Abstract
While androgen ablation remains a mainstay for advanced prostate cancer therapy, nearly all patients will inevitably develop disease escape with time. Upon the development of castration-resistant prostate cancer, other androgen-axis-targeted treatments may be added in an effort to starve the disease of its androgen signaling. Nevertheless, additional androgen-pathway resistance usually develops to these novel hormonal therapies. In this review, we will discuss the resistance mechanisms to modern androgen-axis modulators and how these alterations can influence a patient's response to novel hormonal therapy. We conceptualize these resistance pathways as three broad categories: (1) reactivation of androgen/AR-signaling, (2) AR bypass pathways, and (3) androgen/AR-independent mechanisms. We highlight examples of each, as well as potential therapeutic approaches to overcome these resistance mechanisms.
Keywords: Prostate cancer, Androgen receptor, Splice variants, Resistance, Biomarker
Introduction
Prostate adenocarcinoma afflicts one in six American men over the course of their lifetime and is the second leading cause of cancer-related deaths in US males behind lung cancer [1]. In a cohort of 790 men with metastatic prostate cancer, a landmark trial demonstrated that the combination of hormonal therapy plus docetaxel chemotherapy increased overall survival compared to hormonal therapy alone [2]. However, even with this extended benefit from first-line chemo-hormonal treatment, most prostate cancer patients eventually progress and require treatment with additional second-line hormonal therapy.
Abiraterone acetate and enzalutamide were approved by the FDA in 2011 and 2012, respectively, for the treatment of men with CRPC. Abiraterone blocks extra-gonadal androgen biosynthesis by selectively inhibiting CYP17A1 [3], while enzalutamide directly antagonizes the AR and diminishes AR-signaling [4]. While many patients benefit from these drugs, some men exhibit primary resistance and nearly all eventually develop secondary resistance. The mechanisms underlying this resistance were poorly understood until recently and now are beginning to be elucidated. In this review, we will outline the underlying biology that determines sensitivity or resistance to these modern therapies. We would hope that this review might stimulate new therapeutic combinations to prevent cross-resistance and inspire innovation in the field.
Rediscovering Androgen Receptor Biology
Prostate cancer is an AR-addicted disease whose development and proliferation strongly rely on adequate AR-signaling. Notwithstanding several unique distinctions, AR shares many commonalities with other steroid hormone receptors in its mechanism of action. Unliganded AR primarily resides in the cytoplasm until ligand-binding triggers its translocation to the nucleus by way of a conformational change in the receptor that liberates it from chaperone heat shock proteins [5]. AR subsequently dimerizes via N/C-terminal interactions, namely the interaction of activation function 2 (AF2) of ARLBD with the NTD [6, 7]. Agonist-bound AR also undergoes phosphorylation at several sites prior to nuclear translocation, including S81 in the NTD (the most highly phosphorylated residue on AR) and S650 in the hinge region [5, 8]. Prostate cancer cell growth is limited in the absence of S81 phosphor-ylation, and specific S81 phosphorylation by CDK9 regulates AR transcriptional activity [8]. Once inside the nucleus, dimerized AR recognizes and binds to AREs in the promoter or enhancer region of target genes using two zinc fingers, which then stimulates growth, survival, and differentiation of prostate cancer cells [5–7]. Additional interactions with coactivators and corepressors serve to further facilitate or inhibit transcription of these target genes. While agonist-bound AR recruits coactivators that amplify the transcription signal when AR binds to DNA, antagonist-bound AR recruits a complex of corepressors that attenuates the same signal [5].
AR amplification and gene mutations significantly contribute to the emergence of CRPC and disease progression. The AR gene is located on the human X chromosome and contains eight canonical exons: exon 1 encodes the NTD, exons 2–3 encode the DBD, and exons 4–8 encode the C-terminal LBD of the AR-FL protein [5–7]. AR-DBD comprises considerable homology with the DBD of the GR and the PR, all of which may share common gene targets [7, 9••]. AR also contains a hinge region lying between its DBD and LBD, and this hinge region surrounds a nuclear localization signal with the DBD [6]. The structure of the AR-LBD strongly resembles that of many other steroid hormone receptors. The ligand-binding pocket is formed by the folding of 12 helices in the C-terminal and is subject to conformational changes dependent on ligand-binding [5]. Point mutations in the LBD can thus alter the normal function of the AR, broadening the number of ligands that can bind the LBD [10]. The W742L/W742C mutations, for instance, are linked to bicalutamide resistance, while F877L and T878A mutations have been associated with resistance to novel androgen-directed therapies enzalutamide and abiraterone, respectively [10–13, 14•, 15].
