Abstract
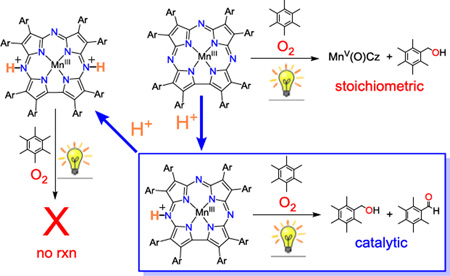
The visible light-driven, catalytic aerobic oxidation of benzylic C-H bonds was mediated by an MnIII corrolazine complex. To achieve catalytic turnovers, a strict selective requirement for the addition of protons was established. The resting state of the catalyst was unambiguously characterized by X–ray diffraction as [MnIII(H2O)(TBP8Cz(H))]+, in which a single, remote site on the ligand is protonated. If two remote sites are protonated, however, reactivity with O2 is shut down. Spectroscopic methods revealed that the related MnV(O) complex is also protonated at the same remote site at −60 °C, but undergoes valence tautomerization upon warming.
Graphical Abstract
The activation of molecular oxygen is a critical transformation in biology, which is mediated by both heme and nonheme metal centers.1 Synthetic, biomimetic transition metal complexes aimed at activating O2 have been targeted by employing both porphyrinoid and nonheme polydentate ligands, and some of these efforts have resulted in important mechanistic insights as well as catalysts for the oxygenation of a range of organic substrates.2 The catalytic, aerobic oxidation of C–H bonds with first–row, biologically relevant metal complexes remains a particularly important, yet challenging goal.3 Although some Fe and Mn metalloporphyrins, and related metallomacrocycles (e.g. phthalocyanines), can catalyze the oxidation of C–H bonds with O2, drawbacks remain in most cases. Such drawbacks include the need for stoichiometric co–reductants, and the involvement of poorly controlled radical–type pathways.2j, 3 The mechanisms of these systems, including the role of high–valent metal–oxo species, is often poorly understood.
Corroles are modified porphyrinoid ligands that stabilize high–valent metal–oxo species, and can exhibit catalytic activity different from their porphyrin analogs. A CrIII corrole catalyzes the aerobic oxidation of PPh3 via a CrV(O) intermediate.4 Under visible light irradiation, diiron(IV) μ–oxo biscorroles have been shown to catalyze the aerobic oxidation of phosphine and C–H substrates, presumably through a disproportionation pathway that involves μ–oxo–bridge cleavage and leads to formation of FeIII/FeV(O) intermediates.5 Diiron(III)–μ–oxo–bridged porphyrins also mediate photoactivated aerobic oxidations through similar μ–oxo cleavage mechanisms.6 Light-driven activation of metalloporphyrinoid complexes for catalytic aerobic oxidation is especially attractive from an environmental and energy perspective.
In previous work, we showed that a stable MnV(O) complex prepared with a corrolazine ligand, which is a meso–N–substituted analog of a corrole, could be synthesized via photoirradiation of a MnIII precursor, O2 (or air), and C–H substrates.7a This work was the first example of the synthesis of a MnV(O) complex from air, light, and a proton/electron source.7a The addition of toluene derivatives to the MnIII/hv/O2 system led to selective C–H hydroxylation of benzylic C–H bonds.7b However, the inherent stability of the MnV(O) complex toward the toluene derivatives limited this chemistry to a strictly stoichiometric process, with the MnV(O) complex as the final Mn product. Only in the case of PPh3, or dihydroacridine, which has a very weak C–H bond (BDFE = 69 kcal mol−1), was catalytic turnover obtained.7a,8
Herein we report the visible light–driven, catalytic aerobic oxidation of benzylic C–H bonds via the proton–controlled activation of a MnIII corrolazine complex. The selective addition of a strong proton donor leads to the formation of new mono– and diprotonated MnIII complexes, which were characterized by single crystal X–ray diffraction (hereafter, XRD). The monoprotonated complex is capable of reacting with air, light and benzylic C–H bonds to give a high–valent Mn–oxo complex. Under the right conditions, the controlled addition of protons provides access to a catalytic cycle. Low–temperature methods were used to trap a novel, proton–activated form of the MnV(O) complex which may play a role in the catalytic cycle.
