Abstract
This year is the tenth anniversary of the publication in this journal of a model suggesting the existence of ‘tumour progenitor genes’. These genes are epigenetically disrupted at the earliest stages of malignancies, even before mutations, and thus cause altered differentiation throughout tumour evolution. The past decade of discovery in cancer epigenetics has revealed a number of similarities between cancer genes and stem cell reprogramming genes, widespread mutations in epigenetic regulators, and the part played by chromatin structure in cellular plasticity in both development and cancer. In the light of these discoveries, we suggest here a framework for cancer epigenetics involving three types of genes: ‘epigenetic mediators’, corresponding to the tumour progenitor genes suggested earlier; ‘epigenetic modifiers’ of the mediators, which are frequently mutated in cancer; and ‘epigenetic modulators’ upstream of the modifiers, which are responsive to changes in the cellular environment and often linked to the nuclear architecture. We suggest that this classification is helpful in framing new diagnostic and therapeutic approaches to cancer.
Ten years ago, it was suggested that, in addition to oncogenes and tumour suppressor genes, epigenetic alterations disrupt the expression of hypothesized ‘tumour progenitor genes’ that mediate stemness at the earliest stage of carcinogenesis, even as a field effect in normal tissues1. Epigenetically altered tumour progenitor genes were proposed to increase the likelihood of cancer when genetic mutations occurred and these same genes were suggested to be involved throughout tumour progression, helping to explain properties such as invasion and metastasis1. In the 10 years since this model was proposed, several discoveries have supported the idea of tumour progenitor genes, including the identification of many of the responsible genes, the role of widespread epigenomic changes involving the nuclear architecture and chromatin compaction, and the parts played by ageing and the environment in these properties.
Nowhere else is the contribution of epigenetic changes to cancer seen more clearly than in paediatric malignancies. Systematic analyses of genetic and epigenetic alterations in a variety of paediatric cancers have surprisingly identified tumour types with few or no mutations, suggesting that epigenetic derangements can themselves drive these cancers. The discovery of the biallelic loss of the chromatin remodeller gene SMARCB1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1; also known as SNF5) in highly malignant paediatric rhabdoid tumours was an early example of the disruption of epigenetic control as a driver of cancer2. Subsequent exome sequencing of these tumours revealed a remarkably simple genome with no other recurrent genetic mutations3. More recently, genome sequencing of paediatric hindbrain ependymomas revealed an absence of any recurrent somatic mutations4. The poor prognosis of patients with hindbrain ependymomas was instead defined by epigenetic changes, with a CpG island methylator phenotype leading to the transcriptional silencing of Polycomb repressive complex 2 (PRC2) targets. Sequencing efforts in retinoblastoma, a childhood cancer that occurs as a result of the inactivation of both copies of the tumour suppressor RB1, found few other genetic alterations5. Instead, epigenetic changes predominate, with changes in the gene expression of known oncogenes driven by alterations in histone modifications and DNA methylation. Similarly, the childhood malignant brain tumour medulloblastoma is driven by key subtype-specific somatic mutations, but has a very low mutation rate overall6. DNA methylation sequencing in medulloblastoma identified highly prevalent epigenetic alterations, most notably consisting of large regions of hypomethylation correlated with increased gene expression7.
In this Review, we revisit the tumour progenitor gene model in the light of our much clearer understanding of the identity of these genes, suggesting the more appropriate term ‘epigenetic mediator’. We suggest that most driver mutations in cancer occur in ‘epigenetic modifiers’ upstream of the mediators, and we integrate the role of upstream ‘epigenetic modulators’ that sense the environment and regulate stemness epigenetically, largely through the structure of chromatin. We suggest that this framework will be useful in organizing approaches to cancer detection and treatment.
Three types of genes in the epigenetics of cancer
There are already two non-epigenetic classification systems for cancer genes: the mutational division into dominant oncogenes and recessive tumour suppressor genes; and the selection division into gene drivers and passengers in tumour development (TABLE 1). The proposed epigenetic functional classification system divides cancer genes into epigenetic modifiers, mediators and modulators. The easiest of these to describe are the epigenetic modifiers — that is, the genes whose products modify the epigenome directly through DNA methylation, the post-translational modification of chromatin or the alteration of the structure of chromatin. These genes are frequently the target of mutations and epimutations in cancer. One of the great surprises of the past few years has been the abundance of mutations in cancer involving such genes, affecting almost all levels of the epigenetic machinery. Also within this group are the genomic sequence changes that affect the binding of chromatin regulators, such as mutations in enhancers or transcription factor binding sites. The epigenetic mediators, which we earlier called tumour progenitor genes, are often the target of epigenetic modification, although they are rarely mutated themselves; importantly, they appear to be responsible for the emergence of cancer stem cells (CSCs). The epigenetic mediators largely overlap with the genes involved in stem cell reprogramming and their role in cancer followed directly from the discovery of their reprogramming role. Epigenetic mediators are those genes whose products are the targets of the epigenetic modifiers. For the most part, these are the genes that drive a tumour or its progenitor cells towards a more stem-like state. As the ultimate mediators of the malignant state, they are attractive targets for novel chemotherapy treatments or biological response modifiers. Last, and perhaps most arguable, are the epigenetic modulators, defined as genes lying upstream of the modifiers and mediators in signalling and metabolic pathways, and serving as the mechanism by which environmental agents, injury, inflammation and other forms of stress push tissues towards a neoplastic propensity and/or increase the likelihood that cancer will arise when a key mutation occurs by chance. We suggest that changes in the structure of chromatin are induced very early in the cancer process by epigenetic modulators and even in the non-mutated normal tissues from which tumours arise. Epigenetic modulator genes include many genes with prominent roles in conventional oncogenic signalling; these are increasingly appreciated to influence the epigenome as part of their function (TABLE 1).
Table 1.
Three classification systems for cancer genes
| Class | Definition | Examples |
|---|---|---|
| Genetic classification | ||
| Oncogene | A gene whose activation by mutation is advantageous to the cancer cell. Acts as dominant | MYC, KRAS, PIK3CA, ABL1, BRAF |
| Tumour suppressor gene | A gene whose inactivation by mutation is advantageous to the cancer cell. Generally acts as recessive | RB1, TP53, WT1, NF1, NF2, VHL, APC, CDKN2A |
| Selection classification | ||
| Driver gene | A gene whose mutation or aberrant expression is subject to selection during tumorigenesis | MYC, KRAS, PIK3CA, ABL1, RB1, TP53, WT1 |
| Passenger gene | A gene mutated in cancer that is not a driver | Estimated as 99.9% of all mutational changes in cancer |
| Epigenetic functional classification | ||
| Epigenetic modulator | A gene, mutated or not, that activates or represses the epigenetic machinery in cancer | IDH1/2, KRAS, APC, TP53, STAT1/3, YAP1, CTCF |
| Epigenetic modifier | A gene, mutated or not, that modifies DNA methylation or chromatin structure or its interpretation in cancer | SMARCA4, PBRM1, ARID1A, ARID2, ARID1B, DNMT3A, TET2, MLL1/2/3, NSD1/2, SETD2, EZH2, BRD4 |
| Epigenetic mediator | A gene regulated by an epigenetic modifier in cancer (mutations rare or absent) that increases pluripotency or survival | OCT4, NANOG, LIN28, SOX2, KLF4 |
APC, adenomatous polyposis coli; ARID, AT-rich interaction domain; BRD4, bromodomain containing 4; CTCF, CCCTC-binding factor; CDKN2A, cyclin-dependent kinase inhibitor 2A; DNMT3A, DNA methyltransferase 3α; EZH2, enhancer of zeste homologue 2; IDH, isocitrate dehydrogenase; KLF4, Kruppel-like factor 4; MLL, mixed-lineage leukaemia; NF, neurofibromin; NSD, nuclear receptor binding SET domain protein; PBRM1, polybromo 1; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; RB1, retinoblastoma 1; SETD, SET domain containing; SMARCA4, SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4; SOX2, sex-determining Y-box 2; STAT, signal transducer and activator of transcription; TET, TET methylcytosine dioxygenase; TP53, tumour protein p53; VHL, von Hippel-Lindau tumour suppressor; WT1, Wilms tumour 1; YAP1, Yes-associated protein 1.
Epigenetic modifiers
A key discovery of large-scale cancer sequencing research has been the widespread occurrence of mutations in epigenetic modifiers (TABLE 2). These consist of components of nearly every level of the epigenetic machinery, including key players in DNA methylation, histone modification and chromatin organization, across a wide variety of cancer types. This has been the subject of other recent reviews8–10, and we limit our discussion here to a number of illustrative examples.
Table 2.
Epigenetic modifier mutations in cancer
| Gene | Tumours | Refs |
|---|---|---|
| Chromatin remodelling | ||
| SMARCB1 | Paediatric malignant rhabdoid tumours | 2 |
| SMARCA4 | Lung adenocarcinoma, Burkitt lymphoma, medulloblastoma | 226–228 |
| PBRM1 | Clear cell renal carcinoma | 30 |
| ARID1A | Ovarian clear cell carcinoma, hepatocellular carcinoma, colorectal cancer, lung adenocarcinoma | 227,229, 230 |
| ARID1B, ARID2 | Hepatocellular carcinoma, melanoma, pancreatic cancer, breast cancer | 231–234 |
| SMARCD1 | Breast cancer | 234 |
| SMARCE1 | Clear cell meningioma | 235 |
| ATRX | Paediatric glioblastoma, pancreatic neuroendocrine tumours | 236,237 |
| DAXX | Paediatric glioblastoma, pancreatic neuroendocrine tumours | 236,237 |
| CHD5 | Neuroblastoma, glioma, breast, lung, colon, ovary, prostate cancers | 238 |
| CHD2 | Chronic lymphocytic leukaemia | 239 |
| CHD1, CHD3, CHD4, CHD6, CHD7, CHD8 | Gastric, colorectal, prostate, breast, bladder, serous endometrial cancers | 240–243 |
| DNA methylation | ||
| DNMT3A | T cell lymphoma, myeloid malignancies including acute myeloid leukaemia | 11–14, 244 |
| DNMT1 | Colorectal cancer | 245 |
| TET2 | T cell lymphoma, myeloid malignancies including acute myeloid leukaemia | 21,22, 246 |
| TET1, TET3 | Colorectal cancer, chronic lymphocytic leukaemia | 247 |
| MBD1, MBD4 | Colorectal cancer, lung adenocarcinoma, breast cancer, melanoma | 227,230, 234,248 |
| Histone acetylation | ||
| EP300 | Diffuse large B cell lymphoma, follicular lymphoma, small-cell lung cancer, transitional cell bladder cancer, serous endometrial cancer, pancreatic cancer | 33,242, 243, 249–251 |
| CREBBP | Diffuse large B cell lymphoma, follicular lymphoma, small-cell lung cancer, transitional cell bladder cancer, ovarian cancer, relapsed acute lymphoblastic leukaemia | 33,242, 249,250, 252,253 |
| HDAC2 | Colorectal cancer | 254,255 |
| HDAC4 | Breast adenocarcinoma | 256 |
| HDAC9 | Prostate adenocarcinoma | 240 |
| Histone methylation | ||
| MLL | Myeloid and lymphoid leukaemias, majority of infant acute lymphoblastic leukaemia, solid tumours (colorectal, lung, bladder, breast) | 257–259 |
| MLL2 | Non-Hodgkin lymphoma (90% of follicular lymphoma, one-third of diffuse large cell lymphoma) | 33,259 |
| MLL3, MLL4 | Solid tumours: bladder, lung, endometrial, hepatocellular | 229,259, 260 |
| SETD1A | Gastric adenocarcinoma, breast cancer, chronic lymphocytic leukaemia | 234,239, 261 |
| PRDM9 | Head and neck squamous cell carcinoma | 38 |
| EZH2 | Gain of function in non-Hodgkin lymphoma and solid tumours | 33,34 |
| Loss-of-function in myeloid malignancies, head and neck squamous carcinoma, T cell leukaemia | 36–39, 262 | |
| NSD1 | Acute myeloid leukaemia, head and neck squamous cell carcinoma, endometrial carcinoma, melanoma, colorectal cancer, multiple myeloma | 44,263 |
| NSD2 | Paediatric acute lymphoblastic leukaemia, colorectal cancer, melanoma | 45,263 |
| SETD2 | Renal cell carcinoma, early T cell precursor acute lymphoblastic leukaemia, high-grade glioma | 47,262, 264 |
| KDM5C (JARID1C) | Renal cell carcinoma | 30,47 |
| KDM6A (UTX) | Multiple myeloma, oesophageal squamous cell carcinoma, renal cell carcinoma, medulloblastoma, prostate, transitional cell bladder cancer | 6,242, 265,266 |
| KDM2B | Diffuse large B cell lymphoma | 34 |
| Readers | ||
| PHF6 | T cell acute lymphoblastic leukaemia, acute myeloid leukaemia | 267–269 |
| PHF23 | Acute myeloid leukaemia | 270 |
| BRD4 | NUT midline carcinoma | 271 |
| BRD8 | Hepatocellular carcinoma | 229 |
| ING1 | Melanoma, oesophageal squamous cell cancer, acute lymphoblastic leukaemia | 272 |
| Histones | ||
| H3F3A | Paediatric glioblastoma, diffuse intrinsic pontine glioma, giant cell tumour of bone | 237,273 |
| H3F3B | Chondroblastoma | 273 |
| HIST1H3B | Paediatric glioblastoma, diffuse intrinsic pontine glioma | 42 |
| HIST1H1B | Chronic lymphocytic leukaemia, follicular lymphoma, colorectal cancer | 239,256, 274 |
ARID, AT-rich interaction domain; ATRX, alpha thalassaemia/mental retardation syndrome X-linked; CHD, chromodomain helicase DNA binding protein; CREBBP, CREB binding protein; BRD4, bromodomain containing 4; DAXX, death-domain associated protein; DNMT3A, DNA methyltransferase 3α; EP300, E1A binding protein p300; EZH2, enhancer of zeste homologue 2; H3F3, H3 histone, family 3; HDAC, histone deacetylase; HIST1H3B, histone cluster 1, H3b; ING1, inhibitor of growth family member 1; KDM2B, lysine (K)-specific demethylase 2B; KDM5C, also known as JARID1C; KDM6A, also known as UTX; MBD1, methyl-CpG binding domain protein 1; MBD4, methyl-CpG binding domain 4 DNA glycosylase; MLL, mixed-lineage leukaemia; NSD, nuclear receptor binding SET domain protein; PBRM1, polybromo 1; PHF, PHD finger protein; PRDM9, PR domain 9; SETD, SET domain containing; SMARC, SWI/SNF related, matrix associated, actin dependent regulator of chromatin; TET, TET methylcytosine dioxygenase.