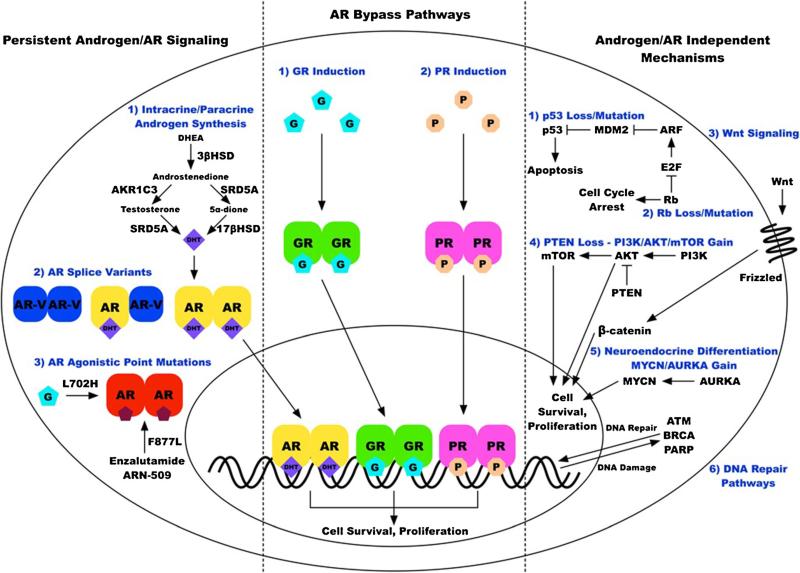
The remainder of this review will discuss specific mechanisms related to clinical androgen-pathway drug-resistance. These resistance mechanisms can be broadly divided into three classes: (1) persistent androgen/AR-signaling, (2) AR bypass pathways, and (3) androgen/AR-independent mechanisms (Fig. 1).
Fig. 1.
Signaling pathways implicated in resistance to novel androgen/AR-directed therapies. These resistance mechanisms are conceptualized in three broad categories: (1) reactivation of androgen/AR-signaling leading to persistent AR-signaling, (2) AR bypass pathways leading to activation of androgen-regulated genes by alternative steroid receptors, and (3) a large number of androgen/AR-independent pathways
Upregulation of Androgen-Synthetic Enzymes
Recent studies have shown that upregulation of enzymes in the androgen synthesis pathway contributes to castration-resistance as well as abiraterone- and enzalutamide-resistance. Extra-gonadal androgen synthesis may occur in the adrenal glands, as well as intratumorally, through upregulation of 3β-hydroxysteroid dehydrogenase (3βHSD), steroid-5α-reductase (SRD5A), and 17β-hydroxysteroid dehydrogenase (17βHSD) [16, 17]. 17α-hydroxylase/17,20-lyase (CYP17A1) itself, the target of abiraterone, has also been shown to be upregulated in prostate cancer patients receiving androgen-axis modulators [16]. Though Salvi et al. [18] demonstrated that AR gene gain in cfDNA had a prognostic effect on PFS in men receiving abiraterone, CYP17A1 gene gain also played a crucial role. Patients with CYP17A1 gain had a median PFS of 2.8 months on abiraterone, while those without such gene gain had a median PFS of 9.2 months [18]. These clinical data suggest that men with copy number gains in the CYP17A1 gene may be less sensitive to abiraterone.
Another androgen-synthetic enzyme that has been implicated in novel hormonal therapy resistance is aldo-keto reductase 1C3 (AKR1C3). This enzyme is responsible for the conversion of Δ4-androstene-3,17-dione to testosterone, and 5α-androstanedione to DHT [19]. Intratumoral upregulation of AKR1C3 has been shown to mediate resistance to both enzalutamide and abiraterone in prostate cancer patients, with this enzyme's ability to convert low levels of circulating androgens into potent AR agonists [20, 21]. Although indomethacin is known to be a weak inhibitor of AKR1C3, the search is on to identify additional more specific AKR1C3 inhibitors for clinical development. In addition, a gain-of-function mutation in the 3βHSD1 gene (N367T) has recently been discovered [22]. This mutation renders 3βHSD1 resistant to ubiquitination and degradation, leading to profound intracellular accumulation of the enzyme and increased levels of DHT [22]. Increased 3βHSD1 activity has been associated with resistance to first-line androgen deprivation therapy, and may even be a mechanism of abiraterone resistance [17, 23].
Notably, there may be promise in new compounds that can antagonize multiple parts of the androgen-axis. A metabolite of abiraterone, called Δ4-abiraterone (D4A), was recently shown to be more potent than abiraterone in vitro [16]. With a chemical structure similar to testosterone, D4A can antagonize the AR directly, in addition to inhibiting CYP17A1, 3βHSD, and SRD5A [16]. Whether D4A will be developed into a clinical drug entity is unclear at this time, but remains of great interest. Galeterone, another CYP17A1 inhibitor, has moved from the lab into the clinic and is currently under investigation in a phase III clinical trial (NCT02438007; Table 1). This compound may have activity against both AR and AR-Vs due to its ability to trigger E3 ubiquitin ligase function and degrade all forms of the AR protein [24]. An analog of galeterone, VNPT55, may also prove more effective in targeting the multitude of androgen-axis resistance pathways [24].
Table 1.
