Abstract
Objectives
The aim of the study was to determine if there had been any change in the number of solid-pseudopapillary neoplasms (SPN) cases detected, their evaluation or the management over time.
Methods
A systematic review of SPN was performed of all articles published in English in PubMed and SCOPUS.
Results
2,744 patients with SPN identified in 484 studies published between 1961-2012; 87.8% cases were reported between 2000-2012. 2,408 (87.8%) females and the mean age was 28.5 years (S.D. ± 13.7). The most common symptom was abdominal pain in 63.6% and incidentally detected in 38.1%. There were 2,285 patients who underwent pancreatic resection. The mean tumor size was 8.6 cm (S.D ± 4.3). Follow-up was reported for 1,952 (90.5%) patients, with mean follow-up of 36.1 months (S.D. ± 32.8). Disease-free survival was documented in 1,866 (95.6%) patients with recurrence in 86 (4.4%); median time to recurrence was 50.5 months.
Conclusions
The number of SPNs reported in the literature has seen a 7-fold increase in the number of cases reported since 2000 compared to before. SPNs continue to be primarily found in young women and present with non-specific symptoms. Surgery remains the mainstay of treatment with an excellent long term prognosis.
Keywords: Pancreas, Solid-pseudopapillary neoplasm, Review, Meta-analysis, Cyst
Introduction
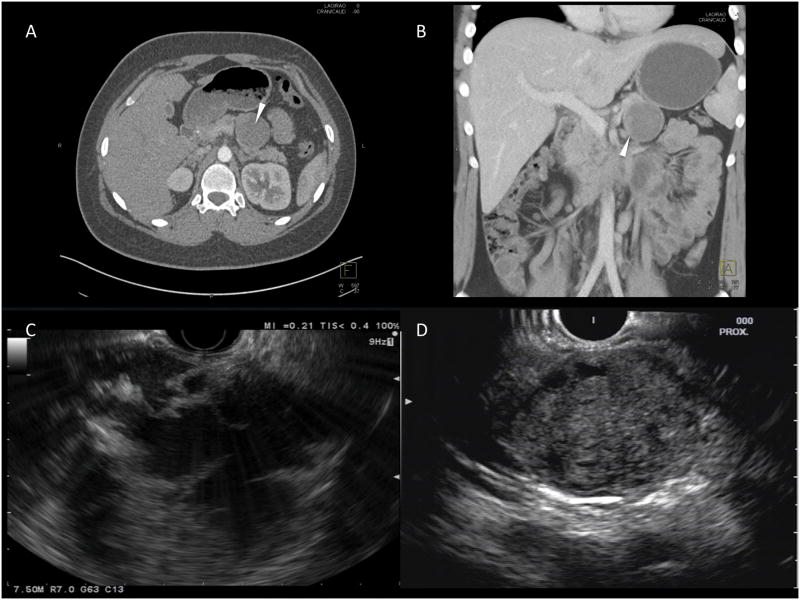
Solid-pseudopapillary neoplasms (SPNs) were first described by Frantz in 19591. SPN have been referred to using a variety of names including papillary epithelial neoplasm of the pancreas, solid and papillary tumors of the pancreas, and Hamoudi or Frantz tumors. In 1996, the World Health Organization (WHO), classified them as a borderline tumor of the exocrine pancreas and named them SPN2. SPN occur most frequently in young women. They classically present as a large, solitary, well-circumscribed lesion, which can have a completely cystic, mixed cystic and solid, or a purely solid appearance on abdominal imaging (Figure 1). The majority of patients have localized disease, with only 9-15% presenting with metastasis or local invasion. The mainstay of treatment is surgical resection, and unlike pancreatic adenocarcinoma, the reported five-year survival rate is as high as 94-97%3,4.
Figure 1. Imaging features of SPN.
This figure demonstrates the classic appearance of a SPN on imaging; (A) and (B) – CT images of mixed solid and cystic appearing lesion in the tail of the pancreas (arrowhead); (C) and (D) – EUS images demonstrating a mixed cystic-solid lesion (C) and a well-defined lesion with a mainly solid component (D).
