DEFINITION OF NAFLD
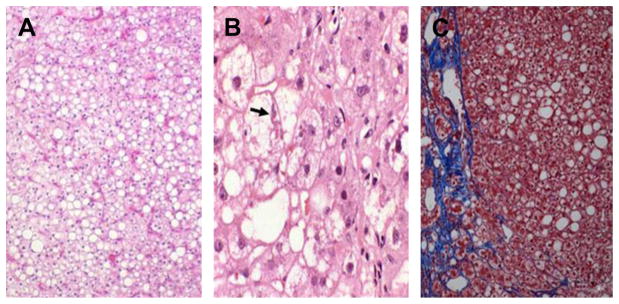
Hepatic steatosis is defined as fat deposition within hepatocytes (Fig. 1). It is seen microscopically as vacuoles in a microvesicular or macrovesicular distribution. Micro-vesicular steatosis is characterized by multiple small, fat vesicles distributed throughout the hepatocyte, whereas macrovesicular steatosis is characterized by a large droplet of fat within the cytoplasm, which pushes the nucleus to the edge of the cell. Although microvesicular steatosis is more commonly seen in Reye syndrome and other forms of mitochondrial injury, nonalcoholic fatty liver disease (NAFLD) typically has macrovesicular distribution of fat deposits. Several drugs can lead to both forms of hepatic steatosis. Although the triggering events differ, each of these insults can lead to excessive hepatic fat deposition, increased reactive oxygen species (ROS) formation, mitochondrial dysfunction, and Endoplasmic Reticulum (ER) stress that induces inflammation, cell death and eventually leads to fibrosis.
Fig. 1.
Histologic appearance of hepatic steatosis and steatohepatitis. (A) Simple steatosis. Vacuoles represent areas of fat accumulation (hematoxylin-eosin). (B) Steatohepatitis demonstrating ballooning degeneration (arrow) and fat deposition (hematoxylin-eosin). (C) Steatohepatitis with hepatic perisinusoidal fibrosis (Prussian blue staining). Original magnification 200×.
Traditionally, NAFLD is defined as fat infiltration in the liver parenchyma in people who do not consume alcohol in quantities that are considered to be hepatotoxic. NAFLD is a manifestation of the metabolic syndrome and it is often associated with obesity, dyslipidemia, and type 2 diabetes mellitus.1,2 With the spread of the obesity epidemic, the disease burden of NAFLD is increasing, both in terms of geography and the age of presentation. It is now the most common cause of chronic liver disease in the United States, affecting about a third of the population.3,4
There are 2 principal phenotypes of NAFLD: (1) nonalcoholic fatty liver (NAFL) and (2) nonalcoholic steatohepatitis (NASH). NAFL is defined by the presence of steatosis without inflammation. NASH is defined by the presence of steatosis, inflammation and hepatocyte ballooning injury.2 NASH progresses to cirrhosis in up to a fifth of patients. Apart from this, several cases of cryptogenic cirrhosis are attributed to NAFLD.2–6
DIAGNOSING DRUG-INDUCED STEATOHEPATITIS
Drug-induced liver injury (DILI) is diagnosed in a person when worsening of the baseline liver function is caused by prescription or nonprescription drugs. The diagnosis of DILI requires consideration of the following:
The biochemical and histologic pattern of liver injury
Lead time between the initiation of the suspected drug and the onset of liver disease
Evidence of improvement of liver function after discontinuation of the drug
The diagnosis of DILI is challenging in many cases because there is no specific maker for DILI. The literature is sparse for newer agents and herbal products. Moreover, the clinical presentation and microscopic appearance of liver injury may be nonspecific and rechallenge is not safe. Hence, several scoring systems have been developed to objectively diagnose DILI, such as the Roussel-Uclaf Causality Assessment Method (RUCAM), the Maria and Victorino method, and the Naranjo scale.7 RUCAM is the most commonly used instrument for the diagnosis of DILI. According to RUCAM, DILI is most likely when it develops within 90 days of the initiation of the drug and improves within 15 to 30 days of discontinuation in cases of hepatocellular and cholestatic patterns of injury, respectively. Drug-induced steatohepatitis may occur after many months of use and may not resolve within 15 days.8,9 RUCAM is, thus, suboptimal for the diagnosis of drug-induced steatohepatitis. The situation is further complicated by a high prevalence of NAFLD in the general population. Hence, even if the disease is previously undiagnosed, several patients have risk factors associated with NAFLD, making it difficult to differentiate drug-induced steatohepatitis from de novo NAFLD. It is possible that drugs may exacerbate preexisting NAFLD.10,11
PATHOGENESIS OF DRUG-INDUCED STEATOHEPATITIS
Mitochondrial Structure: Link Between β-oxidation of Fatty Acids and Fuel Synthesis
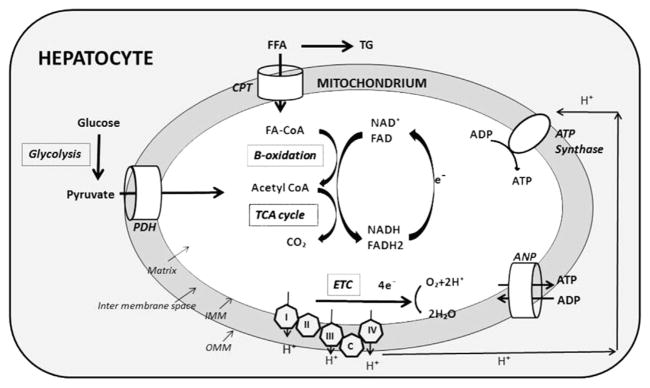