Clinical trials attempting to address divergent mechanisms of androgen/AR resistance
| Upregulation of androgen-synthetic enzymes | |||
| Dutasteride | Phase II | Enzalutamide & Dutasteride as 1st Line Treatment for Patients ≥ 65 Years Old With Prostate Cancer | NCT02213107 |
| Abiraterone Acetate | Phase II | A Phase II Study of Increased-Dose Abiraterone Acetate in Patients With Castration Resistant Prostate Cancer | NCT01637402 |
| Androgen receptor amplification | |||
| Apalutamide/ARN-509 | Phase III | A Study of ARN-509 in Men With Non-Metastatic Castration-Resistant Prostate Cancer (SPARTAN) | NCT01946204 |
| Androgen receptor point mutations | |||
| ODM-201 | Phase III | Efficacy and Safety Study of BAY1841788 (ODM-201) in Men With High-risk Non-metastatic Castration-resistant Prostate Cancer (ARAMIS) | NCT02200614 |
| VT-464 | Phase II | Once-daily Oral VT-464 in Patients With Castration-Resistant Prostate Cancer Progressing on Enzalutamide or Abiraterone | NCT02445976 |
| Androgen receptor splice variants | |||
| Testosterone | Phase II | RE-sensitizing With Supraphysiologic Testosterone to Overcome REsistant (The RESTORE Study) | NCT02090114 |
| Galeterone | Phase III | A Study of Galeterone Compared to Enzalutamide In Men Expressing Androgen Receptor Splice Variant-7 mRNA (AR-V7) Metastatic CRPC (ARMOR3-SV) | NCT02438007 |
| EPI-506 | Phase I/II | Safety and Anti-Tumor Study of Oral EPI-506 for Patients With Metastatic Castration-Resistant Prostate Cancer | NCT02606123 |
| GS-5829 | Phase I/II | Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of GS-5829 as a Single Agent and In Combination With Enzalutamide in Participants With Metastatic Castrate-Resistant Prostate Cancer | NCT02607228 |
| Niclosamide | Phase I | Niclosamide and Enzalutamide in Treating Patients With Androgen Receptor Splice Variant-Positive, Castration-Resistant, Metastatic Prostate Cancer | NCT02532114 |
| Glucocorticoid receptor induction | |||
| Dexamethasone | Phase II | Dexamethasone Prior to Re-treatment With Enzalutamide in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Previously Treated With Enzalutamide and Docetaxel (DEXTER) | NCT02491411 |
| Mifepristone | Phase I/II | Enzalutamide and Mifepristone in Treating Patients With Metastatic Hormone Resistant Prostate Cancer | NCT02012296 |
| Progesterone receptor activation | |||
| Onapristone | Phase I/II | Phase 1-2 Study of Onapristone in Patients With Advanced Castration-resistant Prostate Cancer | NCT02049190 |
| Androgen/AR-independent mechanisms | |||
| Ipilimumab + Nivolumab | Phase II | Biomarker-Driven Therapy With Nivolumab and Ipilimumab in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Expressing AR-V7 (STARVE-PC) | NCT02601014 |
| LY3023414 | Phase II | A Study of Enzalutamide and LY3023414 in Men With Prostate Cancer | NCT02407054 |
| Alisertib | Phase I/II | Alisertib, Abiraterone Acetate and Prednisone in Treating Patients With Hormone-Resistant Prostate Cancer | NCT01848067 |
| Olaparib | Phase II | TOPARP: A Phase II Trial of Olaparib in Patients With Advanced Castration Resistant Prostate Cancer | NCT01682772 |
| Niraparib | Phase I | Enzalutamide and Niraparib in the Treatment of Metastatic Castrate-Resistant Prostate Cancer | NCT02500901 |
Androgen Receptor Amplification
Prostate cancer cells remain responsive to and dependent on androgen-axis signaling, even in the castration-resistant setting [5]. While a temporary halt in disease progression is often observed when these cells are deprived of androgen, AR-signaling adaptations inevitably ensue in response to the selective pressures [5, 25]. One such adaptation is AR gene (and protein) amplification to increase sensitivity to circulating and intratumoral androgens. This heightened sensitivity enables prostate cancer cells to thrive under otherwise limiting conditions [5]. In a study designed to assess the prevalence of AR amplification, Zhang et al. [25] obtained tumor biopsy specimens from 37 patients developing castration-resistant disease, determined AR amplification by FISH analysis, and confirmed the link between AR gene amplification and prostate cancer progression. A clinically significant finding of this study is that patients with AR gene amplification also exhibited increased levels of AR protein, and PFS in these patients was shorter than in patients with normal AR copy numbers, illustrating the significance of AR gene amplification and overexpression in the development of CRPC [25].
AR amplification can also be linked with resistance to enzalutamide and abiraterone. Though not associated with HSPC, AR copy number gain often indicates disease progression in patients with CRPC and is correlated with poor prognosis [14•, 15, 18]. Patients with AR amplification prior to initiating enzalutamide have worse clinical outcomes, exhibited by significantly lower PSA response rates and shorter PFS [14•]. Azad et al. [14•] analyzed cfDNA from 62 metastatic-CRPC (mCRPC) patients progressing on systemic therapy, which revealed that AR amplification occurred at a significantly higher frequency in patients progressing on enzalutamide than on any other agent (53 vs. 21 %). Interestingly, however, a previous study exhibited that bone marrow-specific nuclear AR expression, as well as CYP17A1 expression, was correlated with benefit to enzalutamide therapy [26]. More recently, enzalutamide was shown to trigger a subcellular nuclear-tocytoplasmic shift of AR, thereby suppressing AR-signaling, but also inducing an adaptive feedback mechanism in which testosterone levels are increased in the bone marrow [26]. Combined therapy that directly and indirectly inhibits AR-signaling through AR antagonism and androgen biosynthesis inhibition could evade this feedback mechanism and improve therapeutic efficacy in some CRPC patients.
