Abstract
We examined the impact of antiretroviral treatment adherence among Hepatitis C co-infected HIV patients on survival and clinical outcomes. We analyzed Medicaid claims data from fourteen southern states from 2005-2007, comparing survival and clinical outcomes and cost of treatment for HIV and hepatitis-C co-infected patients (N=4,115) at different levels of adherence to antiretroviral therapy.More than one in five patients (20.5%) showed less than 50% adherence to antiretroviral treatment, but there were no racial-ethnic or gender disparities. Significant survival benefit was demonstrated at each incremental level of adherence to antiretroviral therapy (one-year mortality ranging from 3.5% in the highest adherence group to 26.0% in the lowest). Low adherence patients also had higher rates of hospitalization and emergency department visits. Relative to patients with high (>95%) ART-adherence, those with less than 25% treatment adherence had four-fold greater risk of death (adjusted odds ratio 4.22 [95% CI, 3.03,5.87]). Non-drug Medicaid expenditures were lower for high adherence patients, but cost of medications drove total Medicaid expenditures higher for high-adherence patients. Cost per quality-adjusted life year (QALY) saved (relative to the <25% low-adherence group) ranged from $21,874 for increasing adherence to 25-50% to $37,229 for increasing adherence to 75-95%. Adherence to antiretroviral therapy for patients with HIV and hepatitis C co-infection is associated with lower adverse clinical outcomes at a Medicaid cost per QALY commensurate with other well-accepted treatment and prevention strategies. Further research is needed to identify interventions which can best achieve optimal ART adherence at a population scale.
Keywords: HIV, antiretroviral therapy, adherence, outcomes, Medicaid, Hepatitis C co-infection
Introduction
Similar routes of transmission result in extremely high rates of hepatitis C (HCV) co-infection among patients with human immunodeficiency virus (HIV) in the United States. The prevalence of HCV among HIV infected people is 25%(CDC, 2013). Based on treatment guidelines for HIV and HCV co-infection, the benefits of highly active antiretroviral therapy (ART) outweigh concerns regarding drug induced liver injury in HIV + HCV co-infected individuals.
ART adherence is one factor determining treatment success. Unfortunately, adherence to ART is often sub-optimal. In the context of HIV and HCV co-infection, successful treatment requires nearly perfect adherence to ART in order to reduce viral load and prevent the emergence of drug resistant variants (Moatti et al., 2000; Soriano et al., 2002).
Medicaid is the largest public health insurance program for low-income persons in the United States, and provides health care coverage for nearly half of all people with HIV in the US. Medicaid claims data also provide a detailed longitudinal record of health care utilization and prescription drug usage. Further, Medicaid claim databases are more likely to accurately reflect real-world adherence. The purpose of this study was to quantify the clinical and economic benefits of adherence to ART, with a special focus on the sub-set of Medicaid enrollees with both HIV and HCV co-infection.
Methods
We used a retrospective cohort design to measure adherence rate and assess its clinical and economic benefits for HIV and HCV co-infected patients. We examined the clinical and economic outcomes one year after the initial diagnosis of HCV co-infection in established patients with HIV. The data for this analysis came from 2005-2007 100% of Medicaid paid claims data provided by the Centers for Medicare and Medicaid Services (CMS) as Medicaid Analytic Extract (MAX) files from 14 states: Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Maryland, Missouri, Mississippi, North Carolina, South Carolina, Tennessee, Texas, and Virginia. This project was approved by the Morehouse School of Medicine Institutional Review Board for human subjects research, and our data security and confidentiality protocols were approved by the US/DHHS Center for Medicare and Medicaid Services (CMS) Privacy Board. These studies have been deemed exempt from informed consent requirements, because the protection of the individual's confidentiality would be endangered more by obtaining their name and address to seek informed consent than it is by using the data in a less personally-identifiable format.
Patient selection
Data covered paid claims for activities occurring from 01/01/2005 to 12/31/2007. We identified 102,782 HIV patients in these 14 states, and 13,129 of these HIV-infected individuals had claims consistent with HCV co-infection. We excluded 3,102 patients whose initial diagnosis of co-infection occurred after 01/01/2007 in order to assure that we had a full 365 days for follow-up tracking. Among those eligible enrollees, only 4,115 patients left.