Mitochondria are double-membrane bound organelles involved in several biochemical reactions, including lipid metabolism and ATP synthesis Fig. 2. The outer mitochondrial membrane surrounds the intermembrane space, and the inner mitochondrial membrane (IMM) encloses the mitochondrial matrix. The mitochondrial DNA (mtDNA) is located within the mitochondrial matrix. Respiratory chain complexes I, II, III, and IV and ATP synthase are partially embedded in the IMM. Mitochondrial pyruvate dehydrogenase transforms the pyruvate coming from glucose into acetyl coenzyme A (CoA). Long-chain fatty acids (LCFA) are transported inside the mitochondrial matrix via the carnitine shuttle as long-chain fatty acyl-CoA (LCFA-CoA). Each cycle of β-oxidation yields one molecule of acetyl-CoA. β-oxidation, conversion of pyruvate to acetyl CoA by the pyruvate dehydrogenase complex, and metabolism of acetyl-CoA by the tricarboxylic acid cycle produce NADH and FADH2. These molecules are, in turn, oxidized by transferring their electrons to the mitochondrial respiratory chain. As electrons flow through the electron transport chain (ETC), 3 protons from the mitochondrial matrix are pushed into the intermembrane space at the level of complexes I, III, and IV. This process increases the electrochemical gradient across the IMM. In an energy-depleted state, ADP enters the mitochondrial matrix via the adenine nucleotide translocator (ANT). The increased transmembrane potential allows ADP to drag along protons, which results in the generation of ATP via ATP synthase. ATP can then leave the mitochondrion via the ANT. These processes are well coordinated and ultimately result in the production of oxygen, water, and ATP. However, at the level of complexes I and III, electrons can interact directly with protons and result in the production of ROS.10,12
Fig. 2.
Normal mitochondrial function: long-chain fatty acids (FFA) enter mitochondria via the carnitine palmitoyl shuttle (CPT) and form a complex with coenzyme A (CoA). They undergo β-oxidation, which yields one molecule of acetyl CoA per cycle. Acetyl CoA is also formed from pyruvate via the pyruvate dehydrogenase complex (PDH). Acetyl CoA is finally metabolized to carbon dioxide and water in the tricarboxylic Acid (TCA) cycle. β-oxidation, synthesis of acetyl CoA, and TCA cycle lead to the formation of reduced NAD+ and FAD, which are oxidized by releasing electrons. Electrons low through the electron transport chain (ETC) release protons and result in production of water. In energy-depleted state, ADP enters the mitochondrial matrix by the adenine nucleotide transporter (ANP). Protons are used in synthesis of ATP from ADP by ATP synthase. ATP can then leave the mitochondrium via the ANP. IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane.
Mechanisms of Hepatic Steatosis
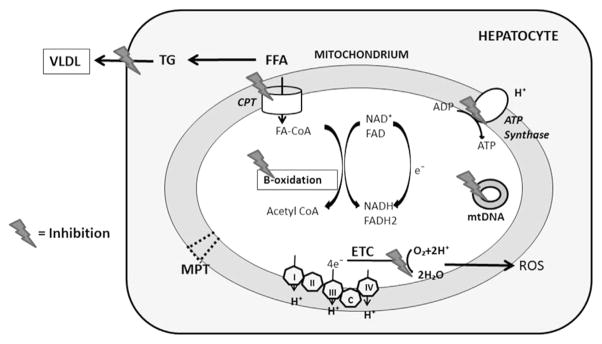
Hepatic steatosis in NAFLD results predominantly from a combination of increased dietary intake, peripheral lipolysis, and de novo fatty acid synthesis Fig. 3.13 Drugs that lead to steatosis and steatohepatitis primarily interfere with mitochondrial respiration, β-oxidation, or both.14 It is important to understand that the ETC and β-oxidation of fatty acids are metabolically inter-connected. Hence, drugs affecting one pathway invariably inhibit the other.15 When hepatic mitochondrial β-oxidation is severely inhibited, the impairment of fatty acyl-CoA β-oxidation increases fatty acyl-CoA and nonesterified fatty acids, which are converted into triglycerides resulting in hepatic steatosis.12 Besides inducing steatosis, the inhibition of β-oxidation and ETC results in increased ROS formation and, in more severe cases, hepatic necrosis.14,16–19
Fig. 3.
Mechanisms of hepatic steatosis and injury in drug-induced steatohepatitis. Inhibition of entry of long-chain fatty acids (FFA) via the carnitine palmitoyl shuttle (CPT) and inhibition of β-oxidation lead to increased free fatty acids, which are esterified into triglycerides. Transport of triglycerides (TG) as very low-density lipoprotein (VLDL) can be blocked by some drugs. Blocking the flow of electrons through the ETC leads to accumulation of electrons. These electrons can directly interact with oxygen to produce ROS. Certain drugs directly damage mtDNA and can induce mitochondrial permeability transition (MPT) pore formation.