In the setting of abiraterone, Carreira et al. [10] observed AR copy number gain in 35 and 37 % of CRPC liquid and tumor biopsy samples, respectively, compared to only in 6 % of precastration samples. Moreover, AR copy number gain was associated with resistance to abiraterone [10]. Similarly, Salvi et al. [18] evaluated AR copy number variations (CNVs) in cfDNA from 53 CRPC patients prior to receiving abiraterone, 16 of which exhibited AR gain. Ten (62.5 %) of those 16 patients exhibited early progression on abiraterone, also suggesting that AR CNVs may predict abiraterone resistance [18]. Likewise, abiraterone-naïve patients with AR gain have been reported to be 4.9 times less likely to have a ≥50 % PSA reduction and also exhibit significantly worse PFS and OS than patients without CNVs [15]. Salvi et al. [18] concordantly found that AR amplification remained predictive of both PFS and OS, as patients with AR gene gain had PFS 3.4 times shorter and OS more than fourfold shorter than patients expressing normal AR copy numbers. Notably, a recent study showed that AR copy number at progression on abiraterone remains relatively unchanged from baseline, suggesting that abiraterone resistance in patients expressing normal AR copy numbers may be explained by other mechanisms [15].
Androgen Receptor Point Mutations
Another way the AR-signaling axis can be rescued in prostate cancer is via point mutations in the LBD that develop in response to treatment with specific antiandrogens. These mutations alter the steric and chemical properties of the ligand-binding pocket, thus conferring AR agonistic activity to alternative ligands and former AR antagonists [5, 27, 28]. Numerous studies have confirmed the importance of these mutations in patients receiving novel hormonal therapies. For example, the L702H point mutation converts AR to a glucocorticoid-activated phenotype, while T878A renders AR progesterone-responsive [10, 27, 28]. L702H often mediates resistance to abiraterone treatment due to the fact that abiraterone is given together with corticosteroids, which can agonize this mutated AR [10, 15]. Similarly, T878A allows for abiraterone resistance since abiraterone inhibits CYP17A1, resulting in an increase in upstream steroids (i.e., progestins) [29].
F877L also appears to mediate resistance to enzalutamide and apalutamide (ARN-509) [10, 12, 13, 14•, 30]. This mutation transforms enzalutamide and apalutamide into AR agonists, and also maintains the AR's sensitivity to androgens. F877L is probably more relevant as an acquired (rather than a primary) mechanism of resistance to enzalutamide/apalutamide and is found in <10 % of patients at the time of progression on these agents [30].
A novel antiandrogen, ODM-201, has been shown to antagonize AR even with the F877L and T878A mutations in pre-clinical studies [31]. These data, combined with the drug's promising phase II clinical results [32], have led to a phase III study using this compound in non-metastatic castration-resistant men (NCT02200614; Table 1). ODM-201's lower risk of seizures and activity against mutant AR may help improve its chances for FDA approval in an ever-crowded antiandrogen market.
Androgen Receptor Splice Variants
Constitutively active AR-Vs contribute yet another mechanism of resistance to novel hormonal therapies, as these truncated molecules have been shown to regulate transcription in an androgen-independent fashion [33]. Upward of 20 AR-Vs have been identified to date [34••], some whose function has been well elucidated, others whose role in advanced prostate cancer remains obscure. AR-Vs usually result from cryptic exon insertions downstream of DBD coding regions or (much more rarely in humans) from deletion of LBD-coding regions [33]. ADT enhances the rate of AR gene transcription and recruitment of critical splicing factors to AR pre-mRNAs, which can result in increased expression of AR-V mRNA and protein [35]. An inverse relationship between canonical AR-FL signaling and AR-V expression has previously been shown [36]: inhibition of AR-FL signaling corresponded with increased expression of AR-Vs in vitro, whereas AR-Vs were not overexpressed during normal AR-FL signaling. Compared to levels in HSPC, AR-V1 and AR-V7 levels have been shown to be increased >20-fold in CRPC [33]. These AR-Vs may serve as transcription factors for enhanced expression of cell-cycle genes, while AR-FL signaling may preferentially activate transcription of genes involved in biosynthesis, cellular metabolism, and differentiation [36]. Most notably, Hu et al. [36] demonstrated a correlation between AR-V7 (and ARV567es) overexpression and upregulation of the cell-cycle gene UBE2C in CRPC patient specimens. Other studies have shown that ARV567es is upregulated in the castration setting, promotes expression and activity of AR-FL, activates a gene set distinct from AR-FL, and is associated with increased nuclear localization of AR and shorter survival [37, 38].
AR-V7 in particular has emerged as an important biomarker, yielding insights into disease prognosis and response to novel hormonal agents. Retrospective studies of human bone and prostate biopsies suggest inferior clinical outcomes for CRPC patients overexpressing AR-V7 [38, 39]. Another study showed that AR-V7 was upregulated in 429 prostate cancer biopsy specimens, and increased nuclear expression of ARV7 was associated with higher risk of disease recurrence following radical prostatectomy [40]. To support this idea of ARV7 driven progression, knockdown of AR-V7 resulted in a weakened propensity for prostate cancer cell growth [40].