Five types of Food and Drug Administration(FDA) approved ARV drugs were recommended or available in Medicaid during the observation period:
nucleoside reverse transcriptase inhibitors (NRTIs), which include zidovudine, didanosine, stavudine, lamivudine, abacavir, emtricitabine,andtenofovir;
non-nucleoside reverse transcriptase inhibitor (NNRTIs) which include efavirenz, nevirapine, delavirdine, and etravirine;
protease inhibitor (PIs) which include atazainavir, darunavir, fosamprenavir, indinavir, nelfinavir, ritonavir, saquinavir, tipranavir, and lopinavir;
entry inhibitors which include maraviroc fusion inhibitors enfuvirtide
integrase inhibitor (raltegravir).
As with ART-naive, HIV-infected persons, a combination ART regimen with at least two nucleoside reverse transcriptase inhibitors plus one non-nucleoside reverse transcriptase inhibitors or one ritonavir-boosted protease inhibitor, or one integrase inhibitor was recommended.
Adherence to ART
Adherence to ART was determined by examining the proportion of prescribed days covered (PPDC) by ART regimens. PPDC was defined as total ART days’ supply divided by the 365 days follow-up period. For example, a person who received a total of 240 days’ supply of ART would have a PPDC of 0.66 (240/365).We stratified person-years adherence to ART on an ordinal scale of five levels (<25%, 25-50%, 50-75%, 75-95%, and >95%).
Clinical Outcomes
One year mortality
Presence of either Medicaid or Medicare date of death in personal summary file was used to flag mortality.
Hospitalization
Hospital admissions were captured in inpatient files and categorized as dichotomous (yes/no). All hospitalization claims were captured from the 365 days after initial diagnosis of HCV co-infection in established HIV patients.
Emergency department (ED) services within the 1 year period after initial diagnosis of HIV-HCV co-infection were identified in inpatient and outpatient files. We categorized ED visit as dichotomous (yes/no) for use in multivariate models.
Quality adjusted life years (QALYs)
QALY is an outcome measure that considers both the quality and the quantity of life lived. Each year in perfect health is assigned the value of 1.0. Each year of less than perfect health is assigned a value less than 1.0 down to a value of 0.0 for death. Studies commonly report a pooled estimated of utility of 0.70 for patients who have AIDS related syndromes and 0.82 for other co-infections(M. A. Chesney et al., 2000). Average QALYs were reported for different adherence groups.
Other covariates
HCV treatment
Any peginterferonalfa or ribavirin claims during the one year tracking period were used as the indicator to define HCV treatment status. We then dichotomized this variable as Yes/No.
AIDS conditions
We captured the presence of AIDS-defining clinical conditions as defined by CDC(Schneider et al., 2008) by searching for corresponding ICD-9-CM codes in the primary or secondary diagnosis fields of each co-infection patient.
Cost Measures
Direct costs paid by Medicaid were obtained from MAX files. ART treatment cost was calculated by the summation of one year of ART related prescription drug costs after the initial co-infection diagnosis. We aggregated outpatient costs, inpatient costs and all prescription costs, and then deducted the ART-associated cost to estimate the total cost excluding drug costs.
Cost effectiveness analysis
Incremental cost-effectiveness ratio (ICER), a standard measure used in cost-effectiveness analysis, was reported in our study. ICER is represented as the ratio of the difference in expected antiretroviral regimen costs among different adherence groups compared with the difference in QALY outcomes among those same adherence groups.
Statistical Analysis
Descriptive analyses comparing sample characteristics, demographics, and socioeconomic status of patients with HCV/HIV co-infection were examined using the Mantel-Haenszel test for categorical variables and ANOVA-test for continuous variables. We used the Kaplan- Meier method to estimate the cumulative probability of mortality for different adherence groups. Participants were censored on the date of death or the end of one year observation if they did not die during this time. We then built a Cox proportional hazard model to estimate hazard ratio for death, with adjustment for covariate effects to obtain adjusted risk ratios. Data were analyzed using SAS 9.2 (Cary,NC).