Drugs that inhibit mitochondrial β-oxidation do so by several mechanisms:
Inhibition of entry of LCFA into the mitochondrial matrix, which is seen with the antidiabetic drug troglitazone, which inhibits mitochondrial acyl-CoA synthase20
Sequestration of CoA in the form of a drug-CoA thioester as seen in valproate toxicity21
Inhibition of enzymes catalyzing β-oxidation, for example, glucocorticoids inhibit acyl-CoA dehydrogenases10,22
Rarely, other mechanisms that result in increased hepatic fat are involved. Some drugs can lead to increased synthesis or decreased secretion of hepatic triglycerides as seen with protease inhibitors and dexamethasone, respectively.23,24 De novo fatty acid synthesis is transiently increased by some antipsychotic medications by an increase in the active form of sterol regulatory element binding protein-1c (SREBP-1c).25
Mechanisms of Steatohepatitis
The causes of the progression of steatosis to steatohepatitis in DILI are not well understood and mostly extrapolated from NASH literature.10–12 One of the most-studied hypothesis is that mitochondrial dysfunction leads to and worsens drug-induced uncoupling of β-oxidation and phosphorylation, which results in the production of ROS. Reduced energy availability and direct damage form ROS, in turn, induce hepatocyte necrosis.12 ROS cause additional damage by interaction with nonesterified polyunsaturated fatty acids to produce lipid peroxidation products, which have a longer half-life than ROS and cause damage by diffusing to surrounding cells. ROS also lead to nuclear translocation of the transcription factor, nuclear factor-κβ (NF-κβ). NF-κβ upregulates genes that promote insulin resistance26 and several inflammatory cytokines, including tumor necrosis factor (TNF)–α 25; interleukin (IL)-8, which promotes polymorphonuclear (PMN) infiltration26; and transforming growth factor (TGF)-β. TGF-β, in turn, stimulates hepatic stellate cell proliferation and fibrosis.16,26 NF-κβ also promotes apoptotic cell death by inducing transcriptional expression of the normally repressed fatty acid synthetase (FAS)-ligand. FAS ligand binds to FAS on adjacent hepatocytes, leading to caspase-9 activation. Caspase-9 then activates other caspases to promote apoptotic hepatocyte cell death.16
PATHOLOGIC SPECTRUM OF DRUG-INDUCED STEATOSIS AND STEATOHEPATITIS
Drugs implicated in hepatic steatosis can be divided on basis of hepatic microscopy into those that cause microvesicular steatosis and those that predominantly lead to macrovesicular steatosis (Table 1). Characteristics and mechanism of injury by individual drugs are described later.
Table 1.
Drugs causing steatosis and steatohepatitis
| Drug | Common Liver Pathology | Mechanism of Liver Injury |
|---|---|---|
| Aspirin | Microvesicular steatosis | Arrest B oxidation by consuming CoA; increase mitochondrial permeability by MPT pore formation |
| Valproate | Microvesicular steatosis | Inhibit CPT-1 activity, sequestering CoA; arresting ETC and ATP synthesis; promotes weight gain |
| Cocaine | Microvesicular steatosis | Undergoes n-oxidation to produce hepatotoxic products and inhibition of β-oxidation |
| NRITs (zidovudine and didanosine) | Microvesicular steatosis | Mitochondrial dysfunction by mtDNA depletion and stimulation of autophagy |
| Tetracycline | Microvesicular steatosis | Inhibit β-oxidation of fatty acids and VLDL secretion |
| NSAIDs (ibuprofen and naproxen) | Microvesicular steatosis | Inhibit β-oxidation of short- and medium-chain fatty acids |
| Glucocorticoids | Macrovesicular steatosis | Promote weight gain and glucose intolerance; inhibit mitochondrial β-oxidation, decrease hepatic triglyceride secretion, and induce lipid peroxidation |
| Amiodarone | Macrovesicular steatosis, steatohepatitis, and phospholipidosis | Decreases β-oxidation of fatty acids; increases lipid peroxidation, ROS generation, and lipogenesis |
| 5-fluorouracil | Macrovesicular steatosis | Unknown |
| Irinotecan | Steatohepatitis | Unknown |
| Methotrexate | Macrovesicular steatosis and steatohepatitis | Inhibits mitochondrial electron transport chain |
| Tamoxifen | Macrovesicular steatosis and steatohepatitis | Promotes de novo fatty acid synthesis and inhibits fatty acid β-oxidation |
| Petrochemicals (eg vinyl chloride) | Microvesicular steatosis and steatohepatitis | Unknown |
Abbreviations: CPT, carnitine palmitoyl shuttle; MPT, mitochondrial permeability transition; NRITs, nucleoside reverse transcriptase inhibitors; NSAIDs, nonsteroidal antiinflammatory drugs; VLDL, very low-density lipoprotein.
Drugs Causing Microvesicular Steatosis
Aspirin
Aspirin is metabolized to salicylic acid and then salicyl-CoA. In aspirin poisoning, this process leads to excessive utilization of CoA, thus blocking the entry of LCFA entry into mitochondria and arresting β-oxidation.27 Aspirin can also directly uncouple respiration. It promotes mitochondrial permeability transition pore formation that leads to mitochondrial death, which triggers cell death by apoptosis and necrosis.28 Aspirin use in children with viral infections has been associated with Reye syndrome. The pathogenesis of Reye syndrome involves widespread arrest of β-oxidation, increased ureagenesis and ketogenesis, and severe hypoglycemia caused by the inability to convert lactate to glucose. Diffuse hepatic microvesicular steatosis is seen in this rapidly fatal disease.19 Since the 1980s, the incidence of Reye syndrome in developed countries has significantly declined.