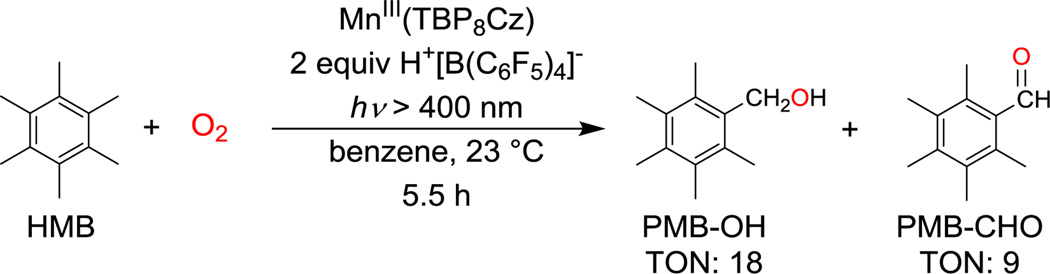
Previously it was shown that photoirradiation (λ > 400 nm) of MnIII(TBP8Cz)(TBP8Cz = octakis(p-tert-butylphenyl corrolazinato3−) in the presence of excess O2 and hexamethylbenzene (HMB) in benzonitrile (PhCN) led to the selective oxidation of HMB to pentamethylbenzyl alcohol (PMB–OH) (87%) with clean formation of MnV(O)(TBP8Cz).7b This MnV(O) complex oxidizes relatively weak C–H bonds such as those found in dihydroanthracene (BDFE = 76 kcal mol−1), but is essentially unreactive toward stronger C–H bonds such as those found in toluene (BDFE = 87 kcal mol−1) or its derivatives.8 The selective hydroxylation of HMB was therefore limited to strictly stoichiometric reactivity.7b We speculated that addition of a strong proton donor might activate the MnV(O) complex toward oxidation of the stronger C–H bonds found in HMB, providing entry into a catalytic oxidation pathway.
Addition of the strong proton donor [H(OEt2)2]+[B(C6F5)4]− (H+[B(C6F5)4]−) to MnIII(TBP8Cz) in benzene (C6H6) caused an immediate color change of the brown MnIII complex to a brown–red solution. The addition of excess HMB (1000 equiv) under ambient conditions to this solution, followed by photoirradiation with visible light (λ > 400 nm), led to the slow bleaching of the solution over 5.5 h. Monitoring of this reaction by UV–vis confirmed the slow decay of the protonated MnIII complex, which exhibited distinct Soret and Q–bands at 446 and 728 nm (Figure S3). Analysis of the reaction mixture by GC–FID revealed the production of PMB–OH and the corresponding aldehyde PMB–CHO (Scheme 1) with 18 turnovers for PMB–OH and 9 turnovers for PMB–CHO. Both oxidation products increase steadily over time, and catalytic activity appears only limited by catalyst stability. In the absence of light, O2, or MnIII complex, no oxidized products were detected. As anticipated, the addition of H+ to the Mn catalyst leads to the catalytic oxidation of HMB with only air and light as additives.
Scheme 1.
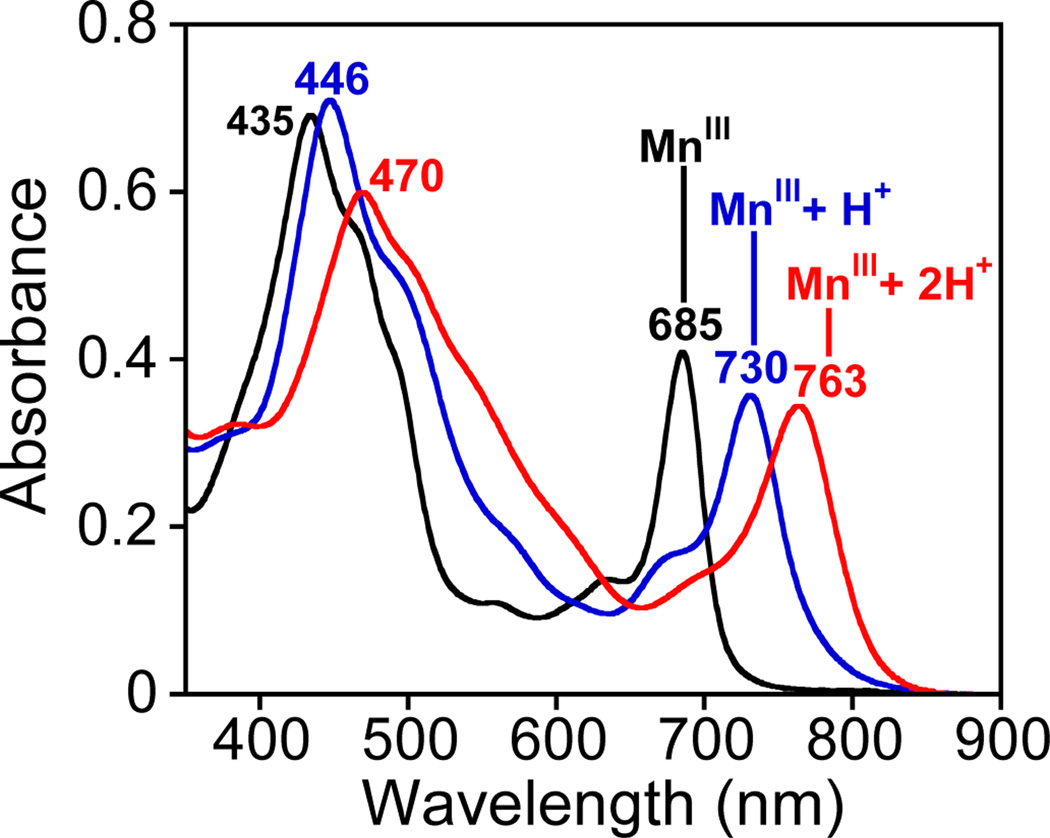
The independent reaction of MnIII(TBP8Cz) with H+[B(C6F5)4]− was investigated to determine the effect of H+ on the MnIII complex. These independent experiments were initially run in CH2Cl2, as opposed to C6H6, because of the available UV-vis data on Mn corrolazine complexes in CH2Cl2.9,10 The starting MnIII complex (435, 685 nm) was converted to a new brown–red species upon addition of one equiv of H+[B(C6F5)4]− (446, 730 nm) (Figure 1). This new spectrum (446, 730 nm) was almost identical to that seen for the resting state of the catalyst in C6H6. Introduction of a second equiv of H+ caused a further change to a second species with further red–shifted Soret and Q–bands at 470 and 763 nm. Spectral titrations (Figure S4) showed that no further changes occur upon addition of up to 5 equiv of H+. Similar spectra were obtained in dry C6H6 (Figure S7, S10).