SPN are rare pancreatic cystic neoplasms, with the largest single institution series to date including only 37 patients who underwent resection of SPN3. Papavramidis et al, performed a review of SPN publications reported in the English language, and identified 718 cases of SPN, which were the sum of all the studies reported up to 20034. Over the last decade there has been a marked increase in the number of incidentally detected pancreatic cysts, with 2.6% of asymptomatic patients undergoing multi-detector computer tomography (CT) scans found to have pancreatic cysts5. We hypothesized that there was likely to have been a similar increase in the number of SPN detected. The aim of this study was to perform a systematic review of all studies on SPN published in the English literature since 1959 and to determine if there have been any changes in the number of cases detected, their evaluation or management over time.
Methods
Literature Search
PubMed and Scopus were queried from the inception of each database to September 19, 2012 using predetermined search strings that included the terms “pancreas” and “pseudopapillary” (see Supplemental Digital Contact - Appendix 1 for full search terms). Because of the various names associated with this lesion, historic names were used in the search strategy which included “Frantz tumor”, “Hamoudi tumor”, and “papillary cystic tumor”. A review of the bibliography of all studies was performed as part of the systematic review.
Inclusion criteria
Full text studies published in the English language were included. Publications of any study design with a confirmed histologic or cytologic diagnosis of SPN of the pancreas and a description of patient characteristics (demographics and/or tumor size and location) were included. Reference list of the relevant review articles identified from the systematic review was reviewed to further identify studies that meet our inclusion criteria.
To avoid duplication of cases, the demographic and patient characteristics were cross-referenced initially by the country of origin, and then by the center from which the case originated. If multiple case series were published from the same center with overlapping study periods, the publication(s) with the largest number of cases was utilized. If a duplicate case was reported in separate case reports, the case report with most pertinent clinical data were used.
Article review and data abstraction
The data were independently screened and reviewed by two reviewers (JKL and AA). Disagreements were reconciled through discussion and consensus with a third investigator (AML). Full text studies that met the inclusion criteria were abstracted by a single reviewer (AA) and reviewed by a second reviewer (JKL). Variables that were recorded included those related to the publication (year, country, hospital from which case(s) originated, and study design), patient demographics and presentation (gender, age, symptoms), investigations (imaging, biopsy), surgery (type and intent), tumor characteristics (size, location, presence of nodal or distant metastases), adjuvant therapy, duration of follow-up, and final outcomes when available. If data were reported for some, but not all patients, it was presumed that the finding was present in the patients reported, and absent in the remaining patients in the paper.
Statistical Analysis
Many studies did not report every field in the data extraction sheet. Therefore, the frequency of findings was calculated based on the total number of patients in whom the finding was reported rather than the total number of patients with SPN. The results are presented as the total number of patients affected, followed by the total number of patients in whom the finding was reported which is reported as (n=X). The number of studies in which these findings were reported is given in the relevant tables.
Descriptive statistics were calculated for all demographic, imaging, clinical and pathological variables and reported as mean ± standard deviation (s.d.) or as proportion. Univariable analysis was performed using Chi-Square test for categorical variables and Student's t-test for continuous variables. A p-value of <0.05 was considered significant. All statistical analysis was conducted using Stata version 12 (StataCorp LP, College Station, TX).