Cocaine
Cocaine-induced hepatotoxicity ranges from hepatic steatosis (both microvesicular and macrovesicular) in milder cases to centrilobular necrosis in more severe ones. Inflammation is characteristically sparse.29 Hepatic n-oxidation of cocaine and its metabolites leads to progressively more hepatotoxic products.30 A recent lipidomic analysis of mice livers has revealed that cocaine use leads to the inhibition of hepatic β-oxidation of fatty acids, which contributes to the accumulation of hepatic triglycerides, long-chain acylcarnitines, and phospholipids.31
Valproate
Valproate is a branched-chain fatty acid that causes microvesicular steatosis and cirrhosis. In one report, up to 60% of patients treated with valproate had ultrasound evidence of hepatic steatosis.32 Valproate is initially metabolized by cytochrome P-450 enzymes to 4-ene-valproate. Both valproate and 4-ene-valproate then form complexes with CoA, sequestering CoA as well as competitively inhibiting carnitine palmitoyl shuttle I activity. Valproate can release protons and, thus, arrest the ETC and ATP synthesis.21 Besides mitochondrial dysfunction, with prolonged use, valproate promotes weight gain and systemic insulin resistance, which may lead to worsening of the underlying NAFLD.32
Nucleoside reverse transcriptase inhibitors, thymidine analogues
Although hepatotoxicity can be seen with all groups of HIV antiretroviral therapy, nucleoside reverse transcriptase inhibitors (NRTIs) have been associated with hepatic steatosis. With prolonged use, thymidine analogues, zidovudine and didanosine (but not the cytidine analogue lamivudine) can lead to hepatic microvesicular steatosis and steatohepatitis. A few cases of acute liver failure have been reported.33,34 These drugs deplete mtDNA and stimulate autophagy, which leads to ROS formation and further worsening of mitochondrial function.33–35 NRTI-related hepatic steatosis is more common in obese patients and women.36 Hence, it is plausible that by inhibition of autophagy, these drugs may worsen and/or unmask underlying NAFLD.35,37
Tetracycline
Intravenous tetracyclines were discontinued in 1991 because of reports of rapid, fulminant, and often fatal hepatotoxicity. Histopathology of tetracycline injury is characterized by generalized microvesicular injury. Tetracyclines inhibit the secretion of hepatic fat as very low-density lipoprotein by inhibiting microsomal triglyceride transfer protein. They also inhibit the mitochondrial β-oxidation of fatty acids.38
Nonsteroidal antiinflammatory drugs (ibuprofen, naproxen)
Nonsteroidal antiinflammatory drugs (NSAIDS) are a leading cause of hepatotoxicity. NSAIDs can cause both cholestatic and hepatocellular patterns of liver injury and, in severe cases, lead to acute liver failure. Only a few NSAIDs have been reported to induce hepatic steatosis. Naproxen and ibuprofen are commonly used NSAIDs in the United States that can lead to microvesicular steatosis. The proposed mechanism is inhibition β-oxidation of short- and medium-chain fatty acids.39,40
Drugs Causing Macrovesicular Steatosis and Steatohepatitis
Most drugs leading to macrovesicular steatosis can also cause steatohepatitis to a varying degree. One exception to this is 5-fluorouracil (5-FU) because its use is associated with isolated macrovesicular steatosis. Individual drugs leading to macrovesicular steatosis and steatohepatitis are summarized later.
Drugs with true cause-effect relationship with steatosis and steatohepatitis
Amiodarone
Amiodarone is a potent antiarrhythmic agent that, over prolonged use, causes several adverse effects, including liver dysfunction; pulmonary fibrosis; neurotoxicity; ocular complications; and, because it is structurally similar to thyroxin, thyroid dysfunction.41,42 These adverse effects are seen in up to 80% of patients taking the drug. Twenty percent to 40% of patients need to discontinue its use because of the adverse effects.42 In some reports, up to 30% of patients taking the drug have an acute elevation of liver enzymes, usually within 24 hours of intravenous infusion. Liver enzymes may be up to 1.5 to 4.0 times the upper limit of normal even in asymptomatic patients.41 Although liver enzyme abnormalities are benign in about a fourth of the patients, 1% to 2% develop symptomatic disease in the form of steatohepatitis. Other more aggressive patterns of injury, including extensive hepatocellular necrosis, Reye syndrome–like illness, and cholestatic hepatitis, have also been reported.41,43,44 With chronic use, amiodarone is concentrated in the liver and can be visualized on imaging studies. With prolonged use, its hepatic levels can be 100 to 500 times higher than serum. Chronic liver injury caused by amiodarone is a function of its cumulative dose. Hence, steatohepatitis can be seen with low daily doses.10,22 The histopathologic appearance of amiodarone-induced hepatotoxicity is similar to classic NASH. Mallory hyaline deposits and neutrophil infiltration with steatosis may be seen. Some patients develop a distinct pattern of lipid deposition inside lysosomes leading to foamy-appearing hepatocytes and Kupffer cells. This condition is referred to as phospholipidosis, and it can be seen in the absence of steatohepatitis.45,46
Amiodarone promotes several enzymes involved in de novo fatty acid synthesis, including SREBP-1c, FAS, and ATP citrate lyase.47 It can inhibit β-oxidation of LCFA by blocking their mitochondrial entry via the carnitine shuttle and by inhibiting long-chain acyl-CoA dehydrogenase.48,49 It arrests mitochondrial respiration by inhibiting enzymes of the ETC as well as by direct inhibition of electron transport by its benzofuran structure.50 It is important to note that because of hepatic concentration and long half-life, amiodarone hepatotoxicity not only takes time to resolve but can also occasionally manifest after drug discontinuation.44
Diethylamioethoxyhexestrol and perhexiline maleate
Perhexiline maleate (Pexid) and diethylamioethoxyhexestrol (Coralgil) caused steatohepatitis and phospholipidosis.51–54 Both drugs have been removed from the market in the United States.