Figure 1.
UV–vis spectral changes for MnIII(TBP8Cz) (12 µM, black line) upon addition of 1 equiv (blue line) and 2 equiv (red line) of H+[B(C6F5)4]− in CH2Cl2 (2 mL) at 25 °C.
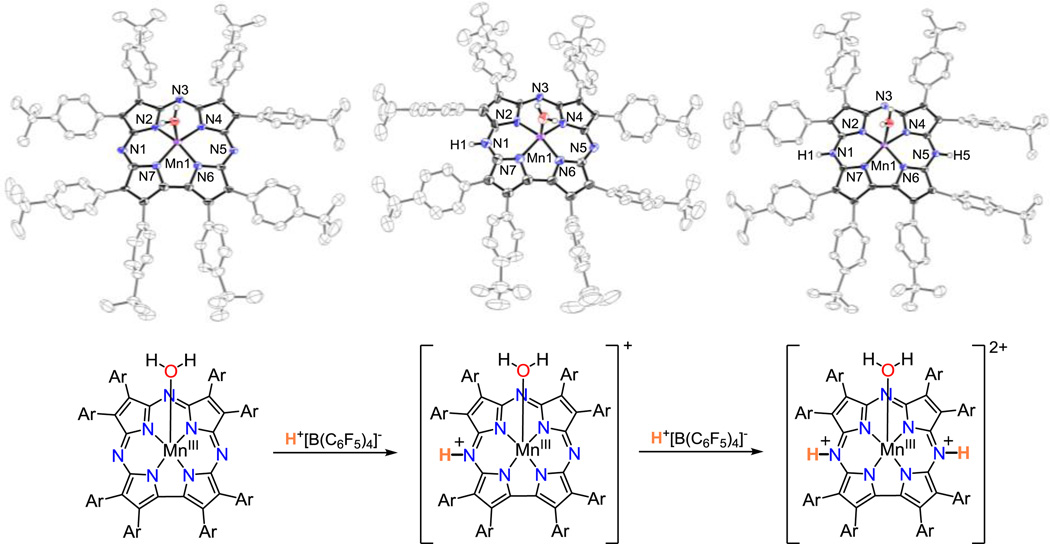
The UV–vis data in Figure 1 clearly show that there are two distinct species accessible via protonation of the starting MnIII corrolazine. The three MnIII complexes identified in Figure 1 were successfully crystallized and unambiguously characterized by XRD. The crystal structures of the neutral, mono– and diprotonated MnIII derivatives are presented in Figure 2. The structure of the neutral MnIII complex contains a 5–coordinate MnIII ion with a water ligand located in the axial position, similar to the structure of a MeOH–bound MnIII(MeOH)(TBP8Cz).9d The axial H2O ligands of two adjacent metallocorrolazines are H–bond donors to the meso–N atoms of the neighboring Cz ligand via a set of 1+1 O—H…N hydrogen bonds (i.e., only one hydrogen bond per axial H2O ligand), resulting in H–bonded dimers of MnIII(H2O) complexes in the solid state. Treatment of the starting MnIII complex with 1 equiv of H+[B(C6F5)4]− in CH2Cl2, followed by removal of solvent and re-crystallization from toluene/heptane, led to dark brown crystals of [MnIII(H2O)(TBP8Cz(H))][B(C6F5)4]. The crystal structure reveals that monoprotonation most likely occurs at one of the meso–N atoms (N1(H)) adjacent to the direct pyrrole–pyrrole bond of the Cz ligand. The axial H2O ligand seen in the neutral MnIII starting complex and H–bonded dimers are retained. Dissolution of crystalline [MnIII(H2O)(TBP8Cz(H))][B(C6F5)4] yields the same UV–vis spectrum as seen for the in situ protonation of the MnIII complex via addition of 1 equiv of H+[B(C6F5)4]− (λmax = 446, 730 nm) (Figure S6). If two equiv of H+[B(C6F5)4]− are added to the neutral MnIII complex, a new complex in the form of dark brown crystals can be isolated. XRD analysis shows that this new species is doubly protonated with the molecular formula [MnIII(H2O)(TBP8Cz(H)2)][B(C6F5)4]2 (Figure 2). Both H–atoms were unambiguously located on the opposite meso–N atoms (N1 and N5). Unlike the neutral and monoprotonated structures, this complex exhibits no H–bonding to nearest neighbors and crystallizes in isolated molecular units. The UV–vis spectrum of crystalline [MnIII(H2O)(TBP8Cz(H)2)][B(C6F5)4]2 redissolved in CH2Cl2 matches that seen for the in situ diprotonation of the MnIII complex following treatment with 2 equiv of H+ (λ = 470, 763 nm) (Figure S7). The metrical parameters for each of the structures show little variation upon mono– and diprotonation, with the exception of a slight shortening (Δd(Mn–O) = 0.028(3) Å) of the MnIII–OH2 distance.