Results
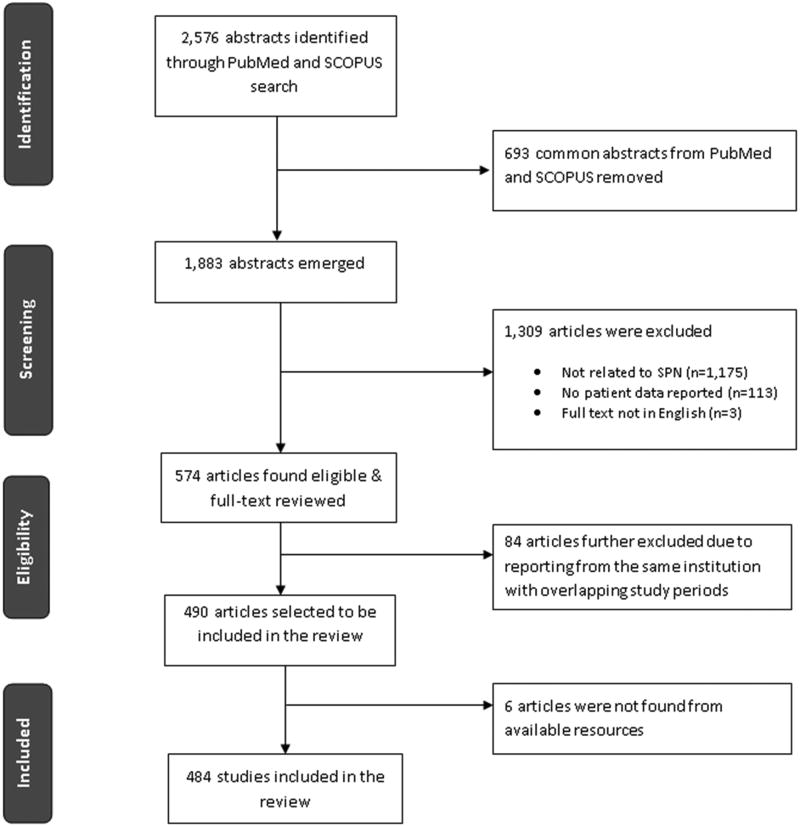
There were 2,576 abstracts identified from the initial search, of which 574 met the inclusion criteria (Figure 2). A total of 84 studies were excluded because of different publications on SPN from the same institution with overlapping study periods and 6 studies were excluded because the full text was not available in English. There were 484 studies (Supplemental Digital Contact - Appendix 2) available for analysis. These included 292 (60.3%) case reports and 191 (39.5%) case series with a mean of 9.9 cases (range 2-114) and 1 (0.2%) literature review. One hundred and forty six (30%) of the studies were published between 1961 and 1999, with 338 (70%) published between 2000 and 2012.
Figure 2. Flow diagram illustrating the search process.
Two literature reviews of SPN, one of English language4 and the other of Chinese language studies6, have previously been published. The English language review was not included4, but a hand search of the references was performed, and the original studies in the references were included in the current study. The Chinese article identified 553 patients in 117 studies, which were found by querying 2 large databases of Chinese language publications6. None of the original publications were available in English, and therefore, the patients from this study were included in the systematic review without a review of the original studies.
Patient presentation
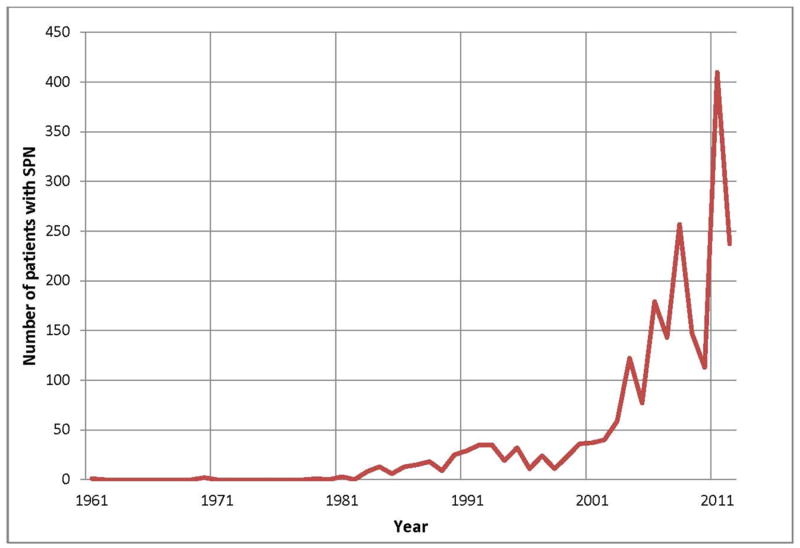
There were 2,744 patients with SPN identified, of whom 6 had synchronous lesions. There were 334 patients (12.2%) and 2,410 patients (87.8%) reported from 1961-1999 and 2000-2012, respectively. The number of patients reported in the literature by year is shown in Figure 3. The results from the Chinese review study6 were excluded from this figure as no data was provided as to the number of patients diagnosed each year. Women accounted for 2,408 (87.8%) of the patients of whom 301 (90.1%, n=334) were diagnosed before 2000 and 2,107 (87.4%, n=2,410) were diagnosed between 2000-2012 (Table 1). The mean age at presentation was 28.5 (S.D. ± 13.7) years. The mean tumor size was 8.6 (S.D. ± 4.3) cm (n=2,750 patients). The size of the SPN detected decreased from a mean of 9.8 cm before 2000, to 8.1 cm in the lesions found between 2000-2012 (P<0.05). The majority of SPN were located in the body and tail of the pancreas (59.3%; n=1,626), with 36.0% (n=988) found in the head or uncinate process with no difference in the location of the lesions between the two time periods.