Results
Table 1 provides descriptive statistics for HIV and HCV co-infected Medicaid enrollees from 14 southern states who had prescription drug claims in 2005-2007 for optimal ART. Based on PPDC levels, 5.9% of patients had low adherence (<25%), 14.6% had 25-50% adherence, 11.5% had 50-75% adherence, 7.9% had 75-95% adherence, and nearly 60% of Medicaid-enrolled HIV and HCV patients had more than 95% ART adherence. More than one in five patients (20.5%) had prescription refill claims indicating less than 50% adherence to antiretroviral treatment. There were no gender, racial/ethnic, or rural/urban differences in adherence.
Table 1.
Characteristic of patients with HIV& HCV co-infections among different ART adherence treatment groups (N=4,115)
| >95% adherent ART | 75-95% adherent ART | 50-75% adherent ART | 25-50% adherent ART | <25% adherent ART | P | |
|---|---|---|---|---|---|---|
| N (%) | 2473(60.1%) | 324(7.9%) | 475(11.5%) | 601(14.6%) | 242(5.9%) | |
| Age | ||||||
| Mean (SD) | 46.3(7.7) | 46.0(8.0) | 45.4(7.4) | 44.5(7.8) | 46.0(7.7) | <.01* |
| Gender | ||||||
| Female | 897(36.6%) | 120(37.6%) | 179(38.3%) | 252(43.0%) | 83(35.9%) | .05^ |
| Male | 1556(63.4%) | 199(62.4%) | 288(61.7%) | 334(57.0%) | 148(64.1%) | |
| Race/Ethnicity | ||||||
| White | 577(23.5%) | 73(22.9%) | 105(22.5.0%) | 151(25.7%) | 47(20.4%) | .99^ |
| African American | 1637(66.7%) | 213(66.8%) | 315(67.5%) | 376(64.0%) | 161(69.7%) | |
| Hispanics | 98(4.0%) | 15(4.7%) | 25(5.4%) | 28(4.8%) | 11(4.8%) | |
| Other | 142(5.8%) | 18(7.9%) | 22(4.7%) | 33(5.6%) | 12(5.2%) | |
| AIDS | 668(27.0%) | 112(5.6%) | 175(36.8%) | 226(37.6%) | 100(41.3%) | <.01 |
| HCV treatment** | ||||||
| Yes | 161(6.5%) | 14(4.3%) | 11(2.3%) | 11(1.8%) | 1(0.4%) | <.01 |
| Rural/Urban Status | ||||||
| Large Metro Area | 117(4.7%) | 12(3.7%) | 20(4.2%) | 34(5.7%) | 5(2.1%) | .25^ |
| Small Metro Area | 176(7.1%) | 15(4.6%) | 15(7.4%) | 33(5.5%) | 15(6.2%) | |
| Rural Area | 2180(88.2°%) | 297(91.7%) | 420(88.4%) | 534(88.9%) | 222(91.7%) | |
| Medicaid Eligible Months per year | ||||||
| Mean (SD) | 10.1(2.8) | 9.4(3.2) | 8.8(3.3) | 8.1(3.7) | 6.7(3.9) | <.01* |
ANOVA
Any Peginterferon Alfa (PegIFN) and Ribavirin (RBV) during the one year tracking period
Mantel-Haenszel test
Table 2 shows the distribution of clinical outcomes including hospitalization, emergency department visit, one year mortality rates, and quality adjusted life years (QALY) by level of adherence to ART. Descriptive statistics also show Medicaid expenditures specific to hospitalization and to ART drug costs, as well as total Medicaid expenditures at different levels of adherence. There was significant survival benefit demonstrated at each incremental level of adherence to antiretroviral therapy (one-year mortality ranging from 3.5% in the highest adherence group to 26.0% in the lowest). Low adherence patients also had higher rates of hospitalization and emergency department visits.
Table 2.