Figure 2.
(top) Displacement ellipsoid plots (50% probability level) for MnIII(TBP8Cz)(H2O) (left), [MnIII(H2O)(TBP8Cz(H))]+ (center) and [MnIII(H2O)(TBP8Cz(H)2)]2+ (right) at 110(2) K. (bottom) Addition of one and two equiv of H+[B(C6F5)4]− to MnIII(TBP8Cz).
The preparation of crystalline mono– and diprotonated MnIII complexes provided pure material for testing as possible catalysts in the observed light–driven, aerobic C–H oxidation. Monoprotonated [MnIII(H2O)(TBP8Cz(H))][B(C6F5)4] was dissolved in CH2Cl2 or C6H6 with excess HMB (1000 equiv) under aerobic conditions. Photoirradiation of this solution for 30 min at 23 °C followed by analysis by GC–FID showed both alcohol and aldehyde products were formed, but only in stoichiometric amounts (PMB–OH: TON = 0.4; PMB–CHO: TON = 0.7), i.e. no catalysis was observed. Monitoring this reaction by UV–vis showed isosbestic conversion of the spectrum for [MnIII(H2O)(TBP8Cz(H))]+ to a new spectrum with features at 418 and 784 nm. This new spectrum (Figure S12) matches that seen previously for the MnIV–oxo π–radical cation complex [MnIV(O)(TBP8Cz•+)(LA)]+ (LA = Lewis acid = ZnII, B(C6F5)3), a valence tautomer of MnV(O)(TBP8Cz) stabilized by Lewis acids.10 In support of this analysis, reaction of crystalline [MnIII(H2O)(TBP8Cz)(H)][B(C6F5)4] with the O–atom donor PhIO leads to isosbestic conversion to [MnIV(O)(TBP8Cz•+)(H)]+ (Figure S13), instead of MnV(O)(TBP8Cz), which is the major product from [MnIII(H2O)(TBP8Cz)] + PhIO.9d These results indicate that generation of the valence tautomer [MnIV(O)(TBP8Cz•+)(H)]+ occurs if 1 equiv of H+ is employed.
The use of crystalline diprotonated [MnIII(H2O)(TBP8Cz(H)2)][B(C6F5)4]2 as starting material, in contrast, leads to catalytic turnover in the light–driven oxidation of HMB, giving PMB–OH (TON = 16) and PMB–CHO (TON = 11) after 5 h in C6H6 at 23 °C. However, the initial spectrum for the diprotonated complex (470, 763 nm) rapidly (≤ 2 min) converts to a spectrum characteristic of the monoprotonated MnIII species (446, 728 nm) under catalytic conditions, and no evidence for the formation of either the MnV(O) or MnIV(O)(π–radical cation)(Figure S14). The 446, 728 nm spectrum slowly bleaches over the course of the catalytic reaction. The spectrum for the diprotonated MnIII complex can be maintained if a third equiv of H+ [B(C6F5)4]− is added prior to photoirradiation, but under these conditions no oxidation products of HMB were obtained. Taken together, these observations indicate that the presence of 2 equiv of H+ are required for catalytic turnover, and it is the monoprotonated MnIII complex that is the catalytically active resting state.
Further insight regarding the role of 2 equiv of H+ came from examining the diprotonated MnIII complex in “wet” C6H6 under aerobic conditions, versus dry C6H6 under inert atmosphere. It was found that residual H2O, present under aerobic conditions, acted as a weak base and led to the deprotonation of [MnIII(H2O)(TBP8Cz(H)2)]2+ to give the monoprotonated complex, while under strictly anaerobic conditions (doubly-distilled C6H6, inert atmosphere), the diprotonated complex was observed (Figures S9–S11). Thus the monoprotonated MnIII complex is formed under the aerobic conditions of the catalytic reaction.