Recent prospective studies have shown that using AR-V7 as a prognostic marker is feasible in the setting of novel hormonal therapy. A myriad of data bolster AR-V7 status as an independent predictive factor in disease development and progression, and as a marker of resistance to enzalutamide and abiraterone. In an early study collecting bone marrow specimens from men embarking on enzalutamide treatment, patients with high levels of pre-treatment androgen signaling demonstrated clinical benefit, while AR-V7 positivity (using immunohistochemistry) was associated with primary resistance [26]. These findings were corroborated by a study from Antonarakis et al. [41••] in 62 mCRPC patients (31 treated with enzalutamide, 31 treated with abiraterone) analyzing baseline CTC-derived AR-V7 status as a predictor of response or resistance to these therapies. Notably, >50 % of patients pre-treated with abiraterone and/or enzalutamide were ARV7-positive as indicated by the CTC-based RT-PCR assay, whereas <15 % of abiraterone-naïve and enzalutamide-naïve patients were found to express AR-V7 [41••]. Men receiving abiraterone or enzalutamide who were positive for AR-V7 had no PSA responses, shorter clinical or radiographic PFS, and shorter OS than their AR-V7 negative counterparts [41••]. Interestingly, AR-V7 negative to positive conversions were noted in four patients receiving enzalutamide and in two patients receiving abiraterone: these men had intermediate clinical outcomes [41••]. In a third study, Steinestel et al. [42] showed that AR-V7 positivity in CTCs most likely emerges under selective therapeutic pressures and is associated with the absolute number of prior hormonal therapies received. That study also showed inferior clinical outcomes to abiraterone and enzalutamide in AR-V7-positive compared to AR-V7-negative men [42]. Of note, no ADT-naïve patient had detectable levels of AR-V7 in their CTCs, supporting the idea that AR-V7 expression may be an adaptive response to first-line and novel androgen-axis therapies.
AR-V7 status has also become an important biomarker in the setting of chemotherapy. Though AR-V7 positivity in CTCs is linked to enzalutamide and abiraterone resistance, taxane chemotherapy may remain an effective therapeutic alternative in these patients. PSA responses have been observed in taxane-treated patients irrespective of AR-V7 status and prior treatment with second-line androgen-axis therapies [43–45], and treatment with cabazitaxel in particular does not appear to be influenced by AR-V7 status [44]. In post hoc analyses, AR-V7-positive men treated with taxane chemotherapy exhibit superior clinical outcomes to those treated with enzalutamide or abiraterone [43]. Interestingly, the authors reported that only one AR-V7-negative patient converted to AR-V7-positive while receiving taxane chemotherapy, whereas nearly 50 % of AR-V7-positive patients became ARV7-negative during taxane treatment [43]. This phenomenon may be due to conversion from CTC-positive to CTC-negative. Nakazawa et al. [45] also observed conversions from AR-V7-negative to positive status in men treated with AR-directed therapies and taxane chemotherapies; whereas, ARV7-positive to negative reversions were observed solely with taxane chemotherapies [45]. Conversions may reflect adaptive induction of and reliance on AR-V7 to maintain AR-axis signaling, while reversions may reflect some disinhibition of canonical AR-signaling and thereby reduced pressure for ARV7 expression [45]. AR-V7 reversions after taxane chemo-therapy may present a unique opportunity for benefit from re-treatment with enzalutamide or abiraterone, although confirmation of this hypothesis is awaited. At this time, the clinical significance of AR-V7 transitions remains unclear.
Some potential therapeutic strategies to overcome AR-V-mediated resistance are listed in Table 1. These include drugs that target and degrade all AR protein (including AR-Vs; e.g., galeterone), agents that inhibit the AR-NTD (e.g., EPI-506), and epigenetic therapies that interfere with AR transcriptional activity (e.g., bromodomain/BET inhibitors). Moreover, combinatorial immunotherapy strategies (e.g., ipilimumab plus nivolumab) may be a fruitful approach in AR-V-expressing patients.
Glucocorticoid Receptor Induction
Another proposed mechanism of resistance to androgen-axis therapies involves upregulation of the GR. The GR may be able to substitute for the AR in various circumstances, binding to AREs and other promoter elements to sustain cell survival [9••]. These two steroid receptors have overlapping transcriptomes, potentially allowing proliferation signals to bypass the AR in patients being treated with enzalutamide or apalutamide [9••]. Of the tissues analyzed in one study, GR mRNA levels were 27-fold higher in enzalutamide- and apalutamide-resistant tumors compared to control [9••]. In vivo, cells expressing high levels of GR were resistant to treatment with enzalutamide and grew aggressively; whereas, LNCaP cells (which express low levels of GR) showed minimal growth [9••]. By knocking down the GR with shRNA, tumor growth was significantly delayed in this previously drug-resistant mouse model [9••]. Concordant results were also seen when assessing human bone marrow biopsies by immunohistochemistry in enzalutamide-treated patients, with GR-positive patients being less likely to have a durable response to therapy [9••]. These data might also imply that enzalutamide should not be given together with corticosteroids, which may be capable of further agonizing an upregulated GR in this setting. Intriguingly, a hypothesis-generating post hoc analysis of the AFFIRM study (which allowed treating physicians to use concurrent steroids, if desired, together with enzalutamide) suggested that enzalutamide produced inferior PFS and OS when combined with steroids [46].
Another hypothesis suggests that GR is negatively regulated by AR, meaning that AR inhibition automatically leads to GR upregulation. ChIP analysis in one study confirmed that an ARE regulates the expression of GR, leading to more questions regarding the high levels of GR in enzalutamide-resistant cells [47]. However, clinical studies have shown PSA reductions in response to dexamethasone and other steroids (GR agonists) [48–51]. This responsiveness may also indicate that PSA levels are not regulated by the GR. To further clarify the GR landscape, a study using dexamethasone in patients developing acquired resistance to enzalutamide is currently underway, and will allow re-treatment with enzalutamide after a period of dexamethasone therapy (NCT02491411; Table 1). Conversely, another trial is analyzing the use of a GR antagonist, mifepristone, in combination with enzalutamide, to block both signaling pathways concurrently (NCT02012296; Table 1). Further studies must be done to show the importance of the GR in maintaining tumor cell populations and inducing proliferation in CRPC. If shown to be a driving force behind drug-resistance to enzalutamide or other novel hormonal agents, the GR has the potential to be therapeutically targeted in combination with other AR-directed strategies.