Mutations in the DNA methylation machinery are common in haematological malignancies. DNA methyltransferase 3α (DNMT3A) is recurrently mutated in myeloid and lymphoid malignancies, especially in acute myeloid leukaemia (AML) and T cell lymphoma11–13. DNMT3A mutation has prognostic value and is associated with poorer outcomes in both AML and T cell lymphoblastic leukaemia14,15. Mouse models evaluating conditional Dnmt3a knockouts in haematopoietic stem cells (HSCs) revealed enhanced self-renewal and impaired differentiation of HSCs16,17. It has been shown that transplantation of Dnmt3a-null HSCs in mice predisposes for a spectrum of malignancies similar to that observed in patients with DNMT3A mutations, confirming that DNMT3A loss confers a pre-leukaemic phenotype in HSCs18,19.
Frequent mutations of the methylcytosine dioxygenase enzyme TET2, a DNA methylation eraser, have likewise been observed in myelodysplastic syndrome, myeloid malignancies and T cell lymphoma20–22 and is recognized as an unfavourable prognostic factor in AML23. Analyses of clonal evolution in myelodysplastic syndrome and chronic myelomonocytic leukaemia have implicated TET2 mutation as an early oncogenic event24–26. Mouse models of TET2 loss exhibit increased HSC self-renewal and myeloproliferation in the context of impaired erythroid differentiation, supporting the functional importance of these mutations20,27,28.
Mutations in the chromatin remodelling machinery are widespread in solid tumours. The initial discovery of the SMARCB1 deletion in paediatric rhabdoid tumours was followed by the identification of patients with germline SMARCB1 mutations and the subsequent loss of the normal allele leading to the development of rhabdoid tumours, confirming a classic tumour suppressor function for this gene29. Cancer sequencing studies have since revealed that genes encoding components of SWI/SNF chromatin remodelling complexes are among the most common targets of mutation. Prominent examples (TABLE 2) include polybromo 1 (PBRM1) mutations in over 40% of clear cell renal carcinomas30 and AT-rich interaction domain 1A (ARID1A) mutations in over half of ovarian clear cell carcinomas31,32. The identification of ARID1A mutations in atypical endometriotic lesions adjacent to an ovarian clear cell carcinoma suggested that ARID1A loss-of-function may occur early in cancer development32.
Mutations to histone-modifying enzymes are common across a diverse range of cancer types. Mutations affecting the SET domain methyltransferase enhancer of zeste homologue 2 (EZH2), a core component of PRC2, appear to have divergent functions in different cancer types. Gain-of-function hotspot mutations and amplifications have been reported in non-Hodgkin lymphomas and a variety of solid tumours, suggesting that these tumours depend on increased H3K27 trimethylation (H3K27me3)33,34. This was supported by mouse studies showing that the conditional expression of activated mutant Ezh2 induces germinal centre hyperplasia and accelerates lymphomagenesis35. Conversely, loss-of-function mutations of EZH2 are frequently seen in myeloid malignancies, head and neck squamous carcinomas, and T cell leukaemia36–40. Further supporting a transforming influence of EZH2 loss is the finding that EZH2 disruption in mice is sufficient to induce T cell acute lymphoblastic leukaemia41. Interestingly, recently described Lys27Met missense mutations in histones H3.3 and H3.1 in the majority of paediatric diffuse intrinsic pontine glioma also serve to inhibit EZH2 enzymatic activity and result in a global decrease in H3K27me3 (REFS 42,43). These observations supporting a function for EZH2 as either an oncogene or tumour suppressor in different tissue types highlights the complexity of epigenetic modifier alterations in cancer.
Epigenetic modifier mutations are also relevant to cancer progression. Translocations and mutations involving the H3K36 methyltransferases (nuclear receptor binding SET domain protein 1 (NSD1), NSD2 and SET domain containing 2 (SETD2)) are common across haematological and solid tumours, including paediatric acute lymphoblastic leukaemia, multiple myeloma and renal cell carcinoma44–47. The mechanistic importance of the SETD2 mutation to cancer progression was illustrated by a study examining intra-tumour heterogeneity in renal cell carcinoma by sequencing spatially separated samples from the same tumour. This revealed that SETD2 underwent multiple distinct inactivating mutations in different parts of a single tumour, suggesting a selective advantage of this alteration to the progression of renal cell carcinoma48. Accordingly, the SETD2 mutation is associated with poorer outcomes in renal cell carcinoma49. In paediatric acute lymphoblastic leukaemia, comparison of matched patient samples from diagnosis and relapse revealed an enrichment of mutations in epigenetic modifiers, including SETD2, in relapsed disease, supporting a role in cancer progression or resistance to treatment50. Epigenetic modifier mutations in cancer may thus be early events driving carcinogenesis (as in the inactivation of SMARCB1 in paediatric rhabdoid tumours or the TET methylcytosine dioxygenase 2 (TET2) mutation in myeloid malignancies), or late mutational changes related to progression (such as SETD2 in renal cell carcinoma).
Epigenetic mediators
Role of stemness and pluripotency factors
Epigenetic modifiers often target regulatory elements that affect the levels of insulin-like growth factor 2 (IGF2) expression and downstream signalling in diverse tumours, such as embryonal tumours of childhood, including Wilms tumour, rhabdomyosarcoma and hepatoblastoma51–54, as well as adult tumours such as colorectal cancer55. Loss of imprinting (LOI) of IGF2 is an epigenetic change that modifies the expression of IGF2, leading to a doubling of dosage. LOI of IGF2 was first identified in embryonal tumours of childhood51–54,56. The dosage of IGF2 is quantitatively related to the growth and number of adenoma57 and increased levels of IGF2 are linked to both hyperproliferation in nephrogenic rests, which predisposes to Wilms tumour58 and the increased proliferation of colon progenitor cells59. This information converges on the observations that the IGF2 signalling pathway is a key mediator of the self-renewal of CSCs in hepatocellular carcinoma60. The LOI of IGF2 in the disorder Beckwith–Wiedemann syndrome provided the first causal argument for the role of epigenetic changes in cancer. Beckwith–Wiedemann syndrome is the canonical disorder for a causal epigenetic risk factor in malignancy, similar to tumour protein p53 (TP53) for conventional mutations, because the epigenetic changes in Beckwith–Wiedemann syndrome precede the development of cancer, are associated with pre-malignant growths (perilobar nephrogenic rests), the epigenetic changes are found in sporadically occurring kidney lesions in newborn infants, and the presence of LOI in Beckwith–Wiedemann syndrome is specifically associated with a substantially increased cancer risk61.
IGF2 and IGF1 receptor (IGF1R) signalling are thus emerging as key, context-dependent regulators of stem cell self-renewal and the proliferation of early progenitor cell pools in normal tissue architectures62–64, tumour tissues59,60 and embryonic stem cell (ESC) cultures65. The properties of IGF2 in promoting stemness and tipping the balance between the stem/progenitor cell pool and differentiated progeny seems to be tightly connected with its role in cancer initiation and progression57,59,60. We suggest that factors contributing to a cell state change towards stem-cell-like phenotypes have central roles in cancer development and we term these factors epigenetic mediators (FIG. 1; TABLE 1). We envisage that epigenetic mediators act at all stages of cancer development by preventing differentiation and eroding barriers against dedifferentiation (FIG. 1). Epigenetic-mediator-induced alterations in the chromatin landscape of cells of origin eventually lead to increased phenotypic flexibility and heterogeneity within the epigenetically altered, precancerous progenitor cell pool; this feature is subsequently selected for and maintained in the tumour tissue during progression.
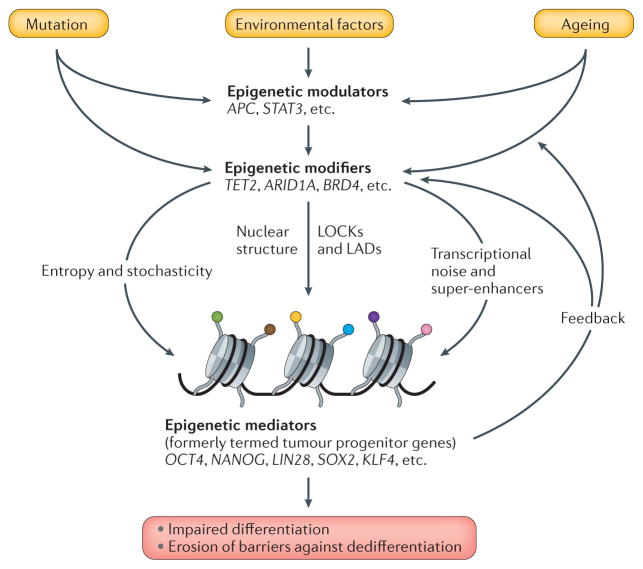
Figure 1. Functional classification of cancer genes and their contribution to malignancy.
Ageing, inflammation and chronic exposure to carcinogens impinge on epigenetic modulators, such as adenomatous polyposis coli (APC) and signal transducer and activator of transcription 3 (STAT3), that fine tune and regulate the function of epigenetic modifiers — for example, TET methylcytosine dioxygenase 2 (TET2) and AT-rich interaction domain 1A (ARID1A) — to bring about changes in the expression of epigenetic mediators — for example, sex-determining Y-box 2 (SOX2) and OCT4 — whose gene products regulate developmental potential. Chronic exposure to a fluctuating, cancer-predisposing environment and ageing promote the selection for epigenetic heterogeneity in vulnerable populations of somatic stem cells and progenitor compartments. Mutations in modulators and modifiers are often selected for during cancer development, which leads not only to increased cell proliferation, but also to the unscheduled expression of mediators that, in turn, inhibit differentiation and promote epigenetic plasticity by affecting the epigenetic modulators and modifiers in a feedback loop. The mechanism of epigenetic instability involves the erosion of barriers against dedifferentiation, such as large organized chromatin K9 modifications (LOCKs) overlapping with lamina-associated domains (LADs), and the emergence of hypomethylated blocks that contain the most variably expressed domains of the tumour genome and interfere with normal differentiation. Increased transcriptional noise at developmentally regulated genes is paralleled by the redistribution of super-enhancers from cell-fate-determining genes to oncogenes that further stabilize the cancer cell state. Stochastic changes in unstable chromatin states lead to the continuous regeneration of epigenetic heterogeneity that manifests as increased cellular entropy and provides the basis for the selection of the fittest during cancer evolution. BRD4, bromodomain containing 4; KLF4, Kruppel-like factor 4.