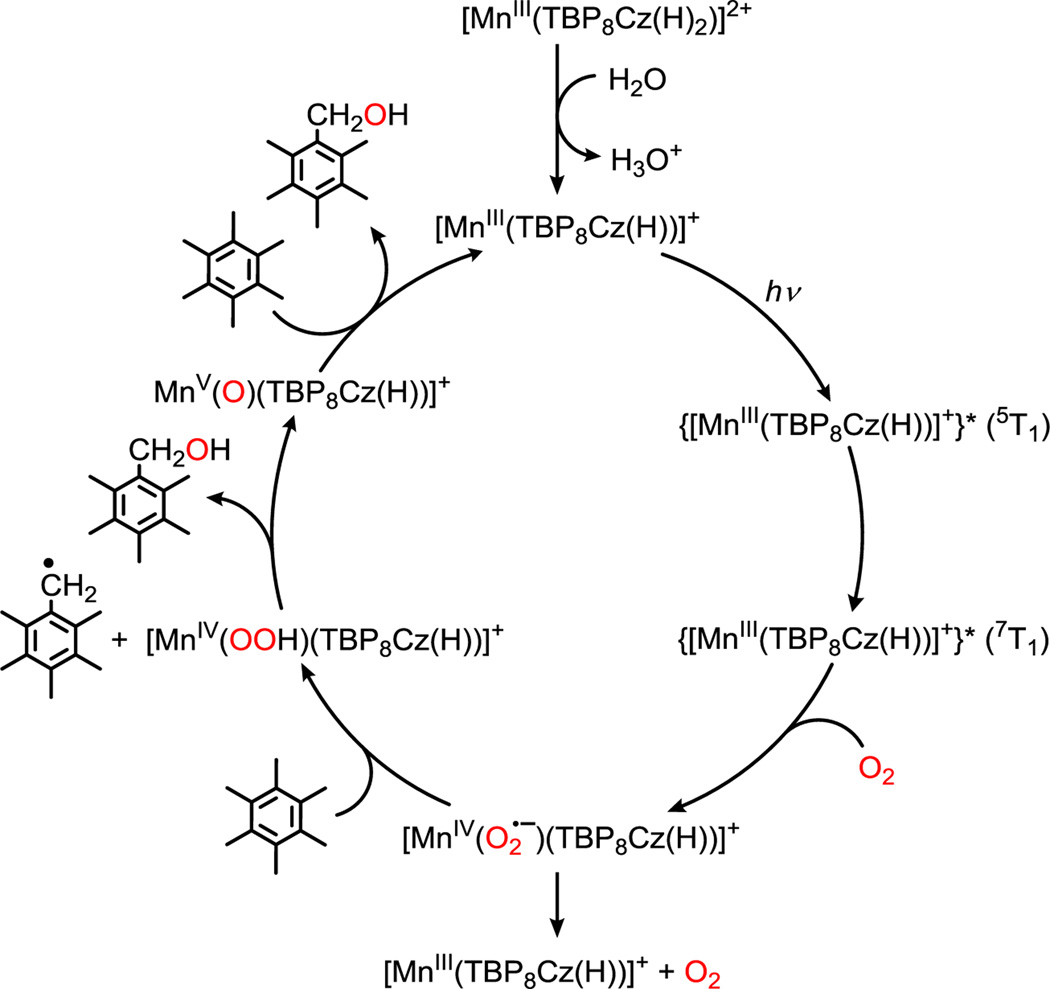
The data presented herein, together with previous femtosecond transient absorption measurements,7b lead to the proposed catalytic cycle shown in Scheme 2. The monoprotonated MnIII complex is the resting state of the catalyst, which is activated by photoexcitation to a tripquintet state (5T1) that rapidly converts to the O2–reactive tripseptet (7T1) state. Entry of O2 gives a putative MnIV(superoxo) complex, which can either undergo rapid back electron–transfer to the ground state and release of O2, or can abstract an H–atom from the HMB substrate. The resulting MnIV(OOH) complex can then hydroxylate the HMB radical via O–O cleavage and rebound of OH•, to yield PMB–OH and the MnV(O) complex. The MnV(O) complex is activated in the presence of acid under the catalytic conditions, leading to oxidation of HMB (or PMB–OH) and completing the catalytic cycle.
Scheme 2.