Chemotherapy-associated steatohepatitis
Irinotecan, 5-FU, and oxaliplatin, along with the biologic agents cituximab (Erbitux) and bevacizumab (Avastin), have improved the survival of patients with colorectal cancer with metastasis.55–57 When used before surgery, they can downsize the tumor and allow resection in carefully selected patients who otherwise have incurable disease.58,59 However, the use of these agents has proved to be challenging because they cause steatosis, steatohepatitis, and sinusoidal obstruction syndrome, collectively referred to as chemotherapy-induced liver injury (CALI). 5-FU causes isolated hepatic steatosis. Steatohepatitis is seen following treatment with irinotecan and is referred to as chemotherapy-associated steatohepatitis. Oxaliplatin has been shown to cause sinusoidal obstruction syndrome.60,61 According to one large study, steatosis involving more than 30% of the hepatocytes was seen in more than 46% of patients and steatohepatitis in about 20% of patients who underwent neoadjuvant chemotherapy for colorectal liver metastasis.62 A recent consensus statement by the International Hepato-Pancreato-Biliary Association noted that hepatic steatosis and steatohepatitis are associated with poor postoperative outcomes, including slower regeneration and increased mortality.63 CALI interferes with decreases the accuracy of the preoperative assessment of metastasis. It has been associated with poor surgical outcomes, such as longer operating time, longer hospital stay, postoperative infections, and perioperative hemorrhage. Liver failure leading to portal hypertension and ascites is possible because of poor functional reserve. However, there is evidence that shows no change in outcomes in patients with isolated hepatic steatosis. Another study has shown worse outcomes to be a function of the amount of resection and blood loss rather than the degree of steatosis.64,65 The problem in interpreting these studies is that it is unclear if those patients who got the drug were somehow different from those who did not. A lack of prechemotherapy data biopsy for diagnoses and the higher prevalence of NAFLD add to the difficulty of establishing causality.63 The mechanism of hepatic fat accumulation and liver injury induced by these drugs remains to be elucidated.
Drugs leading to worsening of underlying NAFLD
Methotrexate
Methotrexate is a folate antagonist. It is used as a chemotherapeutic agent and as an immunosuppressant in the treatment of rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Methotrexate toxicity increases with cumulative doses. Liver pathology ranges from simple steatosis, mild portal inflammation, and focal necrosis to more severe forms of injury, including extensive necrosis, fibrosis, and cirrhosis. Methotrexate can independently cause steatohepatitis and lead to worsening of underlying NASH.66–68 The risk of developing liver disease with methotrexate use is higher in those with underlying liver disease. The American Association of Dermatology’s 2009 guidelines on methotrexate use in psoriasis recommend a liver biopsy after cumulative doses of 3.5 to 4.0 g in patients with no underlying liver disease or risk factors.66 Methotrexate targets mitochondrial respiration to induce steatohepatitis, and scarring may be caused by its effect on the canals of Hering.69,70
Tamoxifen
Tamoxifen is a selective estrogen receptor modulator widely used for the treatment of patients with breast cancer. Several forms of liver injury, both acute and chronic, have been reported with tamoxifen use. Among these, hepatic steatosis and steatohepatitis are the most commonly seen on microscopic examination.71,72 Hepatic steatosis develops within 2 years of therapy in patients with breast cancer who are treated with tamoxifen. Overall, about a third of patients develop steatosis.73,74 Rapid improvement in both steatosis and steatohepatitis is seen with drug withdrawal.73,75 Several of these patients are obese and have other risk factors for metabolic syndrome. Hence, it has been suggested that tamoxifen may accelerate the development of NAFLD.71–76 The mechanisms reported include the promotion of de novo fatty acid synthesis and impairment of fatty acid β-oxidation.11,77
Corticosteroids
Glucocorticoids are used widely as immunosuppressants in a variety of autoimmune diseases. Their use over the long-term commonly leads to weight gain, dyslipidemia, and glucose intolerance. Hence, as expected, glucocorticoids lead to steatosis and steatohepatitis by the worsening of metabolic syndrome. Glucocorticoids also inhibit mitochondrial β-oxidation, decrease hepatic triglyceride secretion, and induce the peroxidation of lipids, thus independently causing steatohepatitis.24,78
MANAGEMENT OF PATIENTS WITH DRUG-INDUCED STEATOHEPATITIS
There are no guidelines and there is little evidence from controlled clinical trials that can be applied in the management of patients with drug-induced steatohepatitis. As in other forms of DILI, if the implicated drug has already been discontinued at the time of diagnosis, it should not be reintroduced because of the risk of developing more aggressive liver injury. However, if patients are still on the drug, stop the drug whenever possible and consider alternative forms of therapy, if available. If not, the risks and benefits of continuing the drug versus stopping it should be carefully weighed. The patients’ background history and risk factors for NAFLD should be reviewed. If no risk factors for NAFLD can be identified, then steatosis/steatohepatitis in those patients can be exclusively attributed to the drug. In such scenarios, stopping the drug should be favored. Current literature supports that steatosis and steatohepatitis both improve after stopping the implicated drug. Liver enzymes and imaging should be used to confirm improvement after stopping the drug. Magnetic resonance imaging (MRI) is more specific than sonography as a marker of hepatic steatosis. The information gained by an MRI may aid in future drug development and to guide therapy in other patients on that particular drug.