Feinberg et al.1 hypothesized the existence of a group of tumour progenitor genes that counteract proper maturation programmes when ectopically expressed or overactive. Such genes, we suggest, belong to the epigenetic mediator category and include, for example, well-known pluripotency factors such as NANOG66, OCT4 (also known as POU5F1)67 and WNT signalling members68. Epigenetically altered genes in induced pluripotent stem cells largely overlap epigenetically altered genes in cancer69. Experimental evidence in mouse model systems has already established that the ectopic expression of NANOG66,70 promotes hyperplastic growth. Furthermore, when challenged with an overactive WNT signalling pathway, the ectopic expression of NANOG in mammary epithelial cells accelerated the development of adenocarcinomas70, demonstrating that the unscheduled expression of pluripotency genes can indeed predispose to and drive cancer development. Further highlighting the ability of mediators to reprogramme chromatin states during the initial phase of tumour development, the premature termination of in vivo reprogramming towards the pluripotent stem cell state led to cancer development in a mouse model system71. Finally, the transient, ectopic expression of OCT4 in vivo induces hyperplastic and dysplastic changes in mouse epithelial tissues and the intestine, with a concomitantly increased progenitor cell pool and increased β-catenin–WNT signalling pathway activity72. The persistent, long-term expression of OCT4, on the other hand, results in the histological features of carcinoma in situ and the emergence of invasive tumours in the skin. Hence, although OCT4 is not essential for somatic stem cell maintenance in the mouse model67, somatic stem cells retain their ability to respond to pluripotency cues that can lead to impaired cellular differentiation72. As the cancer phenotypes in these mouse models depend on the continuous presence of reprogramming factors instead of the presence of irreversible mutations, mediators probably target the epigenome to bring about changes in cell states on the path to cancer71,72.
To destabilize phenotypes and impair differentiation, mediators influence the epigenetic states that define differentiated cell types (FIG. 2). Cellular differentiation is accompanied by the establishment of large blocks of repressive H3K9me2 and H3K9me3 modifications, which, together with DNA methylation73, coordinate the stable, cell-type-specific repression of developmentally regulated genes74. These so-called large organized chromatin K9 modifications (LOCKs) are largely absent from ESCs and cancer cell lines74, which may underlie the phenotypic plasticity of these cell states. In line with the role of LOCKs in the maintenance of differentiated phenotypes, the generation of induced pluripotent stem cells involves the genome-wide reprogramming of DNA methylation and histone modifications75. Reprogramming of chromatin states are induced in part by the OCT4-mediated recruitment of H3K9me2 histone demethylase and chromatin remodelling complexes76.
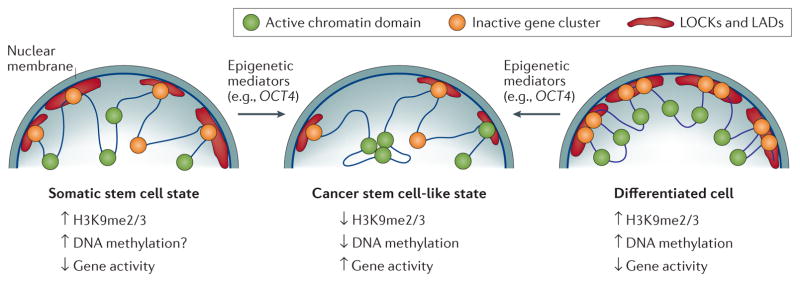
Figure 2. Change in cell state towards cancer stem cell states induced by reprogramming of the 3D epigenome.
This hypothetical scheme explains how epigenetic mediators (for example, OCT4) might reprogramme the epigenome to tip over normal somatic stem cells or differentiated progenitor cells into cancer stem cell states displaying phenotypic heterogeneity. Large organized chromatin K9 modifications (LOCKs) (red cloud) overlapping with lamina-associated domains (LADs) are hypothesized to be largely absent in somatic stem cells (left panel) to ensure epigenetic flexibility associated with the multipotent state. The coordination of cell-type-specific repressed states (right panel) within the LOCKs/LADs is facilitated by epigenetic modifiers establishing multiple layers of epigenetic modifications, such as H3K9me2, H3K9me3, and DNA methylation. The localization of LOCKs/LADs to the lamina leads to the separation of active and inactive domains to reduce transcriptional noise and to provide barriers for dedifferentiation. Conversely, the unscheduled activation of epigenetic mediators leads to the erosion of LADs/LOCKs and the emergence of hypomethylated blocks during the neoplastic process. This, in turn, induces phenotypic heterogeneity by increasing the variability in expression and the probability of switches between the diverse cellular states within the tumour. A loss of LOCKs is postulated, moreover, to interfere with the constraints of enhancer–promoter communication within and between topologically associated domains (TADs), enabling the clustering of oncogenic super-enhancers and expression domains (green circles) to coordinate the expression of oncogenic pathway members (centre panel).
Mediator-induced epigenetic instability and phenotypic plasticity also seem to contribute to tumour evolution during the later stages of tumour development. The expression of OCT4, for example, plays a key part in human testicular germ cell tumour progression and malignant potential77. Similarly, sex-determining Y-box 2 (SOX2), another core pluripotency factor78, is amplified in small-cell lung cancer and squamous cell carcinomas of the lung and oesophagus79,80 and is linked to a poor prognosis in a range of human cancers, such as nasopharyngeal carcinoma81, lung adenocarcinoma82 and breast cancer83. Finally, NANOG and OCT4 have been associated with increased metastatic potential in breast cancer70,83 and lung adenocarcinoma82. The underlying mechanism may in all of these cases relate to the fact that OCT4, NANOG and SOX2 form extensive feed-forward and feedback loops to organize a stem-cell-like transcriptional enhancer circuitry in ESCs that not only prevents proper maturation until it is downregulated84, but may also contribute to the heterogeneity of tumour cell states and phenotypes.
Relevance to cancer stem cells
The presence of immature cell states with self-renewal capacity, occupying the so-called CSC states, is well established in tumours85–87. Although such stem-cell-like cancer cells make up only a minority of the tumour mass, they have the potential to affect tumour heterogeneity via the stochastic initiation of maturation processes85–87 and stochastic transitions between more or less differentiated cellular phenotypes88. Such phenotypic flexibility of tumour cells is further illustrated by experiments showing that, irrespective of the initial differentiation status, cancer cells are able to re-establish the immature–mature tumour cell mix when cultured individually89.
It is important to note that the cell of origin might not be synonymous with CSCs and can be represented by more or less differentiated cell types. For example, mouse model systems have established that the dedifferentiation of mature intestinal epithelial cells precedes the emergence of cancer cells with stem cell features and tumour formation in the intestine90. Furthermore, knocking down tumour suppressor genes in mouse post-mitotic neurons led to the generation of glioblastoma stem cells91. Examples where the specific targeting of somatic stem cells led to the emergence of CSC states include the observations that activation of the WNT pathway in mouse crypt stem cell populations, but not in transit-amplifying progenitor cells, induced the formation of macro-adenomas in the mouse intestine92.
Although the identification of the cell type of origin remains largely elusive in most human cancers, there is good evidence that an initial imbalance between the somatic stem cell and differentiated cell compartments can predispose to cancer, not only in mouse model systems59, but also in human tumours58. Furthermore, although the initial target in chronic myeloid leukaemia is the HSC, CSC features have been ascribed to more mature granulocyte-macrophage progenitor cells, typically with an overactive WNT signalling pathway93. HSCs also seem to be the cell of origin in more mature lymphoid malignancies, such as chronic lymphocytic leukaemia94.
In a similar manner to normal stem cells, which occupy specific compartments within tissues, the so-called stem cell niches, cancer cells displaying stem-like features frequently thrive in ecological niches in which they strike a symbiotic relationship with the microenvironment to support their propagation and phenotypic plasticity. Thus an overactive IGF2 gene in cancer-associated fibroblasts supports the propagation of lung cancer stem cells95, whereas glioblastoma CSCs not only contribute to the endothelial lining, but also gain sustained Notch signalling induced by factors produced by the endothelial lining96,97. Similarly, myofibroblasts produce hepatocyte growth factor to locally support the maintenance of CSC states in the colon and their clonogenicity98. Strikingly, factors secreted from myofibroblasts were also reported to able to induce more differentiated tumour cells to enter into CSC states98. Taken together, these examples suggest that cells with stem-like features thrive due to their ability to instruct their ectopic microenvironments to render them permissive for the expansion of stem-like cells. There is thus a constant flux of information within the expanding tumour and between the tumour and its microenvironment on the path to the increased autonomy of tumour cells87.
The observations that epigenetic mediators contribute not only to the emergence and maintenance of CSC states, but also to tumour progression, indicates that these genes are key players from the very early stages of cancer initiation in cells of origin to metastasis formation (FIG. 2). If correct, the targeting of epigenetic mediator genes should be central in therapeutic interventions to not only reduce cancer risk, but also to antagonize the growth of the primary tumour and metastatic derivatives (see below).
Epigenetic modulators
Given the central role of epigenetic mediators as reprogramming factors in both development and cancer, the two most important questions are: what underlies their unscheduled activation and how do they reprogramme the epigenome? We suggest introducing the term epigenetic modulators to describe the factors that influence the activity and/or localization of the epigenetic modifiers in order to destabilize differentiation-specific epigenetic states. These epigenetic modulators might also indirectly facilitate the unscheduled expression of epigenetic mediators and promote the mediator-induced reprogramming of cellular phenotypes. Epigenetic modulators thus serve to transduce signals from environmental agents, injury, inflammation, ageing and other cellular stressors towards modifiers to alter the chromatin states at tumour suppressors or oncogenes and to promote epigenetic flexibility and the acquisition of stem-like features early during cancer development. Epigenetic modulator genes are often the targets of driver mutations during the late stages of the disease (FIG. 1; TABLE 1).
Oncogenic RAS signalling
Recent reviews have highlighted the importance of chromatin modifications in the spatiotemporal integration of diverse signals from cellular signalling and metabolic pathways99,100. Cancer-relevant signalling pathways thus regulate epigenetic modifiers to indirectly destabilize cellular phenotypes during tumour development (FIG. 1; TABLE 1). A notable example of epigenetic modulators is oncogenic RAS, which orchestrates global101 and local102–104 chromatin modifications that are essential for RAS-mediated transformation. Oncogenic KRAS-induced transformation of non-malignant cell lines thus requires the KRAS-induced downregulation of TET enzymes, leading to an increase in DNA methylation that facilitates the silencing of tumour suppressor genes101. KRAS-mediated silencing of a defined set of tumour suppressor genes, on the other hand, is achieved and maintained by sequence-specific transcriptional repressors that target epigenetic modifiers to regulatory elements102–104. Activated KRAS has thus been shown to increase the level of the ZNF304 transcription factor that binds to the SETDB1–KAP1–DNMT1 repressor complex and targets it to the promoter of tumour suppressor genes located, for example, in the INK4A–ARF (also known as (CDKN2A) locus104. Interestingly, silencing of the same tumour suppressor locus promotes the maintenance of pluripotency in ESCs104 and serves as the rate-limiting factor for the generation of induced pluripotent stem cells105. In line with the profound effects of oncogenic KRAS on the epigenome, lentiviral delivery of mutant KRAS into human basal cells and luminal progenitors isolated from mammary tissue induced their rapid and efficient transformation accompanied by a loss of lineage-specific gene expression. The transformed cells formed and maintained phenotypically heterogeneous, serially transplantable tumours in mice106, indicating the successful establishment of self-renewing CSC states.