Figure 3. The number of patients diagnosed with SPN over time.
This figure shows the number of reported cases of SPN reported in the English literature per year.
Table 1. Patient demographics and tumor characteristics.
| All years | 1961-1999 | 2000-2012 | |
|---|---|---|---|
|
| |||
| Number of studies, n | 484 | 146 | 338 |
|
| |||
| Patients with SPN, n | 2,744 | 334 | 2,410 |
|
| |||
| Female, n (%) | 2,408 (87.8) | 301 (90.1) | 2,107 (87.4) |
|
| |||
| Mean Age, years (± S.D.) | 28.5 (± 13.7) | 26.2 (± 13.1) | 29.5 (± 13.9) |
|
| |||
| Tumor Location, n (%) | |||
| Head | 988 (36.0) | 125 (37.4) | 863 (35.8) |
| Body/Tail | 1,626 (59.3) | 202 (60.5) | 1,424 (59.1) |
| Not documented | 99 (3.6) | 4 (1.2) | 95 (3.9) |
| Extra-pancreatic | 31 (1.1) | 3 (0.9) | 28 (1.2) |
|
| |||
| Number of studies reporting on tumor size, n | 454 | 139 | 315 |
|
| |||
| Total number of patients in studies reporting mean tumor size, n (%) | 2,650 | 325 (12.3) | 2,325 (87.7) |
|
| |||
| Mean tumor size, cm (± S.D.) | 8.6 (± 4.3) | 9.8 (± 4.1) | 8.1 (± 4.4) |
Gender, age and tumor location were reported in all studies.
The most common presenting symptom was abdominal pain or discomfort, which was present in 1,389 (63.6%; n=2,183) patients. Patients also presented with a palpable abdominal mass, nausea or vomiting, and weight loss (Supplemental Digital Contact - Appendix 3). Pancreatitis and jaundice were relatively rare, occurring in 49 (5.0%; n=974) and 10 (10.3%; n=97) patients, respectively. There were 593 (38.1%; n=1,557) patients who were asymptomatic (Supplemental Digital Contact - Appendix 3).
Abdominal Imaging
A total of 3,544 imaging studies were reported with 312 performed prior to 2000 and 3,232 studied performed between 2000-2012 (Table 2). Computed tomography (CT) was the most common form of abdominal imaging and accounted for 1,732 (48.9%) of studies. The second most common form of imaging was transabdominal ultrasound (TAUS), which accounted for 1,225 (34.6%) studies followed by magnetic resonance imaging (MRI), accounting for 457 (12.9%) studies. Endoscopic ultrasound (EUS) accounted for 130 studies (3.7%).
Table 2. Imaging procedures performed on patients with SPN.