SUMMARY
Steatohepatitis is a complex disease with several possible etiologic factors. The clinicopathological picture varies depending on the genetic makeup of an individual and the contributing environmental factors, including nutrition excess and exposure to toxins, such drugs and alcohol. In the real world, several of these exposures are usually present simultaneously in the same individual. Hence, classifying steatohepatitis by the cause, such as alcoholic liver disease, NAFLD, or drug-induced steatohepatitis, creates false barriers that may not allow for a unifying diagnosis in an individual patient. Nevertheless, an understanding of each contributing factor adds to our knowledge into the pathogenesis of the disease and can help us in developing individualized diagnostic and therapeutic tools.
KEY POINTS.
Hepatic steatosis and steatohepatitis can arise from the interplay of several inciting factors, including alcohol, drugs, and metabolic syndrome as nonalcoholic fatty liver disease (NAFLD).
Drugs induce fat deposition in the liver in microvesicular or macrovesicular distribution.
Most drugs implicated in steatosis and steatohepatitis can induce both to a variable extent.
It is difficult to ascertain whether an implicated drug leads to de novo steatosis and/or steatohepatitis versus worsening of underlying NAFLD.
The pathogenesis of drug-induced steatohepatitis often involves mitochondrial dysfunction.
Footnotes
Disclosure: This work has been supported by the NIH T32 Training Grant.
References
- 1.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Dam-Larsen S, Franzmann M, Andersen IB, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–5. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Diagnosing steatohepatitis and predicting liver-related mortality in patients with NAFLD: two distinct concepts. Hepatology. 2011;53:1792–4. doi: 10.1002/hep.24403. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–9. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs–II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 9.Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2003;7:435–51. doi: 10.1016/s1089-3261(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 11.Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185–94. doi: 10.1055/s-2002-30106. [DOI] [PubMed] [Google Scholar]
- 12.Pessayre D, Fromenty B, Berson A, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–54. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 15.Watmough NJ, Bindoff LA, Birch-Machin MA, et al. Impaired mitochondrial beta-oxidation in a patient with an abnormality of the respiratory chain. Studies in skeletal muscle mitochondria. J Clin Invest. 1990;85:177–84. doi: 10.1172/JCI114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297–305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Saudubray JM, Martin D, de Lonlay P, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 18.Fromenty B, Grimbert S, Mansouri A, et al. Hepatic mitochondrial DNA deletion in alcoholics: association with microvesicular steatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 19.Kimura S, Kobayashi T, Tanaka Y, et al. Liver histopathology in clinical Reye syndrome. Brain Dev. 1991;13:95–100. doi: 10.1016/s0387-7604(12)80114-8. [DOI] [PubMed] [Google Scholar]
- 20.Fulgencio JP, Kohl C, Girard J, et al. Troglitazone inhibits fatty acid oxidation and esterification, and gluconeogenesis in isolated hepatocytes from starved rats. Diabetes. 1996;45:1556–62. doi: 10.2337/diab.45.11.1556. [DOI] [PubMed] [Google Scholar]
- 21.Eadie MJ, Hooper WD, Dickinson RG. Valproate-associated hepatotoxicity and its biochemical mechanisms. Med Toxicol Adverse Drug Exp. 1988;3:85–106. doi: 10.1007/BF03259935. [DOI] [PubMed] [Google Scholar]
- 22.Goldman IS, Winkler ML, Raper SE, et al. Increased hepatic density and phospholipidosis due to amiodarone. AJR Am J Roentgenol. 1985;144:541–6. doi: 10.2214/ajr.144.3.541. [DOI] [PubMed] [Google Scholar]
- 23.Lenhard JM, Croom DK, Weiel JE, et al. HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol. 2000;20:2625–9. doi: 10.1161/01.atv.20.12.2625. [DOI] [PubMed] [Google Scholar]
- 24.Letteron P, Brahimi-Bourouina N, Robin MA, et al. Glucocorticoids inhibit mitochondrial matrix acyl-CoA dehydrogenases and fatty acid beta-oxidation. Am J Physiol. 1997;272:G1141–50. doi: 10.1152/ajpgi.1997.272.5.G1141. [DOI] [PubMed] [Google Scholar]
- 25.Lauressergues E, Staels B, Valeille K, et al. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:427–39. doi: 10.1007/s00210-010-0499-4. [DOI] [PubMed] [Google Scholar]
- 26.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deschamps D, Fisch C, Fromenty B, et al. Inhibition by salicylic acid of the activation and thus oxidation of long chain fatty acids. Possible role in the development of Reye’s syndrome. J Pharmacol Exp Ther. 1991;259:894–904. [PubMed] [Google Scholar]