Signalling pathways in chronic inflammation
Another prominent example of cancer-promoting pathways regulating the epigenome is represented by nuclear factor-κB (NF-κB) signalling, which, in part, mediates the effect of chronic inflammation on cancer predisposition107–109. Mouse models of intestinal tumorigenesis uncovered that, in the presence of an overactive WNT signalling pathway, NF-κB induced the dedifferentiation of mature cells, and promoted the acquisition of stem-like characteristics and cancer initiation90. Furthermore, the aberrant activation of NF-κB signalling in the mammary epithelium in doxycycline-inducible mouse models induced altered tissue architecture reminiscent of carcinoma in situ109. On the transient activation of the Src oncogene in vitro, NF-κB participated in a positive feedback loop with the inflammatory cytokine interleukin-6 and transcription factor STAT3, which mediated a stable phenotypic switch from the immortalized mammary epithelial cell state towards a stably transformed, self-renewing state110. Intriguingly, STAT3 (REF. 111) is a key factor in the maintenance of OCT4, NANOG and SOX2 expression by binding to their enhancers during early mouse development112. As STAT3 also promotes proliferation, survival113 and the acquisition of stem cell features in cancer114, one possibility is that chronic inflammation leads to unscheduled activation of epigenetic mediator genes in the cells of origin via STAT3 activation (FIG. 1; TABLE 1). Although STAT3 can interact with epigenetic modifiers, such as the p300 histone acetlytransferase (HAT), SIN3A histone deacetylase (HDAC) complexes or DNMT1 to influence gene expression, cell-type-specific transcriptional effects will probably be influenced by pre-existing chromatin marks115. Signalling pathways activated by chronic inflammation, such as NF-κB signalling, probably directly or indirectly modulate several layers of the epigenome116–118, thereby modulating the effects of STAT3 activation. Using a colitis-induced mouse colon cancer model, single base methylation analyses have revealed that chronic inflammation induces the hypermethylation of several genes important in gastrointestinal homeostasis and repair, a subset of which is also hypermethylated in mouse intestinal adenomas and human colorectal cancer116, further supporting the view that chronic inflammation is a key modulator of epigenetic lesions early during tumour development. Inflammation might contribute to the ectopic expression of epigenetic mediators in tumour-initiating cells by the activation of YAP1, a core member of the Hippo pathway119, which is able to bind p300 (REF. 120) and is a key regulator of intestinal epithelial regeneration in response to inflammation119 as well as an activator of OCT4 and SOX2 (REF. 121) in CSCs of non-small-cell lung cancer.
Tumour suppressor genes as epigenetic modulators
Further examples of epigenetic modulators in cancer include the tumour suppressor protein p53 (FIG. 1; TABLE 1). Gain-of-function p53 mutations in cancer thus endow p53 with the ability to induce genes encoding the histone-modifying enzymes MLL1, MLL2 (mixed-lineage leukaemia) and MOZ, resulting in genome-wide increases in histone H3K9 acetylation and H3K4 trimethylation122. Mutant p53 was likewise recently shown to enact promoter remodelling via a physical interaction with the SWI/SNF chromatin remodelling complex123. Similarly, the adenomatous polyposis coli (APC) tumour suppressor gene has been shown to control intestinal cell differentiation via the regulation of DNA methylation dynamics, as a loss of APC upregulates a DNA demethylase system and leads to the hypomethylation of key intestinal cell fate genes124 (FIG. 1; TABLE 1). Finally, mutations in epigenetic modulators might affect DNA and histone methylation by leading to the production of oncometabolites that inhibit α-ketoglutarate-dependent epigenetic modifiers, such as histone lysine demethylases and TET hydroxylases (FIG. 1; TABLE 1). Mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2 enzymes may, for example, alter the epigenome of tumour cells and block differentiation by causing the accumulation of the D-2-hydroxyglutarate oncometabilite125. Furthermore, mutations in fumarate hydratase (FH) and succinate dehydrogenase (SDH) might lead to the accumulation of their substrates, fumarate and succinate, which serve as competitive inhibitors of histone demethylases and TET enzymes, consequently altering DNA and histone modifications126. Similarly to epigenetic modifiers, modulators are thus often targeted by driver mutations in cancer to promote not only cell proliferation, but also epigenetic instability127.
Effects of ageing
Ageing may influence cancer risk via epigenetic change downstream of epigenetic modulators and mediators. A comparison of newborn infants and centenarians provided a strong suggestion of age-related changes in DNA methylation, subsequently borne out in multiple studies controlling for differences in cell type and exposure128–131, also called epigenetic drift132. Interestingly, a recent comprehensive evaluation of age-associated DNA methylation changes in blood cells identified megabase-scale age-associated hypomethylated blocks that also showed preferential hypomethylation in age-matched cancers131. Analyses of chromatin modifications in ageing have also identified multiple age-associated alterations, including the loss of heterochromatin and a redistribution of activating H3K4me3 marks133,134. A role for epigenetic modifiers in ageing has been reinforced by studies showing that the disruption of histone-modifying enzymes affects lifespan in model systems134. Prominent examples include lifespan extension in Caenorhabditis elegans by the disruption of the H3K4 trimethylation machinery and lifespan extension in Drosophila melanogaster by the heterozygous mutation of PRC2 components135,136. A further link between ageing and chromatin alterations comes from cellular models of premature ageing disorders such as Werner syndrome and Hutchison–Gilford progeria syndrome. In an ESC model of Werner syndrome, the differentiation of ESCs to mesenchymal stem cells recapitulates cellular ageing and is marked by a global loss of H3K9me3 and changes in heterochromatin architecture137. Similarly, in Hutchison–Gilford progeria syndrome, skin fibroblasts show the passage-dependent loss of heterochromatin compartmentalization related to altered H3K27me3 marks138. Epigenetic modulator signalling upstream of age-related chromatin alterations is only beginning to be defined. In C. elegans, the forkhead box O (FOXO) transcription factor DAF-16 serves as an effector of an environmentally responsive insulin-like signalling pathway and regulates longevity via recruitment of the SWI/SNF chromatin remodelling complex to target genes139. Similarly, the energy sensor AMP-activated protein kinase mediates longevity induced by dietary restriction in worms and flies, and impinges on chromatin regulation via the phosphorylation of HDACs and histone H2B140,141. Ageing is characterized by epigenetic change, and a more thorough understanding of the roles of epigenetic modifiers and modulators in this process is likely to inform our understanding of cancer aetiology and risk.
Effects of environmental exposures
A crucial role for the dietary availability of methyl donors in cancer prevention has been demonstrated in animal models and human studies. A methyl-deficient diet is sufficient to induce liver neoplasms in rats142,143. Notably, the dietary deficiency of methyl donors in these animals produced global and gene-specific DNA hypomethylation144,145. Likewise, human studies have shown that a low dietary intake of folate or methionine increases the risk of colon adenomas146. Furthermore, in utero exposure to higher folate and similar one-carbon nutrients has been linked to a reduced risk of childhood acute lymphoblastic leukaemia, brain tumours and neuroblastoma147. Excessive alcohol consumption may increase cancer risk in part via folate depletion. Chronic alcohol consumption in rats results in DNA hypomethylation in the colonic epithelium148. In a human cohort study, low folate and a high alcohol intake were linked to the increased methylation of genes implicated in colorectal cancer149.
Specific carcinogenic exposures have been shown to perturb the DNA methylome150. The aerodigestive tract epithelium of heavy smokers without evidence of cancer displays the aberrant methylation of multiple genes implicated in the pathogenesis of lung cancer151. Similarly, the hypermethylation of genes related to cancer progression was demonstrated in both the bronchial epithelium and peripheral lymphocytes of smokers152. Occupational exposure to airborne benzene in humans has been linked to the hypomethylation of repetitive elements as well as gene-specific hypermethylation, recapitulating changes also found in malignant cells153. Infection with Helicobacter pylori, an aetiological agent in gastric adenocarcinoma and lymphoma, results in increased CpG island methylation in the non-cancerous gastric mucosa154, which is reversible on the eradication of H. pylori infection155. Asbestos, a carcinogen that is not inherently mutagenic, has been suggested to influence cancer risk via an epigenetic mechanism. Accordingly, DNA methylation profiles distinguish pleural mesothelioma from normal pleura and predict the lung burden of asbestos 156. Although the link between epigenetic modifiers, environmental exposure and cancer risk is clearly established, much less is known about the identity of signalling pathways and epigenetic modulators that causally connect carcinogens to the writers of the epigenome.
Deregulated 3D nuclear architecture
Alterations of the epigenome during ageing and in cancer are tightly interconnected with the 3D organization of chromatin157 that modulates chromatin states in both development and cancer (FIG. 2). Hypomethylated blocks thus overlap with lamina-associated domains (LADs)158, which contain repressed, gene-poor regions constitutively localizing to the nuclear periphery, and developmentally repressed genes that are recruited to the lamina in a cell-type-specific manner159. In differentiated cells, a significant fraction of LADs overlaps with large domains enriched in repressive H3K9me2 and H3K9me3 histone modifications called LOCKs74, which expand during differentiation to coordinate cell-type-specific transcriptional repression160–162. Interestingly, downregulation of the epigenetic mediator gene OCT4 coincides with the formation of compact chromatin at the lamina in mice163, suggesting that 3D chromatin compaction itself might contribute to repression164–166 during lineage specification.
Repressive chromatin marks and peripheral localization are functionally intertwined167 and might be particularly sensitive to ageing-related and cancer-predisposing perturbations167–169 (FIG. 2). The recruitment of certain genomic regions to the repressive environment of the nuclear envelope is promoted by sequence-specific transcriptional repressors170, factors that deposit and recognize repressive histone modifications (H3K9me2/me3 (REF. 170) and H3K27me3 (REFS 170,171)), DNA methylation-binding proteins172 and components of the nuclear envelope167. These factors thus act as epigenetic modulators by regulating the position of genomic regions within the 3D nucleus. In turn, the lamina modulates chromatin states by attracting repressive epigenetic modifiers, such as lysine-specific histone demethylase 1A (KDM1A; also known as LSD1)173, histone-lysine N-methyltransferase EHMT2 (also known as G9A)174, HDAC3 (REFS 175,176) and the nuclear co-repressor (N-CoR) complex177 that maintain a repressive environment at the nuclear periphery167,178. These epigenetic modifiers balance self-renewal and differentiation179,180, affect reprogramming into the pluripotent state181 and contribute to ageing-related chromatin changes182,183 and cancer183,184, suggesting that their mechanism of action ties spatiotemporal compartmentalization in the nucleus to the modulation of the epigenome and cellular states (FIG. 2).
In agreement with the role of LOCKs in the maintenance of cellular memories, tumour growth factor-β (TGFβ)-induced epithelial-to-mesenchymal transition (EMT) is preceded by the dual-specific lysine demethylase, LSD1-mediated global loss of H3K9me2 at LOCKs185. Chromatin changes in EMT are reminiscent of ESCs with reduced LOCKs, although the epigenetic modulators that direct LSD1 activity from H3K4me2 demethylation towards H3K9me2 demethylation within LOCKs on treatment with TGFβ and the role of this epigenetic modifier in regulating the levels of H3K9me2 and H3K9me3 in pluripotent cells have not yet been identified74,185,186. Importantly, these experiments might provide mechanistic support for the earlier observations that link EMT phenotypes to the acquisition of stem cell traits74,185,186. Cancer cells might thus gain phenotypic plasticity by acquiring EMT-related chromatin changes leading to the impaired stabilization of cellular memories (FIG. 2). Hence regions displaying a loss of H3K9me2 and H3K9me3 in various cancer cell lines overlap with hypomethylated blocks and the location of increased variability in the gene expression of cancer-relevant and developmentally regulated genes in diverse cancer types157. We envisage that developmental decisions are stabilized by multiple layers of epigenetic modifications, which are established and/or maintained at the lamina. The factors that regulate chromatin–lamina interactions and recruit chromatin-modifying enzymes to the nuclear periphery might thus act as epigenetic modulators by positioning the genome within the nucleus and coordinating the activity of epigenetic modifiers in space and time (FIG. 2). A failure to orchestrate such spatiotemporal crosstalk between repressive chromatin factors will probably lead to the emergence of cells with unstable phenotypes of impaired differentiation. Some of these cells might maintain or regain self-renewal capacity due to epigenetic mediator gene products, representing transition cell fates towards CSCs (FIG. 3).