This table demonstrates the total number of papers in which imaging studies were reported. Below this, the total number of imaging studies reported is provided, followed by the type of study performed. Some patients may have had more than 1 imaging study reported.
| All years | 1961-1999 | 2000-2012 | |
|---|---|---|---|
| Number of papers, n | 420 | 123 | 297 |
| Total number of imaging studies, n | 3,544 | 312 | 3,232 |
| Imaging modality | |||
| Computed tomography (CT), n (%) | 1,732 (48.9) | 176 (56.4) | 1,556 (48.1) |
| Transabdominal ultrasound (TAUS), n (%) | 1,225 (34.6) | 114(36.5) | 1,111 (34.4) |
| Magnetic resonance imaging (MRI), n (%) | 457 (12.9) | 20 (6.4) | 437 (13.5) |
| Endoscopic ultrasound (EUS), n (%) | 130 (3.6) | 2 (0.7) | 128 (4.0) |
Pre-operative tissue diagnosis
A total of 253 pre-operative biopsies were performed either percutaneously (58.5%, n=148) or via EUS guided fine needle aspiration (EUS-FNA) (41.5%; n=105) (Supplemental Digital Contact - Appendix 4), which correctly identified SPN in 164 (64.8%) of the cases. Percutaneous biopsies correctly identified 91 (61.5%, n=148, range 0-100%) SPN. EUS-FNA correctly identified 73 (69.5%, n=105, range 0-100%) SPN; all of which were performed after 2000.
Surgical Intervention
Pancreatic resections were reported in 2,285 patients (85.1%; n=2,685) (Supplemental Digital Contact - Appendix 5), of whom 293 (12.8%) were performed prior to 2000 and 1,992 (87.2%) were performed from 2000-2012. There were 1,111 (48.6%) patients who underwent a distal pancreatectomy, 552 (24.2%) pancreaticoduodenectomy, 202 (8.8%) enucleations, 91 (4.0%) central pancreatectomies, and 18 (0.8%) total pancreatectomies. The type of pancreatic resection was not specified in 311 (13.6%) patients. The surgery was performed via an open procedure in 1,903 (83.3%) cases, while 39 (1.7%) were performed laparoscopically, and in 343 (15.0%) the surgical approach was not indicated. The majority of laparoscopic procedures (n=38, 97.4%) performed between January 2000 and 2012.
Pathological Stage
Detailed pathological staging was infrequently documented, with only 89 (18.4%) studies reporting this data (see Supplemental Digital Contact - Appendix 6 for details) accounting for a total of 1,523 (55.5%) of patients (Table 3). Vascular involvement was identified in 70 patients (4.6%, n=1,523), lymph node metastases was reported in 24 patients (1.6%, n=1,523), and distant spread was noted in 118 patients (7.7%, n=1,523). The study by Yu et al 6 accounted for 36.3% of the patients in this analysis and reported vascular invasion in 11 (2.0%, n=553), lymph node involvement in 3 (0.5%, n=553), distant metastases in 31 (5.6%, n=553) patients. In contrast, the other 88 studies reported on 970 patients and found vascular involvement in 59 (6.1%, n=970) patients, lymph node metastases in 21 (2.2%, n=970) patients, and distant spread in 87 (9.0%, n=970) patients.
Table 3. Pathological staging and outcomes in patients with SPN.
This table demonstrates the total number of studies in which pathological staging was reported and the total number of patients in these studies. The number of patients with vascular, lymph node, and distant metastases are then reported as a percentage of the total number of patients in these studies. The number of studies in which outcomes were reported and the total number of patients in these studies are also demonstrated; however, the follow-up was not reported in all these patients. The number of patients who are disease-free, had recurrence, or died from SPN are reported as a percentage of the number of patients in whom follow-up was reported. The mean duration of follow-up and time to recurrence (when appropriate) are reported as months.