Annual clinical outcomes and cost of treatment in relation to adherence to HHART among HIV & HCV co-infection cohort in Medicaid (N=4,115)
| >95% adherent ART | 75-95% adherent ART | 50-75% adherent ART | 25-50% adherent ART | <25% adherent ART | P | |
|---|---|---|---|---|---|---|
| N (%) | 2473(60.1%) | 324(7.9%) | 475(11.5%) | 601(14.6%) | 242(5.9%) | |
| 1 year mortality | 3.5% (86) | 8.0% (26) | 10.1% (48) | 17.8% (107) | 26.0% (63) | <.01^ |
| Hospitalization | 56.3% (1393) | 63.6% (206) | 68.8 (327) | 72.6% (436) | 76.9% (186) | <.01^ |
| Emergency Dept. Visit | 34.9% (34.9%) | 38.3% (324) | 43.4% (261) | 43.4% (261) | 46.7% (113) | <.01^ |
| QALY(Quality Adjusted Life Years)* | ||||||
| mean(sd) | 0.78 (0.08) | 0.76(0.11) | 0.75(0.13) | 0.71(0.19) | 0.66(0.24) | <.01 |
| Inpatient payment | ||||||
| Mean (95%CI) | $ 7642 (6944,8340) | $ 9997 (8046,11948) | $ 12314 (10005,14263) | $ 12320 (10573,14066) | $ 15386 (11262,19512) | <.01 |
| ARV treatment payment | ||||||
| Mean (95%CI) | $ 9164 (8887,9441) | $ 4912 (4576,5249) | $ 3408 (3198,3618) | $ 2283 (2155,2410) | $ 1189 (1092,1287) | <.01 |
| Total Medicaid Payment (exclude ARV payment | ||||||
| Mean (95%CI) | $ 17407 (15889,18925) | $ 17658 (15046,20269) | $ 18401 (15719,21083) | $ 17368 (15341,19395) | $ 19606 (14878,23735) | .92 |
Quality of life estimates for HIV/AIDS, 0.70 for AIDS patients, and 0.82 for other HIV patients.
Mantel-Haenszel test
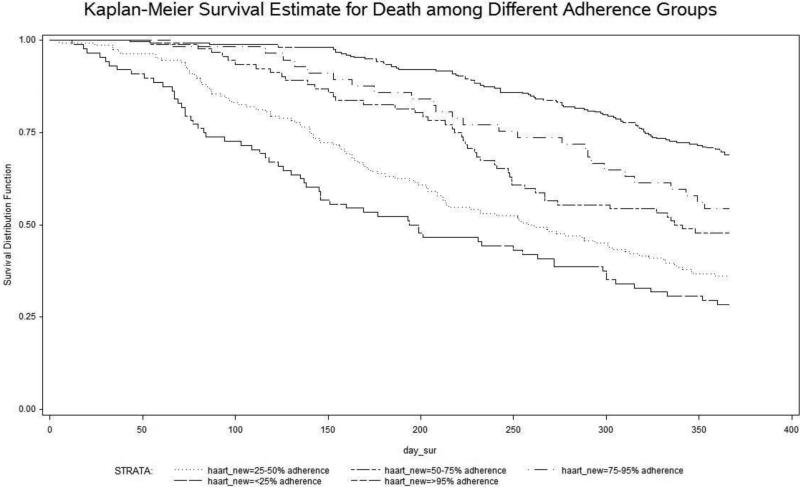
Table 3 shows the crude and adjusted hazard ratio of mortality among different ART groups. After adjusting for age, gender, race/ethnicity, metro/rural residential status, AIDS-related conditions, and HCV treatment status, the association of mortality with lower ART adherence was attenuated, but still significant. Relative to patients with high (>95%) ART-adherence, those with less than 25% treatment adherence still had a greater than four-fold risk of death (adjusted odds ratio 4.22 (95% CI, 3.03, 5.87). Even those with only mild non-adherence (75-95% adherence) had a 1.58 times higher adjusted relative risk (95%CI, 1.02, 2.46) of death (Figure 1).
Table 3.
Relative risk (and 95% CIs) of mortality by different levels adherence of ART treatment in 14 southern states HIV & HCV co-infection Medicaid enrollees
| Treatment | Crude relative risk (95%CI) | Adjusted relative risk (95%CI)** |
|---|---|---|
| >95% adherent ART | 1.00 | 1.00 |
| 75-95% adherent ART | 1.65(1.06,2.55)* | 1.58(1.02,2.46)* |
| 50-75% adherent ART | 2.07(1.45,2.95)* | 2.05(1.44,2.94)* |
| 25-50% adherent ART | 3.06(2.30,4.07)* | 3.21(2.39,4.31)* |
| <25% adherent ART | 4.11(2.97,5.69)* | 4.22(3.03,5.87)* |
P<.05
Cox hazard regression model was adjusted for age, gender, race/ethnicity, metro/rural residential status, AIDS- related conditions, and HCV treatment status.