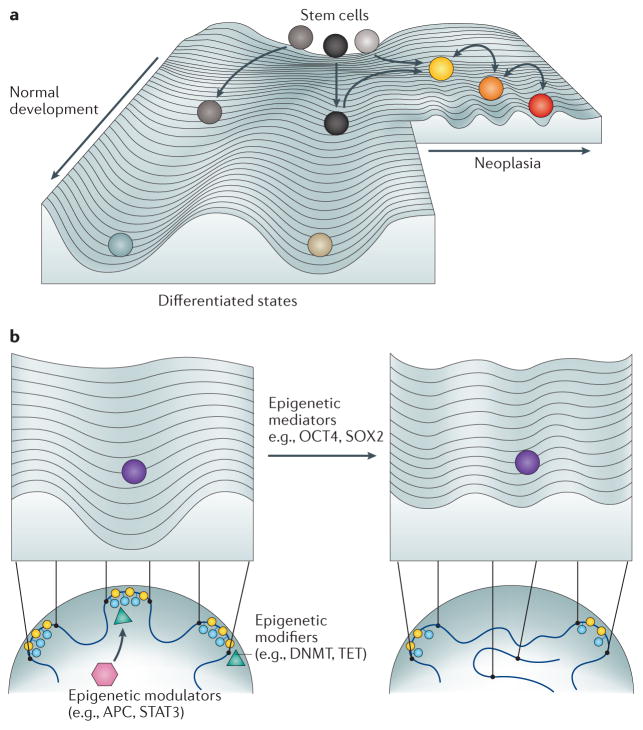
Figure 3. Waddington landscape of phenotypic plasticity in development and cancer.
a. The Waddington landscape of development is adapted to compare cellular states of different entropy during normal differentiation (left side of image) and in cancer (right side of image). The developmental potential of normal somatic stem cells (grey balls) positioned on the top of the hill correlates with high entropy, which is mediated by cellular heterogeneity (different shades of grey). During differentiation, cells are guided towards well-defined cell fates (light blue and brown balls) with lower entropy, paralleled by a decrease in transcriptional noise and the stabilization of cell states (deepening of the valleys or canalization). Cancer stem cell (CSC) states (yellow ball) arise when epigenetic instability interferes with normal differentiation and leads to the erosion of barriers against dedifferentiation — for example, via the erosion of large organized chromatin K9 modifications and the emergence of hypomethylated blocks. In a similar manner to normal differentiation, CSCs with higher entropy occupy higher altitudes on the hill than cancer cells (orange and red balls), although the difference is smaller than between normal stem cells and differentiated progeny. Increased transcriptional noise (shallow valleys) and stochastic switches between diverse cell states (arrows between valleys) are regulated by the interplay between epigenetic modulators, modifiers and mediators, the deregulated epigenome and fluctuating environmental cues (for example, inflammation, repeated exposure to carcinogens, ageing or an overactive WNT pathway). Finally, cellular heterogeneity (yellow, orange and red balls) within the tumour eventually enables selection mechanisms to drive the growth of the fittest clone. b. Illustration of the role of epigenetic modifiers, modulators and mediators on the Waddington landscape described in part a. Epigenetic modulators (pink hexagon) regulate the activity of epigenetic modifiers (green triangles) that induce the ectopic expression of epigenetic mediators. Mediators dynamically alter the contour of the landscape via feedback loops that target epigenetic modifiers such as chromatin modifications (blue circles), lamin proteins (yellow circles) and chromosomal interactions (new loop on right). The expression of epigenetic mediators thus produces a shift in the epigenetic landscape, enabling the sampling of aberrant developmental outcomes displaying increased phenotypic plasticity in neoplastic or pre-neoplastic cells. APC, adenomatous polyposis coli; DNMT, DNA methyltransferase; SOX2, sex-determining Y-box 2; STAT3, signal transducer and activator of transcription 3; TET, TET methylcytosine dioxygenase.
Epigenetic stochasticity
Large domains of epigenetic variability
We have previously suggested that a major driving force for tumour evolution is the emergence of epigenetic stochasticity, allowing rapid selection for growth-favouring tumour traits in a changing microenvironment187–189. Understanding the nature and genomic location of such stochastic variation, as well as the interplay between epigenetic modifiers, epigenetic modulators and epigenetic mediators that destabilize the epigenome to increase stochastic noise, is thus likely to be essential in tackling tumour evolution and resistance to treatment. Experimental evidence has confirmed that stochastic DNA methylation alterations in cancer involve large regions of the epigenome190,191. This stochastic epigenetic change does not occur genome-wide. Rather, genome-wide views of epigenetic variation have shown that large hypomethylated blocks, constituting up to one-third of the genome, contain the most variably methylated regions of the tumour genome190,192. These domains arise early during cancer development191,193,194 and contain the most variably expressed genes regulating cancer-relevant functions190. Moreover, the degree of variation in methylation in early precursor lesions predicts cancer risk193,194, suggesting a causal link between these epigenetic changes and cancer. Hypomethylated blocks in cancer largely correspond to partially methylated domains in normal cells as well as LADs and LOCKs (FIG. 2). These regions underlie much of the reported variation in methylation at CpG islands, shores and distant CpG sites, fuelling phenotypic variation in cancer191,192. In addition, the degree of variation in methylation191, as well as the deviation of the variability in gene expression from the normal corresponding tissue, is a predictor of cancer progression195. The combination of ageing and chronic sun exposure — the two leading causes of skin cancer — induces the widespread formation of hypomethylated blocks in the epidermis at genomic regions that are hypomethylated in squamous cell carcinoma and that overlap with colon cancer-specific hypomethylated blocks196. These same regions are the very ones that show further alterations in methylation in squamous cell cancers arising within the same skin. Given the overlap of these regions with LADs and LOCKs, these data also indicate that the interplay between altered 3D genome organization, stochastic epigenetic change and impaired differentiation mediate the effect of environmental damage with photo-ageing196.
Network entropy and nuclear structure
Recent work has described cellular heterogeneity as network entropy — applied as a measure of signalling pathway promiscuity — and established that the level of network entropy provides an estimate of developmental potential160,197. In other words, the high entropy of a heterogeneous pluripotent stem cell population maintains a diverse range of pathways associated with more mature phenotypes in a poised state for activation. Consistent with the signalling entropy model of cellular differentiation, the variability in the expression of signalling factors and developmental regulators has been experimentally linked to the differentiation potential of ESCs198. In a similar manner to normal differentiation, CSCs display a higher entropy than cancer cells, although the difference is smaller than between normal stem cells and differentiated progeny160. Furthermore, CSCs consistently have a lower entropy than their normal counterparts, indicating the presence of dominating oncogenic pathways. This is in agreement with models suggesting that cancers represent hybrid states between aberrantly increased as well as decreased epigenetic flexibility188 (FIG. 3a).
Importantly, transitions between cellular states of different entropy seem to be regulated epigenetically. Using quantitative RNA fluorescence in situ hybridization in combination with time-lapse movies, the transient stabilization of distinct, noisy expression patterns that predict the potential for differentiation has been linked to changes in the global level of DNA methylation in ESCs199. These findings highlight that changes in DNA methylation might stabilize not only irreversible, but also reversible cell fate transitions, and regulate stochastic switches between states. As opposed to the short timescales of transcription bursts, the long timescales of infrequent state switching follow a stochastic bistable switch model regulated by methylation and demethylation. Interestingly, ESCs and testicular cells display a bimodal and coherent methylation pattern that becomes variable during differentiation and with age200. Further supporting the model in which stochastic variation in fuels aberrantly increased cellular heterogeneity, Epstein–Barr virus immortalization of human B cell cultures induces the emergence of hypomethylated blocks linked with hypervariable DNA methylation and gene expression158. In summary, these experiments are consistent with a model in which inherently stochastic DNA methylation variation unleashed within hypomethylated blocks continuously re-establishes tumour cell heterogeneity and thereby promotes the adaptation of the tumour tissue to changing microenvironments, facilitating the survival and growth of tumour cells outside the context of normal tissue architecture and at metastatic sites188 (FIG. 3a,b).
Mechanism of stochastic epigenetic variation
Recent experiments suggest that the molecular mechanisms of increased stochastic epigenetic variation might involve deregulated spatial separation between active and inactive chromatin environments and/or altered chromatin mobility between different sub-compartments of the nucleus74,157,188,201,202. In accordance with the reversible nature of chromatin modifications, the relocation of LADs and LOCKs away from the lamina has thus been linked to the erosion of repressive marks and an increase in transcriptional activity203. Importantly, the long-term stability of H3K9me2 marks in cycling cells seems to be ensured via the stochastic re-establishment of chromatin–lamina interactions in the G1 phase of the cell cycle203. Compromised recruitment of inactive chromatin domains to the lamina in G1 might thus lead to the heterogeneous erosion of LOCKs within a cell population, leading to stochastic reactivation of genes located within these domains. Similarly, the stochastic relocation of genes to the periphery might contribute to variegated silencing — that is, cell-to-cell variation in gene transcription depending on the subnuclear position, a phenomenon that also includes stochastic allelic exclusion that limits the production of antigen receptors to a single allele per cell204. Moreover, circadian chromatin transitions are also linked to the transient recruitment of clock-controlled loci to lamina205. 3D genome organization itself thus emerges as an epigenetic modulator that fine tunes the spatiotemporal aspects of epigenetic modifier activities to affect phenotypic plasticity in development and cancer (FIG. 3b). We hypothesize that epigenetic mediators promote the emergence of cancer stem-like states and phenotypic flexibility in part by counteracting the formation of repressive subnuclear compartments and the spatial separation between active and inactive chromatin domains. This is likely to require crosstalk between the epigenetic mediators and epigenetic modulators that regulate the dynamics of the 3D nuclear architecture, as well as interaction with epigenetic modifiers to disrupt the multiple layers of epigenetic modifications that establish the differentiated cell state. Very little is known about how different epigenetic perturbations in cancer synergize to deregulate 3D genome organization and influence transcriptional variability. Nonetheless, an interesting opening is provided by the findings that impaired PRC2 function leads to a stochastic loss of repression and increased transcriptional variability at PRC2 target genes, which is linked to a poor prognosis206. H3K27me3 modifications are moreover enriched in LADs close to LAD boundaries207 and have not only been linked to the recruitment of genomic regions to the lamina170, but are also suggested to collaborate with H3K9me3 marks to promote HP1 binding to chromatin208, with potential consequences on the stringency of transcriptional repression genome-wide.
Enhancer usage
Enhancer elements integrate signals from developmental and oncogenic pathways, as well as chromatin organization, to modulate the probability and variability of transcriptional bursts at the associated transcriptional units79,209–211. We envisage that tumour-specific 3D chromatin organization modulates the epigenome and undermines differentiation in part by affecting the specificity and dynamics of enhancer–promoter communication. Global maps of chromatin contacts have thus uncovered long-range enhancer–promoter loops within and between chromosomes212 that fine tune the cell-to-cell variability of gene expression, potentially providing selectable features in a cell population213. Conversely, the robustness of cell-type-specific gene expression is ensured by the local clustering of multiple enhancer elements in cis spanning tens or hundreds of kilobases214. These so-called super-enhancers evolved to integrate signals from multiple cell-fate-determining pathways to ensure a high probability of transcription at genes defining cellular states209. Factors regulating epigenetic modifiers that establish enhancer-specific chromatin states and molecular ties regulating enhancer–promoter interactions might therefore act as epigenetic modulators that influence not only the mean level of transcription, but also its variance213, thereby affecting phenotypic variation (FIG. 3b).
Tumour cells often establish de novo oncogenic super-enhancers that drive proliferation209,215 and are hypersensitive to fluctuations in the level of bromodomain containing 4 (BRD4) and the Mediator complex — an essential cofactor regulating enhancer–promoter contact216. Importantly, the location and activity of super-enhancers is stabilized by the cellular microenvironment of the stem cell niche217, uncovering the surprising sensitivity of super-enhancer formation in stem/progenitor cells to environmental perturbations (FIG. 2).
Enhancer–promoter crosstalk is further constrained by the organization of the genome into topologically associated domains (TADs), which we suggest are categorized as epigenetic modulators based on their role in constituting an additional layer of regulation in setting up gene expression domains218. Importantly, the boundary strength of TADs is linked to the presence of architectural proteins, such as CCCTC-binding factor (CTCF)218,219, an epigenetic modulator (TABLE 1) that binds to DNA in a methylation-sensitive manner220. Reprogramming of such boundaries by widespread DNA methylation alterations present in tumours might further contribute to the loss of cell-type-specific expression domains and might alter the mobility and reach of oncogenic super-enhancers. Developmentally regulated contacts between chromatin fibres thus provide a 3D framework for cell-type-specific enhancer usage that might be reprogrammed in tumours to drive variation in stochastic gene expression and diversify the array of tumour-specific cellular states to enable tumour evolution (FIGS 2,3b). Although the mechanism by which epigenetic mediators, such as OCT4, NANOG and SOX2 (TABLE 1) promote the emergence of stem-like cell states in cancer cells is not fully explored, it is likely to involve the efficient reprogramming of 3D enhancer–promoter crosstalk that maintains differentiated cell states.