| All years | 1961-1999 | 2000-2012 | |
|---|---|---|---|
| Pathologic Stage | |||
| Number of studies, n | 89 | 19 | 70 |
| Total number of patients in studies, n | 1,523 | 72 | 1,451 |
| Vascular Involvement, n (%) | 70 (4.6) | 7 (9.7) | 63 (4.3) |
| Lymph node metastases, n (%) | 24 (1.6) | 3 (4.2) | 21 (1.4) |
| Distant spread, n (%) | 118 (7.7) | 19 (26.4) | 99 (6.8) |
| Outcomes | |||
| Number of studies, n | 320 | 104 | 216 |
| Total number of patients in studies, n | 2,158 | 269 | 1,889 |
| Total number of patients with follow-up, n (%) | 1,952(90.5) | 254 (94.4) | 1,698 (89.9) |
| Follow-up, months (± S.D.) | 36.1 (± 32.8) | 27.4 (± 24.1) | 40.1 (± 35.4) |
| Disease-free, n (%) | 1,866 (95.6) | 242(95.3) | 1,624 (95.6) |
| Recurrence, n (%) | 86 (4.4) | 12 (4.7) | 74 (4.4) |
| Time to recurrence, months (± S.D.) | 50.5 (± 44.6) | 47.9 (± 46.5) | 51.1 (± 44.6) |
| Death due to SPN, n (%) | 29 (1.5) | 5 (1.9) | 24 (1.3) |
Adjuvant Therapy and Overall Outcomes
Adjuvant Therapy
Adjuvant therapy was reported in 47 patients (6.3%, n=749) (Supplemental Digital Contact - Appendix 7). Follow-up was available on 24 patients; 6 died from their disease while the remaining 18 were alive a mean of 51.1 months after diagnosis (S.D. ± 56.2). Of those who received adjuvant therapy, 35 (4.7%, n=749) underwent chemotherapy and 12 (1.6%, n=749) radiotherapy. 5-Fluorouracil (5FU) and gemcitabine were the two most commonly used chemotherapeutic agents. Follow-up was available on 24 patients who received either neoadjuvant or adjuvant chemoradiation therapy and had surgical resection; 6 died from their disease while the remaining 18 were alive a mean of 51.1 months after diagnosis (S.D. ± 56.2). Five patients, who were not surgical candidates, received a combination of chemotherapy and radiation therapy, with 3 patients alive 18-60 months after diagnosis.
Overall outcome following primary resection
Outcomes were reported in 320 studies. Of the 2,158 patients in these studies, follow-up was available in 1,952 (90.5%) patients with a mean follow-up of 36.1 months (S.D. ± 32.8) (Table 3). Recurrence was reported in 86 (4.4%, n=1,952) patients with a mean time to recurrence of 50.5 months (S.D. ± 44.6). There were 66 (3.1%) patients who died, of whom 29 (1.5%) died due to SPN.
Discussion
To our knowledge, the current review represents the largest number of patients with SPN reported in the English literature. Prior to this study, the largest review included 718 patients4. Similar to other types of pancreatic cysts, there has been a marked increase in the number of studies reporting SPN, with 2,744 patients identified in this study. SPN continues to primarily be a tumor of young women, with men accounting for only 12.2% of the cases. Unlike mucinous cystic neoplasms, the other common pancreatic cystic neoplasm in women which occur almost exclusively in the body and tail of the pancreas7, the location of the lesion is not helpful in identifying SPN, which can occur in any part of the pancreas. The most common presentation is with non-specific symptoms such as abdominal pain or discomfort. Unlike other types of pancreatic malignancies, presentation with jaundice, weight loss and pancreatitis are rare, being observed in fewer than 12% of cases.
There has been a dramatic increase in the use of abdominal imaging over the last decade, with 67 million CT scans performed in the United States in 2006 alone8. This increase use of imaging is mirrored by an increase in the number of SPN identified, with almost 90% of SPN cases in this study reported in the last 12 years. As more imaging is performed, one can hypothesize that the number of incidentally detected SPN would increase. In this study, the number of incidentally detected SPN has grown and now accounts for just over 40% of all SPN cases, with 90% of the incidental cases detected in the last 12 years. During the same period the mean tumor size has decreased from almost 10 cm to just over 8 cm.