Figure 1.
ART adherence vs survival function
Incremental cost-effectiveness ratios (ICERs) for different adherence groups are presented in table 4. Non-drug Medicaid expenditures were lower for high adherence patients, but the cost of medications drove total Medicaid expenditures higher for the high-adherence patients. Cost per quality-adjusted life year (QALY) saved (relative to the <25% low-adherence group ranged from $21,874 for increasing adherence to 25-50% to 37,229 for increasing adherence to 75-95%. The highest level of adherence ( >95%) was associated with a cost of $66,455 per QALY.
Table 4.
Cost effectiveness as determined by incremental cost-effectiveness ratios
| Treatment | Cost | QALY | ΔC | ΔE | ICER |
|---|---|---|---|---|---|
| <25% adherent ART | $ 1,189.2 | 0.66 | 0 | 0 | 0 |
| 25-50% adherent ART | $ 2,282.9 | 0.71 | 0.05 | $ 1093.7 | $ 21,874.0/QALY |
| 50-75% adherent ART | $ 3,408.0 | 0.75 | 0.09 | $ 2218.8 | $ 24,653.3/QALY |
| 75-95% adherent ART | $ 4,912.1 | 0.76 | 0.10 | $ 3722.9 | $ 37,229.0/QALY |
| >95% adherent ART | $ 9,163.8 | 0.78 | 0.12 | $ 7974.6 | $ 66,455.0/QALY |
Note: all costs are in US dollars
Discussion
This study demonstrates first that high adherence to ART among Medicaid-enrolled patients with HIV and HCV co-infection is achievable – over 60% of such patients in this dataset had over 95% adherence to their ART. The absence of any significant racial-ethnic or gender disparities also suggests an important role for Medicaid in eliminating disparities and achieving more equitable care and outcomes. Adherence was also associated with significant clinical benefits.
The problem of inadequate adherence to ART can be attributed to both provider and patient behaviors. Hepatotoxicity of ART adds greater complexity to medical decision making. However, barriers to adherence include regimen complexity, side effects, psychological issue, patient belief system, and the patient-provider relationship(M. Chesney, 2003).
Non-adherence also prolongs contagion and increases the risk for AIDS related opportunistic diseases, and death(Kiertiburanakul & Sungkanuparph, 2009; Schneider et al., 2008). The most well-known reason for non-adherence is forgetting(M. A. Chesney et al., 2000; Simoni et al., 2006)which becomes more common as individuals return to work or resume other activities. Confusion over pill schedules and obstacles to medication refills are additional factors in non-adherence(Buchanan et al., 2012; Wolfe, Carrieri, & Shepard, 2010). Personal psychological issues, such as substance abuse, depression, and stress may also contribute(Mellins et al., 2009), as can pain, fatigue, and medication side effects.
A number of non-medical determinants have also been found to impact adherence. Factors such as stable housing, secure employment, and social support all positively predict ART adherence(Godin, Cote, Naccache, Lambert, & Trottier, 2005; Moss et al., 2004). Lack of access to reliable transportation is associated with poor access to ART and poor adherence to ART.
Patient self-management is central for medication adherence. At the provider level, increased physician experience with HIV and AIDS is the most important factor associated with the successful treatment of HIV infected patients(Kitahata et al., 1996). At the practice level, lessons learned from successful chronic disease management programs such as nurse care managers, patient navigators, peer support, group visits, open access scheduling, telephone and text-message follow-up, and other panel-based care management strategies can be applied to the complexity of managing HIV and HCV co-infection(Starr & Springer, 2014; Tu et al., 2013).
Limitations of our study are inherent in the use of administrative claims data. Pharmacy claims are limited by their inability to directly assess whether the patient took the medication. Medicaid claims data do not reflect clinical covariates such as HIV viral load, and duration or severity of illness, or measures of impaired renal function such as GFR or creatinine. Medicaid data also do not provide individual-level information on socioeconomic status, education level, country of origin, length of stay in the US, or degree of social support, all of which may contribute to health care utilization. Finally, any healthcare cost paid for by sources other than Medicaid would not be captured in our data.