Relevance to diagnosis and treatment
Epigenetic chemoprevention to revert or prevent cancer-predisposing polyclonal epigenomic alterations in the progenitor cell compartments might be achieved by inhibiting epigenetic mediators, such as IGF2 signalling. Primary epigenetic changes are thus likely targets for early intervention to prevent tumour progression.
It will be important to consider that mutations in epigenetic modulators and modifiers can arise early in cancer, but a comparatively long time after the polyclonal epigenetic disruption of normal tissue affected by age and the environment through epigenetic modulators. For example, in renal cell carcinoma multiple distinct mutations in different parts of a single tumour converge on the same histone methylation change, suggesting that these mutations arise during progression rather than initiation48,221. These observations thus pinpoint epigenetic modifiers as therapeutic targets of existing tumours to prevent progression. The model also highlights the importance of overlooked approaches to epigenetic drug design and warrants new ways of thinking about assays for drugs rather than half maximal inhibitory concentration (IC50). This is exemplified by pleiotropic, epigenome-wide changes caused by gain-of-function mutations in variant histones, such as H3.3 and H3.1 in paediatric gliomas42,43. The non-linear dynamics of chromatin222,223 thus make the drug dose crucial when attacking epigenetic modifiers. An example is recent work profiling the effects of anthracycline drugs on histone eviction from chromatin224. The authors found that aclarubicin evicts histones from H3K27me3-marked heterochromatin and shows selective toxicity to diffuse large B cell lymphoma cells with increased levels of H3K27me3.
The prominent role of epigenetic instability in the emergence of cancer stem cells and tumour evolution provides an opportunity to reverse drug resistance and deplete CSCs by inhibiting epigenetic mediators. One remarkable opening for such a strategy is offered by the demonstration that tryptophan derivatives regulate OCT4 transcription in stem-like cancer cells225. One of these compounds, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), enhanced the binding of the aryl hydrocarbon receptor to the promoter of OCT4 to suppress its transcription. Accordingly, administration of synthetic ITE reduced the tumorigenic potential of stem-like cells in both subcutaneous and xenograft tumour models225.
Conclusions and future perspectives
The past decade has provided exciting new evidence demonstrating that cancer epigenomes display considerable instability, which leads to the continuous regeneration of epigenetic variation under the selection pressure of the tumour microenvironment190,191. One of the most surprising findings of these experiments is that certain domains of the genome seem to be particularly vulnerable to ageing- and environmental-carcinogen-induced epigenetic alterations, which can then unleash stochastic epigenetic changes within such vulnerable domains early during cancer development190,191. Ten years ago Feinberg et al.1 argued that environmental signals and ageing could affect epigenetic modifiers and lead to the emergence of an epigenetically disrupted progenitor cell pool long before the emergence of oncogenic mutations on the path to cancer. Such epigenetic variation would then drive phenotypic variation during cancer progression and evolution1. Since then, experimental evidence has already accumulated to confirm this prediction, warranting the accurate assessment of the level of transcriptional variation and the contribution of deterministic versus stochastic variation within the epigenome to cancer development. Such an endeavour is likely to require the development of single-cell techniques capable of quantitatively measuring a diverse array of epigenetic modifications at high resolution.
To provide a conceptual framework for the functional characterization of the genes that rewire the epigenome during cancer development and progression (FIG. 1; TABLE 1), we have introduced here a novel classification system that differentiates between epigenetic modifiers and the epigenetic modulators that regulate modifiers, and epigenetic mediators that shape the Waddington landscape of development to shift the phenotype towards stem-like states displaying phenotypic plasticity (FIG. 3). Epigenetic modifiers and epigenetic modulators (TABLE 1) are often mutated in cancer, or transmit signals from oncogenic signalling pathways that indirectly alter local or global chromatin modifications to promote tumour development. We suggest that chromatin states at epigenetic mediator genes are vulnerable targets for cancer-predisposing environmental cues that destabilize the epigenome via signalling and metabolic pathways that impinge on epigenetic modulators. As epigenetic mediators influence phenotypic plasticity during the entire neoplastic process, from the formation of CSC states to malignant derivatives and metastases, these factors should constitute prime targets for both prevention and therapeutic interventions.
The mechanism of increased epigenetic variation in cancer appears to be functionally connected to the perturbations of the 3D organization of the genome and the architecture of the nucleus (FIGS 2,3). Factors that regulate the nuclear architecture and enhancer–promoter communication might thus modulate the epigenome by coordinating the spatiotemporal aspects of epigenetic modifier activity. Moreover, the 3D genome organization in itself seems to affect the epigenome and function as an epigenetic modulator. The physical separation between active and inactive chromatin environments and the formation of TADs constraining enhancer–promoter contacts are thus likely to modulate the level of stochastic variation in epigenetic marks (FIG. 3b). Measuring the impact of deregulated nuclear compartmentalization on phenotypic traits that are selected for on the path to cancer requires the invention of sensitive and quantitative methods that can translate cell-to-cell variations of 3D chromatin organization to transcriptional heterogeneity in small cell populations representing transition cell fates towards CSCs.
We emphasize that the findings of the past 10 years also call for the integration of normal tissue epigenomics into precision medicine funding to promote progress in largely unexplored research areas in the context of cancer progenitors, such as RNA, tumour heterogeneity, transcriptional stochasticity, the contribution of inflammation and cell signalling, and enhancer–promoter interactions, to name but a few.
Acknowledgments
The authors are grateful to R. Ohlsson for his valuable comments on the text and figures. The work discussed here was supported by the US National Institutes of Health grant CA54358 to A.F. and a grant from Karolinska Institutet to A.G.
Glossary
- Field effect
Epigenetic changes in a region of normal cells around a tumour
- CpG island methylator phenotype
The classification of cancers characterized by increased methylation at CpG-rich promoter regions, best characterized in colorectal cancer and glioma and associated with distinct histological and molecular features
- Epimutations
Abnormal epigenetic alterations leading to aberrant gene expression or silencing
- Cancer stem cells
(CSCs). A subpopulation of cancer cells with the ability to propagate the cancer cell population
- Loss of imprinting
(LOI). Loss of parent of origin-specific expression of imprinted genes in cancer
- Epigenetic stochasticity
Non-deterministic changes to epigenetic marks such as DNA methylation, giving rise to epigenetic variation that underlies cellular plasticity in both normal and pathological states, and that can be localized to specific genomic regions
- Canalization
The ability of an organism to produce a consistent developmental outcome despite variations in its environment
- Pleiotropic
Genetic or epigenetic changes that affect multiple seemingly unrelated phenotypic traits
- Non-linear dynamics
The behaviour of a system in which a small change in an input variable can induce a large change in the output. Modelling of chromatin structure and of the impact of chromatin states on transcription has demonstrated non-linear behaviour
- Waddington landscape
A metaphor of development, in which valleys and ridges illustrate the epigenetic landscape that guides a pluripotent cell to a well-defined differentiated state, represented by a ball rolling down the landscape
Footnotes
Competing interests statement
The authors declare competing interests : see Web version for details.
References
- 1.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. This is the model suggesting that some genes are epigenetically disrupted at the earliest stages of malignancies, even before mutations, causing altered differentiation throughout tumour evolution; the current Review revisits this model. [DOI] [PubMed] [Google Scholar]
- 2.Versteege I, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 3.Lee RS, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122:2983–2988. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack SC, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. This paper reported an absence of recurrent mutations in a subtype of paediatric posterior fossa ependymoma, suggesting the existence of alternative, non-mutational mechanisms for cancer initiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugh TJ, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hovestadt V, et al. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature. 2014;510:537–541. doi: 10.1038/nature13268. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153:38–55. doi: 10.1016/j.cell.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plass C, et al. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat Rev Genet. 2013;14:765–780. doi: 10.1038/nrg3554. [DOI] [PubMed] [Google Scholar]
- 10.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ley TJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan XJ, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 13.Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366:95–96. doi: 10.1056/NEJMc1111708. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann V, et al. The molecular profile of adult T-cell acute lymphoblastic leukemia: mutations in RUNX1 and DNMT3A are associated with poor prognosis in T-ALL. Genes Chromosomes Cancer. 2013;52:410–422. doi: 10.1002/gcc.22039. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro AF, et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood. 2012;119:5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- 16.Challen GA, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challen GA, et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell. 2014;15:350–364. doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayle A, et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2015;125:629–638. doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celik H, et al. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood. 2015;125:619–628. doi: 10.1182/blood-2014-08-594564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Wahab O, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114:144–147. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langemeijer SM, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41:838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 23.Chou WC, et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood. 2011;118:3803–3810. doi: 10.1182/blood-2011-02-339747. [DOI] [PubMed] [Google Scholar]
- 24.Papaemmanuil E, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itzykson R, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013;121:2186–2198. doi: 10.1182/blood-2012-06-440347. [DOI] [PubMed] [Google Scholar]
- 26.Busque L, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran-Crusio K, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madzo J, et al. Hydroxymethylation at gene regulatory regions directs stem/early progenitor cell commitment during erythropoiesis. Cell Rep. 2014;6:231–244. doi: 10.1016/j.celrep.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biegel JA, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 30.Varela I, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones S, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiegand KC, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morin RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasqualucci L, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beguelin W, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst T, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 37.Nikoloski G, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–667. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 38.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ntziachristos P, et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 2012;18:298–301. doi: 10.1038/nm.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannum G, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon C, et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 2012;26:651–656. doi: 10.1101/gad.186411.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu G, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis PW, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Garcia E, et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood. 2011;117:211–220. doi: 10.1182/blood-2010-07-298349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaffe JD, et al. Global chromatin profiling reveals NSD2 mutations in pediatric acute lymphoblastic leukemia. Nat Genet. 2013;45:1386–1391. doi: 10.1038/ng.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalgliesh GL, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hakimi AA, et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network. Clin Cancer Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mar BG, et al. Mutations in epigenetic regulators including SETD2 are gained during relapse in paediatric acute lymphoblastic leukaemia. Nat Commun. 2014;5:3469. doi: 10.1038/ncomms4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rainier S, et al. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. doi: 10.1038/362747a0. This and the next paper represent the discovery of altered imprinting in cancer. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa O, et al. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms’ tumour. Nature. 1993;362:749–751. doi: 10.1038/362749a0. This and the previous paper represent the discovery of altered imprinting in cancer. [DOI] [PubMed] [Google Scholar]
- 53.Zhan S, Shapiro DN, Helman LJ. Activation of an imprinted allele of the insulin-like growth factor II gene implicated in rhabdomyosarcoma. J Clin Invest. 1994;94:445–448. doi: 10.1172/JCI117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rainier S, Dobry CJ, Feinberg AP. Loss of imprinting in hepatoblastoma. Cancer Res. 1995;55:1836–1838. [PubMed] [Google Scholar]
- 55.Levine AJ, et al. Genetic variation in insulin pathway genes and distal colorectal adenoma risk. Int J Colorectal Dis. 2012;27:1587–1595. doi: 10.1007/s00384-012-1505-8. [DOI] [PubMed] [Google Scholar]
- 56.Falls JG, Pulford DJ, Wylie AA, Jirtle RL. Genomic imprinting: implications for human disease. Am J Pathol. 1999;154:635–647. doi: 10.1016/S0002-9440(10)65309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hassan AB, Howell JA. Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Res. 2000;60:1070–1076. [PubMed] [Google Scholar]
- 58.Bjornsson HT, et al. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst. 2007;99:1270–1273. doi: 10.1093/jnci/djm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakatani T, et al. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 60.Shan J, et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56:1004–1014. doi: 10.1002/hep.25745. [DOI] [PubMed] [Google Scholar]