Progesterone Receptor Activation
The PR also shares significant homology with the AR, especially in the DBD (>80 % identity in this region). Therefore, it is also possible that PR (like GR) may become induced during androgen ablation, thereby restoring expression of AR-regulated genes [52, 53]. In one study, PR was detected using immunohistochemical staining in about 30 % of mCRPC biopsy specimens [54], and preliminary clinical data from another study suggest that activated (nuclear) PR may be associated with development of castration-resistance [55]. While the role of PR in castration-resistant progression admittedly remains unclear, a phase I/II study is currently being conducted using the oral PR antagonist, onapristone, in men with abiraterone- or enzalutamide-refractory CRPC (NCT02049190; Table 1) [56]. In that study, all patients will undergo baseline tumor biopsies to evaluate activated PR using an analytically validated immunohistochemical assay.
Androgen/AR-Independent Mechanisms
While the androgen/AR pathways are important in understanding progression on novel hormonal therapies, many other resistance mechanisms have also been implicated (Fig. 1; Table 2). With the addition of modern androgen/AR-directed therapies to the treatment regimen, refractory prostate cancer can develop increasingly more mutations and lead to an aggressive and lethal phenotype. For example, neuroendocrine differentiation can result from the loss or mutation of tumor suppressors like Rb and/or p53 (Table 2) [34••, 57]. PTEN is also a commonly lost tumor suppressor gene in prostate cancer, and its loss has been shown to drive metastasis (Table 2) [34••, 58, 59]. Recent studies have linked both canonical and non-canonical Wnt signaling to therapeutic resistance and disease progression (Fig. 1) [34••, 60•, 61]. Similarly, amplification of two oncogenes, N-Myc (MYCN) and Aurora kinase A (AURKA), can lead to neuroendocrine differentiation (Table 2) [62]. MYCN appears to be upregulated in 40 % of neuroendocrine prostate cancer cases and approximately 5 % of prostate adenocarcinomas [63]. Each of these pathways presents a potential therapeutic target for the future, and ongoing clinical trials will determine their clinical relevance moving forward. For a more detailed review of androgen/AR-independent mechanisms of escape, we refer the reader to several excellent recent reviews [64, 65].
Table 2.
Androgen/AR-independent mechanisms of resistance
| Substrate | Mechanism of resistance in CRPC |
|---|---|
| Src-1 | Stimulation of MAPK signaling |
| IL-6 | Stimulation of MAPK signaling; Resistance to bicalutamide via upregulation of TIF2 |
| HER2/HER3 | Stablization of AR and increased binding to AREs |
| PTEN loss | Activation of PI3K/Akt signaling pathway |
| Akt/PI3K | Inhibition of AR degradation via increased interaction of AR with p300 |
| EZH2 | Epigenetic silencing of tumor suppressor genes |
| STAT3 | Resistance to enzalutamide; Promotion of PCa stemlike cells |
| c-Met | Enhanced PCa cell proliferation, motility, and invasion |
| RB1 deletion | Increased cell growth via disinhibition of cell-cycle progression |
| TP53 deletion or mutation | Dysregulation of cell division |
| MYCN gain | Dysregulation of some cell proliferation genes |
| AURKA gain | Dysregulation of cell-cycle progression |
| PARP1 overexpression | Disruption of proper DNA damage repair; Disruption of transcriptional regulation |
| IGF1 and FGF | Increased cell growth and proliferation/inhibition of apoptosis via Akt pathway |
DNA repair pathways have also emerged recently as a clinically relevant and exploitable avenue for therapeutic manipulation, and it is becoming increasingly understood that PARP enzymes may have a dual role in DNA damage repair and AR transcriptional regulation [66]. Since prostate cancer cells are rapidly multiplying, they need to upregulate DNA repair enzymes due to the constant stress of proliferation. A recent study showed that patients with defects in certain DNA repair enzymes (including BRCA1/2, ATM, Fanconi's anemia genes, and CHEK2) had an 88 % response rate to the PARP inhibitor olaparib [67••]. These results may lead to biomarker-driven precision medicine trials using biopsies to determine treatment selection. This study has led to a subsequent phase II trial in mCRPC patients who have failed taxane chemotherapy (NCT01682772; Table 1). As we begin to have a better understanding of this disease's underlying biology, we can develop more rational therapeutics and pragmatic clinical trials.
Conclusions
The resistance mechanisms behind androgen-axis therapies are numerous and our knowledge of them is ever expanding. New agents are entering clinical trials that hope to block AR-signaling even further than the compounds currently approved by the FDA. EPI-506, with its ability to bind the NTD of AR-FL and AR-Vs in the preclinical setting, is currently being tested in a phase I clinical trial (NCT02606123; Table 1). Galeterone has entered a pivotal phase III clinical trial in AR-V7-positive patients and may prove superior to enzalutamide in that population (NCT02438007; Table 1). In regard to the current therapies on the market, our best strategy may be to use biomarkers to determine a patient's ideal opportunity for responsiveness. However, even those patients with response to these therapies will develop progressive disease.