- 28.Oh KW, Qian T, Brenner DA, et al. Salicylate enhances necrosis and apoptosis mediated by the mitochondrial permeability transition. Toxicol Sci. 2003;73:44–52. doi: 10.1093/toxsci/kfg045. [DOI] [PubMed] [Google Scholar]
- 29.Wanless IR, Dore S, Gopinath N, et al. Histopathology of cocaine hepatotoxicity. Report of four patients. Gastroenterology. 1990;98:497–501. doi: 10.1016/0016-5085(90)90845-r. [DOI] [PubMed] [Google Scholar]
- 30.Roberts SM, Harbison RD, James RC. Human microsomal N-oxidative metabolism of cocaine. Drug Metab Dispos. 1991;19:1046–51. [PubMed] [Google Scholar]
- 31.Shi X, Yao D, Gosnell BA, et al. Lipidomic profiling reveals protective function of fatty acid oxidation in cocaine-induced hepatotoxicity. J Lipid Res. 2012;53:2318–30. doi: 10.1194/jlr.M027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luef GJ, Waldmann M, Sturm W, et al. Valproate therapy and nonalcoholic fatty liver disease. Ann Neurol. 2004;55:729–32. doi: 10.1002/ana.20074. [DOI] [PubMed] [Google Scholar]
- 33.Brivet FG, Nion I, Megarbane B, et al. Fatal lactic acidosis and liver steatosis associated with didanosine and stavudine treatment: a respiratory chain dysfunction? J Hepatol. 2000;32:364–5. doi: 10.1016/s0168-8278(00)80088-4. [DOI] [PubMed] [Google Scholar]
- 34.Chariot P, Drogou I, de Lacroix-Szmania I, et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatol. 1999;30:156–60. doi: 10.1016/s0168-8278(99)80020-8. [DOI] [PubMed] [Google Scholar]
- 35.Stankov MV, Panayotova-Dimitrova D, Leverkus M, et al. Autophagy inhibition due to thymidine analogues as novel mechanism leading to hepatocyte dysfunction and lipid accumulation. AIDS. 2012;26:1995–2006. doi: 10.1097/QAD.0b013e32835804f9. [DOI] [PubMed] [Google Scholar]
- 36.Osler M, Stead D, Rebe K, et al. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 2010;11:121–9. doi: 10.1111/j.1468-1293.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 37.Neuman MG, Schneider M, Nanau RM, et al. HIV-antiretroviral therapy induced liver, gastrointestinal, and pancreatic injury. Int J Hepatol. 2012;2012:760706. doi: 10.1155/2012/760706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letteron P, Sutton A, Mansouri A, et al. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38:133–40. doi: 10.1053/jhep.2003.50309. [DOI] [PubMed] [Google Scholar]
- 39.Freneaux E, Fromenty B, Berson A, et al. Stereoselective and nonstereoselective effects of ibuprofen enantiomers on mitochondrial beta-oxidation of fatty acids. J Pharmacol Exp Ther. 1990;255:529–35. [PubMed] [Google Scholar]
- 40.Victorino RM, Silveira JC, Baptista A, et al. Jaundice associated with naproxen. Postgrad Med J. 1980;56:368–70. doi: 10.1136/pgmj.56.655.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis JH, Ranard RC, Caruso A, et al. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9:679–85. doi: 10.1002/hep.1840090504. [DOI] [PubMed] [Google Scholar]
- 42.Podrid PJ. Amiodarone: reevaluation of an old drug. Ann Intern Med. 1995;122:689–700. doi: 10.7326/0003-4819-122-9-199505010-00008. [DOI] [PubMed] [Google Scholar]
- 43.Kalantzis N, Gabriel P, Mouzas J, et al. Acute amiodarone-induced hepatitis. Hepatogastroenterology. 1991;38:71–4. [PubMed] [Google Scholar]
- 44.Chang CC, Petrelli M, Tomashefski JF, Jr, et al. Severe intrahepatic cholestasis caused by amiodarone toxicity after withdrawal of the drug: a case report and review of the literature. Arch Pathol Lab Med. 1999;123:251–6. doi: 10.5858/1999-123-0251-SICCBA. [DOI] [PubMed] [Google Scholar]
- 45.Guigui B, Perrot S, Berry JP, et al. Amiodarone-induced hepatic phospholipidosis: a morphological alteration independent of pseudoalcoholic liver disease. Hepatology. 1988;8:1063–8. doi: 10.1002/hep.1840080514. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JH, Mullick F, Ishak KG, et al. Histopathologic analysis of suspected amiodarone hepatotoxicity. Hum Pathol. 1990;21:59–67. doi: 10.1016/0046-8177(90)90076-h. [DOI] [PubMed] [Google Scholar]
- 47.Antherieu S, Rogue A, Fromenty B, et al. Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology. 2011;53:1895–905. doi: 10.1002/hep.24290. [DOI] [PubMed] [Google Scholar]
- 48.Fromenty B, Fisch C, Labbe G, et al. Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J Pharmacol Exp Ther. 1990;255:1371–6. [PubMed] [Google Scholar]
- 49.Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol. 1996;52:273–80. doi: 10.1016/0006-2952(96)00204-3. [DOI] [PubMed] [Google Scholar]
- 50.Fromenty B, Fisch C, Berson A, et al. Dual effect of amiodarone on mitochondrial respiration. Initial protonophoric uncoupling effect followed by inhibition of the respiratory chain at the levels of complex I and complex II. J Pharmacol Exp Ther. 1990;255:1377–84. [PubMed] [Google Scholar]