Proposed Catalytic Cycle for the Oxidation of HMB
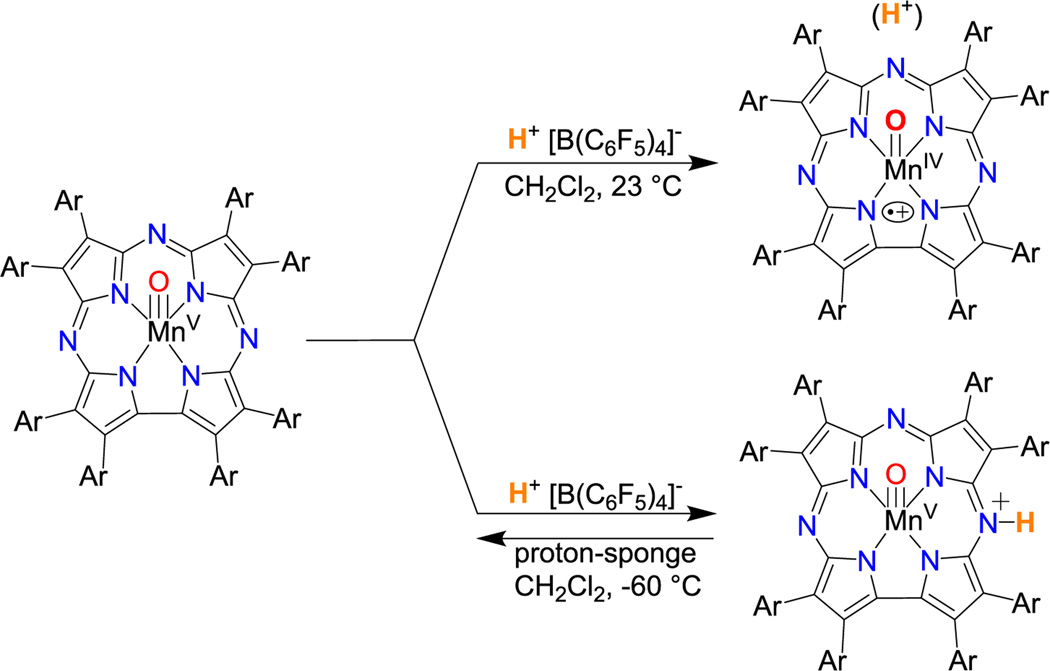
Attempts were made to gain insights into the final step of the catalytic cycle by reacting isolated MnV(O)(TBP8Cz) with H+[B(C6F5)4]− in CH2Cl2 or benzene. This reaction, when run with one equiv of H+ at 23 °C, afforded the valence tautomer [MnIV(O)(TBP8Cz•+)(H)]+ (Scheme 3, Figure S15). This species was unreactive toward either excess HMB or PMBOH (1000 equiv) for up to 2 h at 23 °C, and photoirradiation of this solution caused significant decomposition (~40% bleaching). The addition of 2 equiv of H+[B(C6F5)4]− to isolated MnV(O)(TBP8Cz) in the presence of light and excess HMB caused rapid (<10 s) formation of MnIII, although organic oxidation products could not be identified. The exact nature of the catalytically active intermediate cannot be assigned, but it is clear that 2 equiv of H+ rapidly destabilizes the isolated MnV(O) complex, and these observations suggests that H+ may assist in activating the MnV(O) complex to induce turnover under the appropriate catalytic conditions.
Scheme 3.
Addition of H+ to MnV(O)(TBP8Cz)
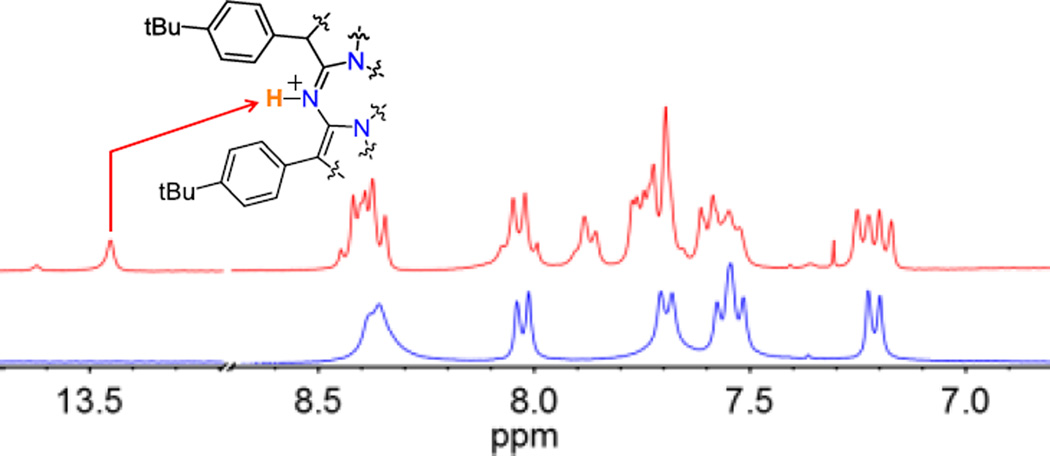
Further examination of the reaction of MnV(O)(TBP8Cz) and H+[B(C6F5)4]− at low temperature (−60 °C) led to an isosbestic change together with the formation of a distinct, new species with λmax = 436, 650 nm (Figure S16). This spectral change is reversible upon addition of one equiv of l,8–bis(dimethylamino)naphthalene (proton sponge), consistent with an acid–base transformation (Figure S17). The structure of the new MnV species (436, 650 nm) was characterized by low–temperature ID and 2D NMR spectroscopy. The 1H NMR spectra (−50 to −60 °C) of the starting MnV(O) complex and the monoprotonated derivative are compared in Figure 3. Both spectra are diamagnetic, confirming that the low–spin (d2) MnV oxidation state is retained at −60 °C (Figure 3). The most notable difference between the two species is the appearance of a new peak at 13.45 ppm following addition of the H+[B(C6F5)4]−. The chemical shift of this resonance is consistent with protonation of a meso–N atom, as seen for the MnIII complexes, and is shifted downfield due to a ring current effect. This new peak disappeared upon exchange with D2O, while the other peaks remained intact (Figure S21). The protonated complex also exhibits a new multiplet at 7.87 ppm, along with additional splitting in both the aromatic and tBu regions (Figures S20). These perturbations