The prevalence of HCV co-infection in our study may seem low, when compared to prevalence figures generated from population-based or birth-cohort screening(Sherman, Rouster, Chung, & Rajicic, 2002). However, prior to the recent CDC recommendation of birth cohort screening for HCV, the recommendation was to screen for HCV based on risk factors. Most providers screened patients only if they have risk factors such as IV drug use or had liver function abnormalities. Thus the overall prevalence of HCV/HIV co-infection in our initial study sample (15%) is similar to previously reported risk based screening prevalence measures (16%).
The recent approval of effective directly-acting antiviral treatment for HCV by the FDA is expected to dramatically change the treatment paradigm, reducing the rate of liver complications and providing additional benefits to HIV/HCV co-infected patients who are adherent to treatment. Reducing the cost of ART to state Medicaid programs could substantially lower the cost per QALY associated with increased ART adherence, or even provide absolute cost savings from increasing adherence.
Acknowledgments
Funding sources: Agency for Healthcare Research and Quality (AHRQ) mid-senior Career Development grant # K18HS022444; NIH/NIMHD RCMI Infrastructure for Clinical and Translational Research grant # U54MD007588; NIH/NIMHD Reducing Health Disparities in Vulnerable Populations (P20) grant # 1P20MD006881-02; DHHS Office of Minority Health Cooperative Agreement #5MPCMP121069-02-00.
References
- Buchanan AL, Montepiedra G, Sirois PA, Kammerer B, Garvie PA, Storm DS, Nichols SL. Barriers to medication adherence in HIV-infected children and youth based on self-and caregiver report. Pediatrics. 2012;129(5):e1244–e1251. doi: 10.1542/peds.2011-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. [Nov 20, 2013];HIV and Viral Hepatitis Fast Fact. 2013 from http://www.cdc.gov/hepatitis/Populations/PDFs/HIVandHep-FactSheet.pdf.
- Chesney M. Adherence to HAART regimens. AIDS patient care and STDs. 2003;17(4):169–177. doi: 10.1089/108729103321619773. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Adherence Working Group Of The Outcomes Committee Of The Adult Aids Clinical Trials, G. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Godin G, Cote J, Naccache H, Lambert LD, Trottier S. Prediction of adherence to antiretroviral therapy: a one-year longitudinal study. AIDS care. 2005;17(4):493–504. doi: 10.1080/09540120412331291715. [DOI] [PubMed] [Google Scholar]
- Kiertiburanakul S, Sungkanuparph S. Emerging of HIV drug resistance: epidemiology, diagnosis, treatment and prevention. Current HIV research. 2009;7(3):273–278. doi: 10.2174/157016209788347976. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. New England Journal of Medicine. 1996;334(11):701–707. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, Bell J. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS care. 2009;21(2):168–177. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J, Manif Study G. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. Aids. 2000;14(2):151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, Bangsberg DR. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clinical Infectious Diseases. 2004;39(8):1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT, Centers for Disease Control and, P. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged< 18 months and for HIV infection and AIDS among children aged 18 months to< 13 years, United States, 2008. MMWR Recomm Rep. 2008;57(10):1–12. [PubMed] [Google Scholar]
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clinical infectious diseases. 2002;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V, Sulkowski M, Bergin C, Hatzakis A, Cacoub P, Katlama C, Moreno S. Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. Aids. 2002;16(6):813–828. doi: 10.1097/00002030-200204120-00001. [DOI] [PubMed] [Google Scholar]
- Starr WM, Springer LB. CE: Nursing in the Fourth Decade of the HIV Epidemic. AJN The American Journal of Nursing. 2014;114(3):38–47. doi: 10.1097/01.NAJ.0000444491.93733.53. [DOI] [PubMed] [Google Scholar]
- Tu D, Belda P, Littlejohn D, Pedersen JS, Valle-Rivera J, Tyndall M. Adoption of the chronic care model to improve HIV care In a marginalized, largely aboriginal population. Canadian Family Physician. 2013;59(6):650–657. [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. The Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]