- 61.DeBaun MR, et al. Epigenetic alterations of H19 and LIT1 distinguish patients with Beckwith-Wiedemann syndrome with cancer and birth defects. Am J Hum Genet. 2002;70:604–611. doi: 10.1086/338934. This paper showed that epigenetic changes can precede and specifically confer risk of cancer, providing epigenetic epidemiological causal evidence similar to Li-Fraumeni and p53 mutations for genetic epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferron SR, et al. Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis. Nat Commun. 2015;6:8265. doi: 10.1038/ncomms9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatraman A, et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–349. doi: 10.1038/nature12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ziegler AN, et al. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30:1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischedick G, et al. Nanog induces hyperplasia without initiating tumors. Stem Cell Res. 2014;13:300–315. doi: 10.1016/j.scr.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Lengner CJ, et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marucci L, et al. Beta-catenin fluctuates in mouse ESCs and is essential for Nanog-mediated reprogramming of somatic cells to pluripotency. Cell Rep. 2014;8:1686–1696. doi: 10.1016/j.celrep.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Doi A, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu X, Mazur SJ, Lin T, Appella E, Xu Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene. 2014;33:2655–2664. doi: 10.1038/onc.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohnishi K, et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell. 2014;156:663–677. doi: 10.1016/j.cell.2014.01.005. This paper provided a link between reprogramming and oncogenic transformation, showing that transient expression of reprogramming factors in an in vivo mouse model leads to tumour development in various tissues in the absence of irreversible genetic transformation. [DOI] [PubMed] [Google Scholar]
- 72.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015;16:519–532. doi: 10.1038/nrm4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen B, Wu H, Shinkai Y, Irizarry RA, Feinberg AP. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat Genet. 2009;41:246–250. doi: 10.1038/ng.297. This paper showed that large areas of heterochromatin expand during differentiation and can distinguish cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tonge PD, et al. Divergent reprogramming routes lead to alternative stem-cell states. Nature. 2014;516:192–197. doi: 10.1038/nature14047. [DOI] [PubMed] [Google Scholar]
- 76.Shakya A, et al. Pluripotency transcription factor Oct4 mediates stepwise nucleosome demethylation and depletion. Mol Cell Biol. 2015;35:1014–1025. doi: 10.1128/MCB.01105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 78.Lee BK, et al. Tgif1 counterbalances the activity of core pluripotency factors in mouse embryonic stem cells. Cell Rep. 2015;13:52–60. doi: 10.1016/j.celrep.2015.08.067. [DOI] [PubMed] [Google Scholar]
- 79.Rudin CM, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bass AJ, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo W, et al. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiou SH, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, et al. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5:10803–10815. doi: 10.18632/oncotarget.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 86.Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep. 2014;15:244–253. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 88.Kai K, et al. Maintenance of HCT116 colon cancer cell line conforms to a stochastic model but not a cancer stem cell model. Cancer Sci. 2009;100:2275–2282. doi: 10.1111/j.1349-7006.2009.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta PB, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 90.Schwitalla S, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Friedmann-Morvinski D, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 93.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 94.Kikushige Y, et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell. 2011;20:246–259. doi: 10.1016/j.ccr.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 95.Chen WJ, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 96.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Borovski T, et al. Tumor microvasculature supports proliferation and expansion of glioma-propagating cells. Int J Cancer. 2009;125:1222–1230. doi: 10.1002/ijc.24408. [DOI] [PubMed] [Google Scholar]
- 98.Vermeulen L, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 99.Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. 2013;14:211–224. doi: 10.1038/nrm3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolybaba A, Classen AK. Sensing cellular states – signaling to chromatin pathways targeting Polycomb and Trithorax group function. Cell Tissue Res. 2014;356:477–493. doi: 10.1007/s00441-014-1824-x. [DOI] [PubMed] [Google Scholar]
- 101.Wu BK, Brenner C. Suppression of TET1-dependent DNA demethylation is essential for KRAS-mediated transformation. Cell Rep. 2014;9:1827–1840. doi: 10.1016/j.celrep.2014.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gazin C, Wajapeyee N, Gobeil S, Virbasius CM, Green MR. An elaborate pathway required for Ras-mediated epigenetic silencing. Nature. 2007;449:1073–1077. doi: 10.1038/nature06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wajapeyee N, Malonia SK, Palakurthy RK, Green MR. Oncogenic RAS directs silencing of tumor suppressor genes through ordered recruitment of transcriptional repressors. Genes Dev. 2013;27:2221–2226. doi: 10.1101/gad.227413.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Serra RW, Fang M, Park SM, Hutchinson L, Green MR. A KRAS-directed transcriptional silencing pathway that mediates the CpG island methylator phenotype. eLife. 2014;3:e02313. doi: 10.7554/eLife.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li H, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen LV, et al. Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature. 2015;528:267–271. doi: 10.1038/nature15742. This paper describes the development of phenotypically heterogeneous, serially transplantable tumours from basal cells and luminal progenitors transduced with oncogenic KRAS. [DOI] [PubMed] [Google Scholar]
- 107.Trosko JE, Tai MH. Adult stem cell theory of the multi-stage, multi-mechanism theory of carcinogenesis: role of inflammation on the promotion of initiated stem cells. Contrib Microbiol. 2006;13:45–65. doi: 10.1159/000092965. [DOI] [PubMed] [Google Scholar]
- 108.Suman S, et al. Current perspectives of molecular pathways involved in chronic Inflammation-mediated breast cancer. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.10.133. http://dx.doi.org/10.1016/j.bbrc.2015.10.133. [DOI] [PubMed]
- 109.Barham W, et al. Aberrant activation of NF-κB signaling in mammary epithelium leads to abnormal growth and ductal carcinomain situ. BMC Cancer. 2015;15:647. doi: 10.1186/s12885-015-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 112.Do DV, et al. A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 2013;27:1378–1390. doi: 10.1101/gad.221176.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tyagi N, et al. p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett. 2016;370:260–267. doi: 10.1016/j.canlet.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hutchins AP, Diez D, Miranda-Saavedra D. Genomic and computational approaches to dissect the mechanisms of STAT3’s universal and cell type-specific functions. JAKSTAT. 2013;2:e25097. doi: 10.4161/jkst.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abu-Remaileh M, et al. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015;75:2120–2130. doi: 10.1158/0008-5472.CAN-14-3295. [DOI] [PubMed] [Google Scholar]
- 117.De Santa F, et al. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 118.Lee J, et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. The paper showed that the activation of innate immunity enhances the reprogramming efficiency of Yamanaka factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strano S, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 121.Bora-Singhal N, et al. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33:1705–1718. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu J, et al. Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature. 2015;525:206–211. doi: 10.1038/nature15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pfister NT, et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015;29:1298–1315. doi: 10.1101/gad.263202.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rai K, et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell. 2010;142:930–942. doi: 10.1016/j.cell.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. This paper is a good example of metabolic epigenetic modulation by a tumour suppressor gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiao M, et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heyn H, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci USA. 2012;109:10522–10527. doi: 10.1073/pnas.1120658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Florath I, Butterbach K, Muller H, Bewerunge-Hudler M, Brenner H. Cross-sectional and longitudinal changes in DNA methylation with age: an epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Hum Mol Genet. 2014;23:1186–1201. doi: 10.1093/hmg/ddt531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan T, et al. An integrative multi-scale analysis of the dynamic DNA methylation landscape in aging. PLoS Genet. 2015;11:e1004996. doi: 10.1371/journal.pgen.1004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Teschendorff AE, West J, Beck S. Age-associated epigenetic drift: implications, and a case of epigenetic thrift? Hum Mol Genet. 2013;22:R7–R15. doi: 10.1093/hmg/ddt375. The authors intriguingly argue that epigenetic drift has an evolutionary advantage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun D, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16:593–610. doi: 10.1038/nrm4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Siebold AP, et al. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance. Proc Natl Acad Sci USA. 2010;107:169–174. doi: 10.1073/pnas.0907739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greer EL, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang W, et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015;348:1160–1163. doi: 10.1126/science.aaa1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCord RP, et al. Correlated alterations in genome organization, histone methylation, and DNA-lamin A/C interactions in Hutchinson-Gilford progeria syndrome. Genome Res. 2013;23:260–269. doi: 10.1101/gr.138032.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Riedel CG, et al. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol. 2013;15:491–501. doi: 10.1038/ncb2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mihaylova MM, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bungard D, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science. 2010;329:1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilson MJ, Shivapurkar N, Poirier LA. Hypomethylation of hepatic nuclear DNA in rats fed with a carcinogenic methyl-deficient diet. Biochem J. 1984;218:987–990. doi: 10.1042/bj2180987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ghoshal AK, Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis. 1984;5:1367–1370. doi: 10.1093/carcin/5.10.1367. [DOI] [PubMed] [Google Scholar]
- 144.Bhave MR, Wilson MJ, Poirier LA. c-H-ras and c-K-ras gene hypomethylation in the livers and hepatomas of rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1988;9:343–348. doi: 10.1093/carcin/9.3.343. [DOI] [PubMed] [Google Scholar]
- 145.Pogribny IP, et al. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–1901. [PubMed] [Google Scholar]
- 146.Giovannucci E, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 147.Ciappio ED, Mason JB, Crott JW. Maternal one-carbon nutrient intake and cancer risk in offspring. Nutr Rev. 2011;69:561–571. doi: 10.1111/j.1753-4887.2011.00424.x. [DOI] [PubMed] [Google Scholar]
- 148.Choi SW, et al. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr. 1999;129:1945–1950. doi: 10.1093/jn/129.11.1945. [DOI] [PubMed] [Google Scholar]
- 149.van Engeland M, et al. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res. 2003;63:3133–3137. [PubMed] [Google Scholar]
- 150.Cortessis VK, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012;131:1565–1589. doi: 10.1007/s00439-012-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zochbauer-Muller S, et al. Aberrant methylation of multiple genes in the upper aerodigestive tract epithelium of heavy smokers. Int J Cancer. 2003;107:612–616. doi: 10.1002/ijc.11458. [DOI] [PubMed] [Google Scholar]
- 152.Russo AL, et al. Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res. 2005;11:2466–2470. doi: 10.1158/1078-0432.CCR-04-1962. [DOI] [PubMed] [Google Scholar]
- 153.Bollati V, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 154.Maekita T, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 155.Chan AO, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55:463–468. doi: 10.1136/gut.2005.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Christensen BC, et al. Epigenetic profiles distinguish pleural mesothelioma from normal pleura and predict lung asbestos burden and clinical outcome. Cancer Res. 2009;69:227–234. doi: 10.1158/0008-5472.CAN-08-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pujadas E, Feinberg AP. Regulated noise in the epigenetic landscape of development and disease. Cell. 2012;148:1123–1131. doi: 10.1016/j.cell.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hansen KD, et al. Large-scale hypomethylated blocks associated with Epstein-Barr virus-induced B-cell immortalization. Genome Res. 2014;24:177–184. doi: 10.1101/gr.157743.113. This paper showed the link between hypomethylated blocks, variable gene expression, and heterochromatin LOCKs/LADs in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meuleman W, et al. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res. 2013;23:270–280. doi: 10.1101/gr.141028.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Banerji CR, et al. Cellular network entropy as the energy potential in Waddington’s differentiation landscape. Sci Rep. 2013;3:3039. doi: 10.1038/srep03039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen X, et al. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev. 2012;26:2499–2511. doi: 10.1101/gad.200329.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu N, et al. Recognition of H3K9 methylation by GLP is required for efficient establishment of H3K9 methylation, rapid target gene repression, and mouse viability. Genes Dev. 2015;29:379–393. doi: 10.1101/gad.254425.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahmed K, et al. Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS One. 2010;5:e10531. doi: 10.1371/journal.pone.0010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Therizols P, et al. Chromatin decondensation is sufficient to alter nuclear organization in embryonic stem cells. Science. 2014;346:1238–1242. doi: 10.1126/science.1259587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eskeland R, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vallot C, Herault A, Boyle S, Bickmore WA, Radvanyi F. PRC2-independent chromatin compaction and transcriptional repression in cancer. Oncogene. 2015;34:741–751. doi: 10.1038/onc.2013.604. [DOI] [PubMed] [Google Scholar]
- 167.Lemaitre C, Bickmore WA. Chromatin at the nuclear periphery and the regulation of genome functions. Histochem Cell Biol. 2015;144:111–122. doi: 10.1007/s00418-015-1346-y. [DOI] [PubMed] [Google Scholar]