are consistent with a lowering of the molecular symmetry, as expected for protonation at a single meso-N position adjacent to the direct pyrrole–pyrrole bond of the Cz ligand. Definitive assignment of the 13.45 ppm peak comes from 1H–1H 2D nuclear Overhauser effect spectroscopy (NOESY) (Figure S22). Well–defined cross–peaks are observed between the new peak at 13.45 ppm and peaks at 8.04 and 7.70 ppm, indicating an interaction between the meso N–H and two nearby (<5 Å) aryl C–H resonances. From the 1H–1H 2D correlation (COSY) spectrum, we can clearly assign the aryl C–H resonances at 8.04 and 7.70 ppm as arising from protons on separate benzene rings (Figure S23). Taken together, the NMR data show that a meso–N atom closest to the direct pyrrole–pyrrole bond is protonated upon addition of H+[B(C6F5)4]− to the MnV(O) complex at −60 °C (Scheme 3). Thus the MnV(O) complex can be protonated at the meso-N position as seen for MnIII, and this protonation site, together with possible protonation at the oxo position, may play a role in the proton–assisted, light–driven, catalytic aerobic oxidation of HMB.
Figure 3.
Comparison of 1H NMR spectra (aryl region) for [MnV(O)(TBP8Cz(H))]+ (red) at −60 °C and MnV(O)(TBP8Cz) (blue) at −50 °C.
In summary we have shown that a MnIII porphyrinoid complex can catalyze the visible light-driven, aerobic oxidation of benzylic C–H bonds. Strict control of the proton content is necessary to achieve catalytic turnover. The MnIII resting state of the catalyst was definitively characterized by XRD. Low temperature spectroscopic methods have identified a new form of an MnV(O) corrolazine, in which a remote site is selectively protonated, and suggests a potential binding site for H+ along the catalytic pathway. Although we cannot definitively characterize the precise protonation state of the MnV complex under catalytic conditions, taken together, the data indicate that 2 equiv of acid are necessary for catalysis. This work furthers our knowledge regarding the criteria for developing biomimetic heme complexes for O2 activation catalysts, and suggests a promising strategy in catalyst design could involve the incorporation of remote ligand sites for modification by non-covalent interactions.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM101153 to D.P.G) and an ALCA and SENTAN project (to S.F.) from JST, Japan. We thank Prof. K. D. Karlin for instrumentation use and we thank Dr. A. Majumdar and Dr. J. Tang for helpful NMR discussions.