With this in mind, one new approach is to target pathways that are less variable and cannot be mutated in response to therapy, perhaps using immune-directed strategies. To this end, a biomarker-driven immunotherapy trial in prostate cancer is going to test ipilimumab (an anti-CTLA-4 antibody) combined with nivolumab (an anti-PD-1 antibody) in AR-V7–positive CRPC patients (NCT02601014; Table 1). The hypothesis is that the cancers from these patients will have higher mutational burden and will respond better to therapies that help the immune system recognize self from non-self (i.e., cancer). If successful, this trial could change the prostate cancer landscape from that of novel hormonally targeted therapies to one of immunotherapies. Immunotherapy has been successful in many other cancer types, and remains the only systemic therapy that can produce lasting responses after treatment has been completed.
In conclusion, treatment selection using androgen-axis modulators should ideally be guided by biomarkers, including AR-Vs and AR mutations as well as others. The use of such biomarkers (whether derived from biopsies, CTCs, or circulating nucleic acids) promises to play a critical role in determining which patients will respond best to certain therapies. Only by further prospectively validating these biomarkers in the clinic and developing new ones can we create a superior experience for prostate cancer patients.
Abbreviations
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ARE
androgen response element
- AR-FL
full-length androgen receptor
- AR-V
androgen receptor splice variant
- cfDNA
cell-free DNA
- CRPC
castration-resistant prostate cancer
- CTC
circulating tumor cell
- CYP17A1
cytochrome P450 17A1
- DBD
DNA-binding domain
- DHT
dihydrotestosterone
- GR
glucocorticoid receptor
- HSPC
hormone-sensitive prostate cancer
- LBD
ligand-binding domain
- NTD
N-terminal domain
- PFS
progression-free survival
- PR
progesterone receptor
- OS
overall survival
Footnotes
Compliance with Ethical Standards
Conflict of Interest John L. Silberstein and Maritza N. Taylor declare no potential conflicts of interest. Emmanuel S. Antonarakis has served as a paid consultant/advisor for Janssen, Astellas, Sanofi, Dendreon, Essa, and Medivation; he has received research funding from Janssen, Johnson & Johnson, Sanofi, Dendreon, Exelixis, Genentech, Novartis, and Tokai; he is a co-inventor of a technology that has been licensed to Tokai.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Howlander N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. 2015 Available from: http://seer.cancer.gov/csr/1975_2012/
- 2.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–15. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–70. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan A, Dong Y, Zhang H, Qi Y, Balk SP, Sartor O. Castration-resistant prostate cancer: adaptive responses in the androgen axis. Cancer Treat Rev. 2014;40:426–33. doi: 10.1016/j.ctrv.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Gordon V, Bhadel S, Wunderlich W, Zhang J, Ficarro SB, Mollah SA, et al. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol Endocrinol. 2010;24:2267–80. doi: 10.1210/me.2010-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [This article is one of the first studies to show the clinical relevance of GR in prostate cancer, especially in the setting of enzalutamide resistance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3:1030–43. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 12.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 13.Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [This study showed the feasibility and prognostic impact of using cfDNA as a biomarker to analyze androgen receptor copy number variations and mutations.] [DOI] [PubMed] [Google Scholar]
- 15.Romanel A, Tandefelt DG, Conteduca V, Jayaram A, Casiraghi N, Wetterskog D, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–51. doi: 10.1038/nature14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Evaul K, Sharma KK, Chang KH, Yoshimoto J, Liu J, et al. Abiraterone inhibits 3beta-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin Cancer Res. 2012;18:3571–9. doi: 10.1158/1078-0432.CCR-12-0908. [DOI] [PubMed] [Google Scholar]
- 18.Salvi S, Casadio V, Conteduca V, Burgio SL, Menna C, Bianchi E, et al. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer. 2015;112:1717–24. doi: 10.1038/bjc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 alpha-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 alpha/17 beta-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–84. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N, Gaikwad NW, et al. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75:1413–22. doi: 10.1158/0008-5472.CAN-14-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW, et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem Biol Interact. 2015;234:332–8. doi: 10.1016/j.cbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearn JWD, AbuAli G, Magi-Galluzzi C, Reddy CA, Chang KH, Klein EA, et al. HSD3B1 and resistance to androgen deprivation therapy in prostate cancer. J Clin Oncol. 2015;33(suppl 7) doi: 10.1016/S1470-2045(16)30227-3. abstr 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwegyir-Afful AK, Senthilmurugan R, Purushottamachar P, Ramamurthy VP, Njar VC. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget. 2015;6:27440–60. doi: 10.18632/oncotarget.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Hong SZ, Lin EJ, Wang DY, Li ZJ, Chen LI. Amplification and protein expression of androgen receptor gene in prostate cancer cells: fluorescence hybridization analysis. Oncol Lett. 2015;9:2617–22. doi: 10.3892/ol.2015.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 28.Steketee K, Timmerman L, Ziel-van der Made AC, Doesburg P, Brinkmann AO, Trapman J. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002;100:309–17. doi: 10.1002/ijc.10495. [DOI] [PubMed] [Google Scholar]
