and Women's Hospital, Harvard Medical School
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.England LJ, Dietz PM, Njoroge T, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(4):365 e361–368. doi: 10.1016/j.ajog.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Hedderson M, Ehrlich S, Sridhar S, et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35(7):1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24(5):441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Ratner RE. Prevention of type 2 diabetes in women with previous gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S242–245. doi: 10.2337/dc07-s223. [DOI] [PubMed] [Google Scholar]
- 7.Bentley-Lewis R, Levkoff S, Stuebe A, et al. Gestational diabetes mellitus: postpartum opportunities for the diagnosis and prevention of type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2008;4(10):552–558. doi: 10.1038/ncpendmet0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C. Gestational diabetes mellitus and risk of future maternal cardiovascular disease. Expert Rev Cardiovasc Ther. 2010;8(12):1639–1641. doi: 10.1586/erc.10.167. [DOI] [PubMed] [Google Scholar]
- 9.McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013 Feb 7;3(2) doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Delft K, Schwertner-Tiepelmann N, Thakar R, et al. Recruitment of pregnant women in research. J Obstet Gynaecol. 2013;33(5):442–446. doi: 10.3109/01443615.2013.767787. [DOI] [PubMed] [Google Scholar]
- 12.Tooher RL, Middleton PF, Crowther CA. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC Pregnancy Childbirth. 2008;8:36. doi: 10.1186/1471-2393-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute of Health. Recruitment and retention of women in clinical studies. A Report of the Workshop Sponsored by the Office of Research on Women's Health 1993 [Google Scholar]
- 14.Rosemann T, Szecsenyi J. General practitioners'attitudes towards research in primary care: qualitative results of a cross sectional study. BMC Fam Pract. 2004;5:31. doi: 10.1186/1471-2296-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swan W, Kilmartin G, Liaw ST. Assessment of readiness to prevent type 2 diabetes in a population of rural women with a history of gestational diabetes. Rural Remote Health. 2007;7(4):802. [PubMed] [Google Scholar]
- 16.Ferrara A, Hedderson MM, Albright CL, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care. 2011;34(7):1519–1525. doi: 10.2337/dc10-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicklas JM, Zera CA, Seely EW, et al. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23. doi: 10.1186/1471-2393-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C, Ferrara A. Gestational diabetes during and after pregnancy. 1st. London: Springer; 2010. [Google Scholar]
- 19.Manca DP, O'Beirne M, Lightbody T, et al. The most effective strategy for recruiting a pregnancy cohort: a tale of two cities. BMC Pregnancy Childbirth. 2013;13:75. doi: 10.1186/1471-2393-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Khorazaty MN, Johnson AA, Kiely M, et al. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnunen TI, Aittasalo M, Koponen P, et al. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC Pregnancy Childbirth 2008. 2008;8:37. doi: 10.1186/1471-2393-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight BA, Wyatt K. Barriers encountered when recruiting obese pregnant women to a dietary intervention. Nurs Times. 2010;106(32):20–22. [PubMed] [Google Scholar]
- 23.Patten CA, Windsor RA, Renner CC, et al. Feasibility of a tobacco cessation intervention for pregnant Alaska Native women. Nicotine Tob Res. 2010;12(2):79–87. doi: 10.1093/ntr/ntp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chasan-Taber L, Fortner RT, Hastings V, et al. Strategies for recruiting Hispanic women into a prospective cohort study of modifiable risk factors for gestational diabetes mellitus. BMC Pregnancy Childbirth. 2009;9:57. doi: 10.1186/1471-2393-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih ST, Davis-Lameloise N, Janus ED, et al. Mothers After Gestational Diabetes in Australia Diabetes Prevention Program (MAGDA-DPP) post-natal intervention: an update to the study protocol for a randomized controlled trial. Trials. 2014;15:259. doi: 10.1186/1745-6215-15-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Infanti JJ, O'Dea A, Gibson I, et al. Reasons for participation and non-participation in a diabetes prevention trial among women with prior gestational diabetes mellitus (GDM) BMC Med Res Methodol. 2014;14:13. doi: 10.1186/1471-2288-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Infanti JJ, Dunne FP, O'Dea A, et al. An evaluation of Croi MyAction community lifestyle modification programme compared to standard care to reduce progression to diabetes/pre-diabetes in women with prior gestational diabetes mellitus (GDM): study protocol for a randomised controlled trial. Trials. 2013;14:121. doi: 10.1186/1745-6215-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stendell-Hollis NR, Laudermilk MJ, West JL, et al. Recruitment of lactating women into a randomized dietary intervention: successful strategies and factors promoting enrollment and retention. Contemp Clin Trials. 2011;32(4):505–511. doi: 10.1016/j.cct.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Macleod M, Craigie AM, Barton KL, et al. Recruiting and retaining postpartum women from areas of social disadvantage in a weight-loss trial--an assessment of strategies employed in the WeighWell feasibility study. Matern Child Nutr. 2013;9(3):322–331. doi: 10.1111/j.1740-8709.2011.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicklas JM, Zera CA, England LJ, et al. A Web-Based Lifestyle Intervention for Women With Recent Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstetrics & Gynecology. 2014;124(3):563–70. doi: 10.1097/AOG.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levkoff S, Levy B, Weitzman P. The matching model of recruitment. J Ment Health and Aging. 2000;6(1):29–38. [Google Scholar]
- 32.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist. 2003;43(1):18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Promislow JH, Makarushka CM, Gorman JR, et al. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004;18(2):143–152. doi: 10.1111/j.1365-3016.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 35.Coleman-Phox K, Laraia BA, Adler N, et al. Recruitment and retention of pregnant women for a behavioral intervention: lessons from the maternal adiposity, metabolism, and stress (MAMAS) study. Prev Chronic Dis. 2013;10 doi: 10.5888/pcd10.120096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkin JA, Marshall SJ, Larson KA, et al. A comparison of methods of recruitment to a health promotion program for university seniors. Prev Med 1998. 1998;27(4):562–571. doi: 10.1006/pmed.1998.0327. [DOI] [PubMed] [Google Scholar]
- 37.Tzelepis F, Paul CL, Walsh RA, et al. Proactive telephone counseling for smoking cessation: meta-analyses by recruitment channel and methodological quality. J Natl Cancer Inst. 2011;103(12):922–941. doi: 10.1093/jnci/djr169. [DOI] [PubMed] [Google Scholar]
- 38.Lee RE, McGinnis KA, Sallis JF, et al. Active vs. passive methods of recruiting ethnic minority women to a health promotion program. Ann Behav Med. 1997;19(4):378–384. doi: 10.1007/BF02895157. [DOI] [PubMed] [Google Scholar]
- 39.Chang MW, Brown R, Nitzke S. Participant recruitment and retention in a pilot program to prevent weight gain in low-income overweight and obese mothers. BMC Public Health. 2009;9:424. doi: 10.1186/1471-2458-9-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rukstalis M, C H. Early childhood obesity prevention in primary care: Opt-in versus opt-out recruitment strategies. Clin Med Res. 9(3-4):163–164. [Google Scholar]