CT, TAUS, MRI and EUS have all been used to detect SPN. CT is by far the dominant imaging modality, accounting for almost 50% of the procedures performed. The use of MRI and EUS has increased substantially in the United States, with a 791% and 517% increase respectively in the number of procedures performed between 2000-20109. This increased utilization has been mirrored in SPN for MRI but not EUS. Although the number of EUS procedures increased between the two time periods, EUS is still infrequently used, accounting for only 3.6% of imaging procedures performed during the last 12 years. In this series the overall accuracy of EUS-FNA was 69.5%. This is lower than that routinely reported for EUS-FNA for pancreatic adenocarcinoma, which is in the range of 86-91%10,11. This could be due to the presence of on-site evaluation for adequacy of samples obtained by EUS-FNA can affect results. Operator and/or institutional experience may also play a role in adequacy of tissue sampling by EUS-FNA, with a recent study demonstrating a trend towards increasing diagnostic yield with time12. Given the wide variation in accuracy reported (0% to 100%) for SPN, it is likely that a number of these factors may have been involved.
Surgery is associated with an improved overall survival and continues to be the standard of care for localized SPN. In comparison to pancreatic adenocarcinoma and pancreatic neuroendocrine neoplasms, the survival of resected SPN is excellent. However it should be noted however that SPN is clearly a malignant neoplasm, with local and metastatic potential. Thus, there is debate about the optimum extent and type of surgical operation for SPN. Laparoscopic pancreas surgery is a relatively new approach. Although laparoscopic distal pancreatectomies are performed in most high volume centers, laparascopic pancreaticoduodenectomies or central pancreatectomies, are only performed by a very small number of surgeons13,14. This, in addition to low experience with SPN, is likely to influence the relatively low number of cases approached laparoscopically. Although still relatively rare, the number of pancreatic parenchyma sparing operations performed for SPN has doubled over the last 12 years as compared to previously and now account for almost 15% of all pancreatic resections. One of the critical questions when considering parenchymal sparing surgery in patients with SPN is the risk of lymph node metastases. Overall, lymph node involvement was reported to be almost 2.0% in this study. However, over a third of these patients were from a study by Yu et al 6 in which the lymph node involvement was 0.5%, compared with a rate of 2.2% in the other 88 studies that reported on lymph node involvement. The number of lymph nodes examined at surgery has been shown to influence the staging in patients with pancreatic ductal adenocarcinoma, where patients with fewer than 12 lymph nodes were found to be understaged15. No details of the total number of lymph nodes evaluated were provided in the Yu study, and it is possible that the risk of lymph node metastases may have been underestimated.
There are few studies that have addressed the use of adjuvant therapy for patients with SPN. These include case reports of successful management of otherwise non-surgical patients with SPN managed by radiotherapy alone21 and in patients who underwent initial curative resection with subsequent recurrence managed by chemotherapy with subsequent disease-free survival16. However, the small numbers of cases makes it difficult to draw any conclusions on the role of adjuvant therapy or the optimal type of therapy for SPN.
Overall, SPN has a very good prognosis, with just over 95% of patients reported as disease free after surgical resection and with less than 2% mortality. The mean time to recurrence of the tumor in this study was just over 4 years; however, the mean follow-up reported for patients was only 3 years. These findings suggest that the recurrence rate may be underestimated in the literature. The data from this study suggest that all patients with SPN should be followed for a minimum of 5 years.
The primary limitation of the present systematic review is that the quality of the included studies is poor, since the majority of publications are case reports or case series involving less than 10 patients, with no prospective or randomized studies. Many of the studies failed to report on all of the outcomes evaluated in the present study. Therefore, we analyzed the results based on the total number of cases reported in the literature. Additionally, care was taken to avoid duplication of cases based on review of patient demographics, presentation, and source of each case. Some cases were excluded that were not duplicates but were nested in small case series of which other patients were published in another larger case series. Despite these limitations, this is the largest systematic review published to date of this exceedingly rare tumor.
In conclusion, SPN is a rare pancreatic tumor with just under 2,800 cases reported in the English literature over 51 years. There has been a 7 fold increase in the number of cases detected since 2000, most likely secondary to improvement in the quality and use of cross sectional imaging. SPN typically occurs in young women with imaging demonstrating a solitary lesion in the pancreas. Surgery is the mainstay of treatment, with an overall excellent prognosis.