- 29.Chen EJ, Sowalsky AG, Gao S, Cai C, Voznesensky O, Schaefer R, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–80. doi: 10.1158/1078-0432.CCR-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathkopf DE, Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, et al. AACR Annual Meeting. 15 suppl. Vol. 75. Cancer Res; Philadelphia, PA: 2015. Androgen receptor mutations in patients with castration-resistant prostate cancer with and without prior abiraterone acetate treatment. abstr CT134. [Google Scholar]
- 31.Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fizazi K, Massard C, Bono P, Jones R, Kataja V, James N, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15:975–85. doi: 10.1016/S1470-2045(14)70240-2. [DOI] [PubMed] [Google Scholar]
- 33.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [This study is one of the most comprehensive genomic analyses of castration-resistant prostate cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33:3140–50. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu Y, Dai B, Ye D, Kong Y, Chang K, Jia Z, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. doi: 10.1038/srep07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [This study showed that AR-V7 might be a clinically relevant biomarker for enzalutamide and abiraterone resistance that can be tested from CTCs through non-invasive means.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinestel J, Luedeke M, Arndt A, Schnoeller TJ, Lennerz JK, Wurm C, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2015 doi: 10.18632/oncotarget.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, Eisenberger MA, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–65. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scher HI, Fizazi K, Saad F, Chi KN, Taplin ME, Sternberg CN, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide, an androgen receptor inhibitor. J Clin Oncol. 2013;31(suppl 6) abstr 6. [Google Scholar]
- 47.Xie N, Cheng H, Lin D, Liu L, Yang O, Jia L, et al. The expression of glucocorticoid receptor is negatively regulated by active androgen receptor signaling in prostate tumors. Int J Cancer. 2015;136:E27–38. doi: 10.1002/ijc.29147. [DOI] [PubMed] [Google Scholar]
- 48.Storlie JA, Buckner JC, Wiseman GA, Burch PA, Hartmann LC, Richardson RL. Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone-refractory meta-static prostate carcinoma. Cancer. 1995;76:96–100. doi: 10.1002/1097-0142(19950701)76:1<96::aid-cncr2820760114>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura K, Nonomura N, Yasunaga Y, Takaha N, Inoue H, Sugao H, et al. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer. 2000;89:2570–6. doi: 10.1002/1097-0142(20001215)89:12<2570::aid-cncr9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 50.Shamash J, Powles T, Sarker SJ, Protheroe A, Mithal N, Mills R, et al. A multi-centre randomised phase III trial of Dexamethasone vs Dexamethasone and diethylstilbestrol in castration-resistant prostate cancer: immediate vs deferred Diethylstilbestrol. Br J Cancer. 2011;104:620–8. doi: 10.1038/bjc.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkitaraman R, Thomas K, Huddart RA, Horwich A, Dearnaley DP, Parker CC. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 2008;101:440–3. doi: 10.1111/j.1464-410X.2007.07261.x. [DOI] [PubMed] [Google Scholar]
- 52.Miyahira AK, Simons JW, Soule HR. The 20th Annual Prostate Cancer Foundation Scientific Retreat report. Prostate. 2014;74:811–9. doi: 10.1002/pros.22808. [DOI] [PubMed] [Google Scholar]
- 53.Grindstad T, Andersen S, Al-Saad S, Donnem T, Kiselev Y, Nordahl Melbo-Jorgensen C, et al. High progesterone receptor expression in prostate cancer is associated with clinical failure. PLoS One. 2015;10:e0116691. doi: 10.1371/journal.pone.0116691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mateo J, Nowakowska K, Jayaram A, Rodrigues DN, Riisnaes R, Zukiwski A, et al. Prostate Cancer Foundation Scientific Retreat. Carlsbad, CA: 2014. Phase 1 study of onapristone, a progesterone receptor (PR) antagonist, in castration-resistant prostate cancer. abstract 60. [Google Scholar]
- 55.Zukiwski A, Bosq J, Gilles EM, Belldegrun A. Progesterone receptor (PR), a potential mechanism of resistance and target in AIPC. Prostate Cancer Foundation Scientific Retreat. 2014 abstract 47. [Google Scholar]
- 56.Mateo J, Rodrigues DN, Lopez RP, Flohr P, Riisnaes R, Lokiec FM, et al. A phase 1–2 study of the type I progesterone receptor (PR) antagonist, onapristone, in patients with advanced castration-resistant prostate cancer.. J Clin Oncol; ASCO Annual Meeting; 2014; abstract TPS5097. [Google Scholar]
- 57.Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890–903. doi: 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mithal P, Allott E, Gerber L, Reid J, Welbourn W, Tikishvili E, et al. PTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancer. Int J Urol. 2014;21:1209–14. doi: 10.1111/iju.12571. [DOI] [PubMed] [Google Scholar]
- 59.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–89. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–6. doi: 10.1126/science.aab0917. [This study was the first to accomplish single-cell RNA sequencing and uncovered a new potential mechanism of enzalutamide resistance related to non-canonical Wnt signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–68. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosquera JM, Beltran H, Park K, MacDonald TY, Robinson BD, Tagawa ST, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beltran H. The N-myc oncogene: maximizing its targets, regulation, and therapeutic potential. Mol Cancer Res. 2014;12:815–22. doi: 10.1158/1541-7786.MCR-13-0536. [DOI] [PubMed] [Google Scholar]
- 64.Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67:470–9. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng FY, de Bono JS, Rubin MA, Knudsen KE. Chromatin to clinic: the molecular rationale for PARP1 inhibitor function. Mol Cell. 2015;58:925–34. doi: 10.1016/j.molcel.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [This study showed the first potential genetic signature predicting response to the PARP inhibitor, olaparib, in men with CRPC.] [DOI] [PMC free article] [PubMed] [Google Scholar]