- 51.Pessayre D, Mansouri A, Haouzi D, et al. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15:367–73. doi: 10.1023/a:1007649815992. [DOI] [PubMed] [Google Scholar]
- 52.Pessayre D, Bichara M, Degott C, et al. Perhexiline maleate-induced cirrhosis. Gastroenterology. 1979;76:170–7. [PubMed] [Google Scholar]
- 53.Kubo M, Hostetler KY. Metabolic basis of diethylaminoethoxyhexestrol-induced phospholipid fatty liver. Am J Physiol. 1987;252:E375–9. doi: 10.1152/ajpendo.1987.252.3.E375. [DOI] [PubMed] [Google Scholar]
- 54.Le Gall JY, Guillouzo A, Glaise D, et al. Perhexiline maleate toxicity on human liver cell lines. Gut. 1980;21:977–84. doi: 10.1136/gut.21.11.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 56.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 57.Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997;350:681–6. doi: 10.1016/s0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- 58.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–57. doi: 10.1097/01.sla.0000141198.92114.f6. discussion: 657–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–20. doi: 10.1097/00000658-199610000-00009. discussion: 520–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentilucci UV, Santini D, Vincenzi B, et al. Chemotherapy-induced steatohepatitis in colorectal cancer patients. J Clin Oncol. 2006;24:5467. doi: 10.1200/JCO.2006.08.1828. author reply: 5467–8. [DOI] [PubMed] [Google Scholar]
- 61.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–6. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 62.Brouquet A, Benoist S, Julie C, et al. Risk factors for chemotherapy-associated liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery. 2009;145:362–71. doi: 10.1016/j.surg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Schwarz RE, Berlin JD, Lenz HJ, et al. Systemic cytotoxic and biological therapies of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:106–15. doi: 10.1111/j.1477-2574.2012.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho JY, Suh KS, Kwon CH, et al. Mild hepatic steatosis is not a major risk factor for hepatectomy and regenerative power is not impaired. Surgery. 2006;139:508–15. doi: 10.1016/j.surg.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–44. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–85. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Langman G, Hall PM, Todd G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol. 2001;16:1395–401. doi: 10.1046/j.1440-1746.2001.02644.x. [DOI] [PubMed] [Google Scholar]
- 68.Berends MA, van Oijen MG, Snoek J, et al. Reliability of the Roenigk classification of liver damage after methotrexate treatment for psoriasis: a clinicopathologic study of 160 liver biopsy specimens. Arch Dermatol. 2007;143:1515–9. doi: 10.1001/archderm.143.12.1515. [DOI] [PubMed] [Google Scholar]
- 69.Hytiroglou P, Tobias H, Saxena R, et al. The canals of Hering might represent a target of methotrexate hepatic toxicity. Am J Clin Pathol. 2004;121:324–9. doi: 10.1309/5HR9-0TNC-4Q4J-RXWX. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto N, Oliveira MB, de Campello AP, et al. Methotrexate: studies on the cellular metabolism. I. Effect on mitochondrial oxygen uptake and oxidative phosphorylation. Cell Biochem Funct. 1988;6:61–6. doi: 10.1002/cbf.290060110. [DOI] [PubMed] [Google Scholar]
- 71.Ogawa Y, Murata Y, Nishioka A, et al. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351:725. doi: 10.1016/S0140-6736(05)78493-2. [DOI] [PubMed] [Google Scholar]
- 72.Pratt DS, Knox TA, Erban J. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1995;123:236. doi: 10.7326/0003-4819-123-3-199508010-00018. [DOI] [PubMed] [Google Scholar]
- 73.Murata Y, Ogawa Y, Saibara T, et al. Unrecognized hepatic steatosis and non-alcoholic steatohepatitis in adjuvant tamoxifen for breast cancer patients. Oncol Rep. 2000;7:1299–304. doi: 10.3892/or.7.6.1299. [DOI] [PubMed] [Google Scholar]
- 74.Bruno S, Maisonneuve P, Castellana P, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishino M, Hayakawa K, Nakamura Y, et al. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180:129–34. doi: 10.2214/ajr.180.1.1800129. [DOI] [PubMed] [Google Scholar]
- 76.Saphner T, Triest-Robertson S, Li H, et al. The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer. 2009;115:3189–95. doi: 10.1002/cncr.24374. [DOI] [PubMed] [Google Scholar]
- 77.Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 2010;52:1258–65. doi: 10.1002/hep.23813. [DOI] [PubMed] [Google Scholar]
- 78.Letteron P, Fromenty B, Terris B, et al. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–8. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]