Supplementary Material
Acknowledgments
Disclosures: Support from Cancer Center grant P30 CA006973
Abbreviations
- CT
computed tomography
- EUS
endoscopic ultrasound
- MRCP
magnetic resonance cholangiopancreatography
- MRI
magnetic resonance imaging
- SPN
solid-pseudopapillary neoplasm, TAUS, transabdominal ultrasound
Footnotes
Conflict Statement: Mouen A. Khashab is a consultant for Boston Scientific and Olympus America and has received research support from Cook Medical
Vikesh K. Singh is a consultant for Abbvie, Santarus, D-Pharm, and Boston Scientific.
Joanna K Law, Aadil Ahmed, Venkata S. Akshintala, Matthew T. Olson, Siva P. Raman, Syed Z. Ali, Elliot K. Fishman, Ihab Kamel, Marcia I. Canto, Marco Dal Molin, Robert A. Moran, Nita Ahuja, Michael Goggins, Ralph H. Hruban, Christopher L. Wolfgang, and Anne Marie Lennon have nothing to disclose.
Contributor Information
Joanna K Law, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Aadil Ahmed, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Vikesh K Singh, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Venkata S Akshintala, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Matthew T Olson, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD.
Siva P Raman, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD.
Syed Z Ali, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD.
Elliot K Fishman, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD.
Ihab Kamel, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD.
Marcia I Canto, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Marco Dal Molin, Department of Pathology, Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD.
Robert A Moran, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Mouen A Khashab, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
Nita Ahuja, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD.
Michael Goggins, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD.
Ralph H Hruban, Department of Pathology, Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine, Baltimore, MD.
Christopher L Wolfgang, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD.
Anne Marie Lennon, Division of Gastroenterology, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Frantz V. Tumor of the pancreas Atlas of Tumor Pathology, 1st series. Washington, DC: US Armed Forces Institute of Pathology; 1959. pp. 32–33. [Google Scholar]
- 2.Kloppel G, Heitz PU, Capella C, et al. Pathology and nomenclature of human gastrointestinal neuroendocrine (carcinoid) tumors and related lesions. World journal of surgery. 1996;20(2):132–141. doi: 10.1007/s002689900021. [DOI] [PubMed] [Google Scholar]
- 3.Reddy S, Cameron JL, Scudiere J, et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. Journal of the American College of Surgeons. 2009;208(5):950–957. doi: 10.1016/j.jamcollsurg.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. Journal of the American College of Surgeons. 2005;200(6):965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191(3):802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu PF, Hu ZH, Wang XB, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World journal of gastroenterology : WJG. 2010;16(10):1209–1214. doi: 10.3748/wjg.v16.i10.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamao K, Yanagisawa A, Takahashi K, et al. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40(1):67–71. doi: 10.1097/MPA.0b013e3181f749d3. [DOI] [PubMed] [Google Scholar]
- 8.Mettler FA, Jr, Thomadsen BR, Bhargavan M, et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95(5):502–507. doi: 10.1097/01.HP.0000326333.42287.a2. [DOI] [PubMed] [Google Scholar]
- 9.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. e1171–1173. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. Journal of gastroenterology. 2012 doi: 10.1007/s00535-012-0695-8. epub ahead of print October 24, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2011;23(Suppl 1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 12.Olson MT, Ali SZ. Cytotechnologist on-site evaluation of pancreas fine needle aspiration adequacy: comparison with cytopathologists and correlation with the final interpretation. Acta cytologica. 2012;56(4):340–346. doi: 10.1159/000338646. [DOI] [PubMed] [Google Scholar]
- 13.Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. Journal of hepato-biliary-pancreatic surgery. 2009;16(6):726–730. doi: 10.1007/s00534-009-0142-2. [DOI] [PubMed] [Google Scholar]
- 14.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Archives of surgery (Chicago, Ill : 1960) 2010;145(1):19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 15.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Annals of surgical oncology. 2008;15(1):165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 16.Ansari D, Elebro J, Tingstedt B, et al. Single-institution experience with solid pseudopapillary neoplasm of the pancreas. Scandinavian journal of gastroenterology. 2011;46(12):1492–1497. doi: 10.3109/00365521.2011.627448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.