- 168.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 169.Shah PP, et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harr JC, et al. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol. 2015;208:33–52. doi: 10.1083/jcb.201405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Towbin BD, et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–947. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 172.Guarda A, Bolognese F, Bonapace IM, Badaracco G. Interaction between the inner nuclear membrane lamin B receptor and the heterochromatic methyl binding protein, MeCP2. Exp Cell Res. 2009;315:1895–1903. doi: 10.1016/j.yexcr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 173.Schooley A, Moreno-Andres D, De Magistris P, Vollmer B, Antonin W. The lysine demethylase LSD1 is required for nuclear envelope formation at the end of mitosis. J Cell Sci. 2015;128:3466–3477. doi: 10.1242/jcs.173013. [DOI] [PubMed] [Google Scholar]
- 174.Montes de Oca R, Shoemaker CJ, Gucek M, Cole RN, Wilson KL. Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS One. 2009;4:e7050. doi: 10.1371/journal.pone.0007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Demmerle J, Koch AJ, Holaska JM. The nuclear envelope protein emerin binds directly to histone deacetylase 3 (HDAC3) and activates HDAC3 activity. J Biol Chem. 2012;287:22080–22088. doi: 10.1074/jbc.M111.325308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Somech R, et al. The nuclear-envelope protein and transcriptional repressor LAP2β interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci. 2005;118:4017–4025. doi: 10.1242/jcs.02521. [DOI] [PubMed] [Google Scholar]
- 177.Holaska JM, Wilson KL. An emerin “proteome”: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46:8897–8908. doi: 10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Finlan LE, et al. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4:e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Melcer S, et al. Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat Commun. 2012;3:910. doi: 10.1038/ncomms1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adamo A, et al. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 181.Ma DK, Chiang CH, Ponnusamy K, Ming GL, Song H. G9a and Jhdm2a regulate embryonic stem cell fusion-induced reprogramming of adult neural stem cells. Stem Cells. 2008;26:2131–2141. doi: 10.1634/stemcells.2008-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sadaie M, et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 2013;27:1800–1808. doi: 10.1101/gad.217281.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zane L, Sharma V, Misteli T. Common features of chromatin in aging and cancer: cause or coincidence? Trends Cell Biol. 2014;24:686–694. doi: 10.1016/j.tcb.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shankar SR, et al. G9a, a multipotent regulator of gene expression. Epigenetics. 2013;8:16–22. doi: 10.4161/epi.23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol. 2011;18:867–874. doi: 10.1038/nsmb.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feinberg AP, Irizarry RA. Evolution in health and medicine Sackler colloquium: stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1757–1764. doi: 10.1073/pnas.0906183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohlsson R, et al. Epigenetic variability and the evolution of human cancer. Adv Cancer Res. 2003;88:145–168. doi: 10.1016/s0065-230x(03)88306-9. [DOI] [PubMed] [Google Scholar]
- 190.Hansen KD, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Timp W, et al. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. Genome Med. 2014;6:61. doi: 10.1186/s13073-014-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Berman BP, et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat Genet. 2012;44:40–46. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Teschendorff AE, et al. Epigenetic variability in cells of normal cytology is associated with the risk of future morphological transformation. Genome Med. 2012;4:24. doi: 10.1186/gm323. This paper showed that epigenetic variability in normal tissue predicts the development of later cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Teschendorff AE, Widschwendter M. Differential variability improves the identification of cancer risk markers in DNA methylation studies profiling precursor cancer lesions. Bioinformatics. 2012;28:1487–1494. doi: 10.1093/bioinformatics/bts170. [DOI] [PubMed] [Google Scholar]
- 195.Dinalankara W, Bravo HC. Gene expression signatures based on variability can robustly predict tumor progression and prognosis. Cancer Inform. 2015;14:71–81. doi: 10.4137/CIN.S23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vandiver AR, et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015;16:80. doi: 10.1186/s13059-015-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Teschendorff AE, Banerji CR, Severini S, Kuehn R, Sollich P. Increased signaling entropy in cancer requires the scale-free property of protein interaction networks. Sci Rep. 2015;5:9646. doi: 10.1038/srep09646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kumar RM, et al. Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature. 2014;516:56–61. doi: 10.1038/nature13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Singer ZS, et al. Dynamic heterogeneity and DNA methylation in embryonic stem cells. Mol Cell. 2014;55:319–331. doi: 10.1016/j.molcel.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Landan G, et al. Epigenetic polymorphism and the stochastic formation of differentially methylated regions in normal and cancerous tissues. Nat Genet. 2012;44:1207–1214. doi: 10.1038/ng.2442. This paper demonstrated stochastic epipolymorphisms in cancer. [DOI] [PubMed] [Google Scholar]
- 201.Reddy KL, Feinberg AP. Higher order chromatin organization in cancer. Semin Cancer Biol. 2013;23:109–115. doi: 10.1016/j.semcancer.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carone DM, Lawrence JB. Heterochromatin instability in cancer: from the Barr body to satellites and the nuclear periphery. Semin Cancer Biol. 2013;23:99–108. doi: 10.1016/j.semcancer.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kind J, et al. Single-cell dynamics of genome-nuclear lamina interactions. Cell. 2013;153:178–192. doi: 10.1016/j.cell.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 204.Schlimgen RJ, Reddy KL, Singh H, Krangel MS. Initiation of allelic exclusion by stochastic interaction of Tcrb alleles with repressive nuclear compartments. Nat Immunol. 2008;9:802–809. doi: 10.1038/ni.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao H, et al. PARP1- and CTCF-mediated interactions between active and repressed chromatin at the lamina promote oscillating transcription. Mol Cell. 2015;59:984–997. doi: 10.1016/j.molcel.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 206.Wassef M, et al. Impaired PRC2 activity promotes transcriptional instability and favors breast tumorigenesis. Genes Dev. 2015;29:2547–2562. doi: 10.1101/gad.269522.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guelen L, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 208.Boros J, Arnoult N, Stroobant V, Collet JF, Decottignies A. Polycomb repressive complex 2 and H3K27me3 cooperate with H3K9 methylation to maintain heterochromatin protein 1alpha at chromatin. Mol Cell Biol. 2014;34:3662–3674. doi: 10.1128/MCB.00205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hnisz D, et al. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol Cell. 2015;58:362–370. doi: 10.1016/j.molcel.2015.02.014. The paper showed that super-enhancers integrate developmental cues and oncogenic signalling pathways to regulate genes that control cell identity or tumour development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bahar Halpern K, et al. Bursty gene expression in the intact mammalian liver. Mol Cell. 2015;58:147–156. doi: 10.1016/j.molcel.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lam A, Deans TL. A noisy tug of war: the battle between transcript production and degradation in the liver. Dev Cell. 2015;33:3–4. doi: 10.1016/j.devcel.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 212.Li G, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Noordermeer D, et al. Variegated gene expression caused by cell-specific long-range DNA interactions. Nat Cell Biol. 2011;13:944–951. doi: 10.1038/ncb2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Whyte WA, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482:221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Loven J, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20:4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Adam RC, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521:366–370. doi: 10.1038/nature14289. This paper showed that the microenvironment dynamically reprogrammes the location of super-enhancers in follicular stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gomez-Diaz E, Corces VG. Architectural proteins: regulators of 3D genome organization in cell fate. Trends Cell Biol. 2014;24:703–711. doi: 10.1016/j.tcb.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lupianez DG, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. This paper showed that disruption of TAD structure can result in pathological long-range enhancer–promoter interactions and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kanduri C, et al. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr Biol. 2000;10:853–856. doi: 10.1016/s0960-9822(00)00597-2. [DOI] [PubMed] [Google Scholar]
- 221.Gerlinger M, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. doi: 10.1038/ng.2891. This paper used multi-region sequencing to analyse tumour evolution in renal cell carcinoma and reported distinct, spatially separated mutations converging on modifiers of specific histone marks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yildirim E, Sadreyev RI, Pinter SF, Lee JT. X-chromosome hyperactivation in mammals via nonlinear relationships between chromatin states and transcription. Nat Struct Mol Biol. 2012;19:56–61. doi: 10.1038/nsmb.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stephens AD, et al. Pericentric chromatin loops function as a nonlinear spring in mitotic force balance. J Cell Biol. 2013;200:757–772. doi: 10.1083/jcb.201208163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pang B, de Jong J, Qiao X, Wessels LF, Neefjes J. Chemical profiling of the genome with anti-cancer drugs defines target specificities. Nat Chem Biol. 2015;11:472–480. doi: 10.1038/nchembio.1811. [DOI] [PubMed] [Google Scholar]
- 225.Cheng J, et al. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209. doi: 10.1038/ncomms8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Love C, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Imielinski M, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Robinson G, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fujimoto A, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 230.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li M, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011;43:828–829. doi: 10.1038/ng.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hodis E, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stephens PJ, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Smith MJ, et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45:295–298. doi: 10.1038/ng.2552. [DOI] [PubMed] [Google Scholar]
- 236.Jiao Y, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 238.Kolla V, Zhuang T, Higashi M, Naraparaju K, Brodeur GM. Role of CHD5 in human cancers: 10 years later. Cancer Res. 2014;74:652–658. doi: 10.1158/0008-5472.CAN-13-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Puente XS, et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature. 2015;526:519–524. doi: 10.1038/nature14666. [DOI] [PubMed] [Google Scholar]
- 240.Berger MF, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tahara T, et al. Colorectal carcinomas with CpG island methylator phenotype 1 frequently contain mutations in chromatin regulators. Gastroenterology. 2014;146:530–538e5. doi: 10.1053/j.gastro.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gui Y, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Le Gallo M, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Neumann M, et al. Whole-exome sequencing in adult ETP-ALL reveals a high rate of DNMT3A mutations. Blood. 2013;121:4749–4752. doi: 10.1182/blood-2012-11-465138. [DOI] [PubMed] [Google Scholar]
- 245.Kanai Y, Ushijima S, Nakanishi Y, Sakamoto M, Hirohashi S. Mutation of the DNA methyltransferase (DNMT) 1 gene in human colorectal cancers. Cancer Lett. 2003;192:75–82. doi: 10.1016/s0304-3835(02)00689-4. [DOI] [PubMed] [Google Scholar]
- 246.Delhommeau F, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 247.Scourzic L, Mouly E, Bernard OA. TET proteins and the control of cytosine demethylation in cancer. Genome Med. 2015;7:9. doi: 10.1186/s13073-015-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Krauthammer M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pasqualucci L, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Peifer M, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ward R, Johnson M, Shridhar V, van Deursen J, Couch FJ. CBP truncating mutations in ovarian cancer. J Med Genet. 2005;42:514–518. doi: 10.1136/jmg.2004.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mullighan CG, et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471:235–239. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ropero S, et al. A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat Genet. 2006;38:566–569. doi: 10.1038/ng1773. [DOI] [PubMed] [Google Scholar]
- 255.Hanigan CL, et al. An inactivating mutation in HDAC2 leads to dysregulation of apoptosis mediated by APAF1. Gastroenterology. 2008;135:1654–1664. doi: 10.1053/j.gastro.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 256.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 257.Thirman MJ, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993;329:909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- 258.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 259.Rao RC, Dou Y. Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat Rev Cancer. 2015;15:334–346. doi: 10.1038/nrc3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zang ZJ, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 262.Zhang J, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vougiouklakis T, Hamamoto R, Nakamura Y, Saloura V. The NSD family of protein methyltransferases in human cancer. Epigenomics. 2015;7:863–874. doi: 10.2217/epi.15.32. [DOI] [PubMed] [Google Scholar]
- 264.Fontebasso AM, et al. Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol. 2013;125:659–669. doi: 10.1007/s00401-013-1095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.van Haaften G, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Van Vlierberghe P, et al. PHF6 mutations in adult acute myeloid leukemia. Leukemia. 2011;25:130–134. doi: 10.1038/leu.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Van Vlierberghe P, et al. PHF6 mutations in T-cell acute lymphoblastic leukemia. Nat Genet. 2010;42:338–342. doi: 10.1038/ng.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Huether R, et al. The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat Commun. 2014;5:3630. doi: 10.1038/ncomms4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang GG, et al. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature. 2009;459:847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.French CA, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am J Pathol. 2001;159:1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pena PV, et al. Histone H3K4me3 binding is required for the DNA repair and apoptotic activities of ING1 tumor suppressor. J Mol Biol. 2008;380:303–312. doi: 10.1016/j.jmb.2008.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Behjati S, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45:1479–1482. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li H, et al. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; and ARID1A underlying the pathogenesis of follicular lymphoma. Blood. 2014;123:1487–1498. doi: 10.1182/blood-2013-05-500264. [DOI] [PMC free article] [PubMed] [Google Scholar]