Footnotes
ASSOCIATED CONTENT
Supporting Information. CIF, experimental procedures, Table S1, and Figures S1–S23. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.(a) Sono M, Roach MP, Coulter ED, Dawson JH. Chem. Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]; (b) Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]; (c) Kovaleva EG, Lipscomb JD. Nat. Chem. Biol. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Ellis PE, Lyons JE. Coord. Chem. Rev. 1990;205:181–193. [Google Scholar]; (b) O'Reilly ME, Del Castillo TJ, Falkowski JM, Ramachandran V, Pati M, Correia MC, Abboud KA, Dalai NS, Richardson DE, Veige AS. J. Am. Chem. Soc. 2011;133:13661–13673. doi: 10.1021/ja2050474. [DOI] [PubMed] [Google Scholar]; (c) Park YJ, Ziller JW, Borovik AS. J. Am. Chem. Soc. 2011;133:9258–9261. doi: 10.1021/ja203458d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jaafar H, Vileno B, Thibon A, Mandon D. Dalton Trans. 2011;40:92–106. doi: 10.1039/c0dt00756k. [DOI] [PubMed] [Google Scholar]; (e) Lionetti D, Day MW, Agapie T. Chem. Sci. 2013;4:785–790. doi: 10.1039/C2SC21758A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) de Torres M, van Hameren R, Nolte RJM, Rowan AE, Elemans JAAW. Chem. Commun. 2013;49:10787–10789. doi: 10.1039/c3cc45608k. [DOI] [PubMed] [Google Scholar]; (g) Saha B, Gupta D, Abu-Omar MM, Modak A, Bhaumik A. J. Catal. 2013;299:316–320. [Google Scholar]; (h) Zhang Q, Gorden JD, Goldsmith CR. Inorg. Chem. 2013;52:13546–13554. doi: 10.1021/ic402501k. [DOI] [PubMed] [Google Scholar]; (i) Dai F, Yap GPA, Theopold KH. J. Am. Chem. Soc. 2013;235:16774–16776. doi: 10.1021/ja408357x. [DOI] [PubMed] [Google Scholar]; (j) Sorokin AB. Chem. Rev. 2013;113:8152–8191. doi: 10.1021/cr4000072. [DOI] [PubMed] [Google Scholar]; (k) Ray K, Pfaff FF, Wang B, Nam W. J. Am. Chem. Soc. 2014;236:13942–13958. doi: 10.1021/ja507807v. [DOI] [PubMed] [Google Scholar]
- 3.(a) Grinstaff MW, Hill MG, Labinger JA, Gray HB. Science. 1994;264:1311–1313. doi: 10.1126/science.8191283. [DOI] [PubMed] [Google Scholar]; (b) Punniyamurthy T, Velusamy S, Iqbal J. Chem. Rev. 2005;205:2329–2363. doi: 10.1021/cr050523v. [DOI] [PubMed] [Google Scholar]; (c) Roduner E, Kaim W, Sarkar B, Urlacher VB, Pleiss J, Glaser R, Einicke WD, Sprenger GA, Beifuss U, Klemm E, Liebner C, Hieronymus H, Hsu SF, Plietker B, Laschat S. ChemCatChem. 2013;5:82–112. [Google Scholar]
- 4.Mahammed A, Gray HB, Meier-Callahan AE, Gross Z. J. Am. Chem. Soc. 2003;125:1162–1163. doi: 10.1021/ja028216j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Harischandra DN, Lowery G, Zhang R, Newcomb M. Org. Lett. 2009;11:2089–2092. doi: 10.1021/ol900480p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang R, Vanover E, Chen TH, Thompson H. Appl. Catal., A. 2013;464:95–100. [Google Scholar]
- 6.Rosenthal J, Luckett TD, Hodgkiss JM, Nocera DG. J. Am. Chem. Soc. 2006;128:6546–6547. doi: 10.1021/ja058731s. [DOI] [PubMed] [Google Scholar]
- 7.(a) Prokop KA, Goldberg DP. J. Am. Chem. Soc. 2012;134:8014–8017. doi: 10.1021/ja300888t. [DOI] [PubMed] [Google Scholar]; (b) Jung J, Ohkubo K, Prokop-Prigge KA, Neu HM, Goldberg DP, Fukuzumi S. Inorg. Chem. 2013;52:13594–13604. doi: 10.1021/ic402121j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jung J, Ohkubo K, Goldberg DP, Fukuzumi S. J. Phys. Chem. A. 2014;118:6223–6229. doi: 10.1021/jp505860f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren JJ, Tronic TA, Mayer JM. Chem. Rev. 2010;110:6961–7001. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Neu HM, Yang TH, Baglia RA, Yosca TH, Green MT, Quesne MG, de Visser SP, Goldberg DP. J. Am. Chem. Soc. 2014;136:13845–13852. doi: 10.1021/ja507177h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Prokop KA, Neu HM, de Visser SP, Goldberg DP. J. Am. Chem. Soc. 2011;133:15874–15877. doi: 10.1021/ja2066237. [DOI] [PubMed] [Google Scholar]; (c) Prokop KA, de Visser SP, Goldberg DP. Angew. Chem., Int. Ed. 2010;49:5091–5095. doi: 10.1002/anie.201001172. [DOI] [PubMed] [Google Scholar]; (d) Lansky DE, Mandimutsira B, Ramdhanie B, Clausen M, Penner-Hahn J, Zvyagin SA, Telser J, Krzystek J, Zhan R, Ou Z, Kadish KM, Zakharov L, Rheingold AL, Goldberg DP. Inorg. Chem. 2005;44:4485–4498. doi: 10.1021/ic0503636. [DOI] [PubMed] [Google Scholar]
- 10.(a) Leeladee P, Baglia RA, Prokop KA, Latifi R, de Visser SP, Goldberg DP. J. Am. Chem. Soc. 2012;134:10397–10400. doi: 10.1021/ja304609n. [DOI] [PubMed] [Google Scholar]; (b) Baglia RA, Durr M, Ivanovic-Burmazovic I, Goldberg DP. Inorg. Chem. 2014;53:5893–5895. doi: 10.1021/ic500901y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.